Abstract
Rationale
De-differentiation of vascular smooth muscle cells (VSMC) leading to a proliferative cell phenotype significantly contributes to the development of atherosclerosis. Mitogen activated protein kinase (MAPK) phosphorylation of proteins including connexin 43 (Cx43) has been associated with VSMC proliferation in atherosclerosis.
Objective
To investigate whether MAPK phosphorylation of Cx43 is directly involved in VSMC proliferation.
Methods and Results
We show in vivo that MAPK phosphorylated Cx43 forms complexes with the cell cycle control proteins cyclin E and CDK2 in carotids of apolipoprotein-E receptor null (ApoE−/−) mice and in C57Bl/6 mice treated with platelet-derived growth factor–BB (PDGF). We tested the involvement of Cx43 MAPK phosphorylation in vitro using constructs for full length Cx43 (Cx43) or the Cx43 C-terminus (Cx43CT) and produced null phosphorylation Ser>Ala (Cx43MK4A/ Cx43CTMK4A) and phospho-mimetic Ser>Asp (Cx43MK4D/Cx43CTMK4D) mutations. Co-immunoprecipitation studies in primary VSMC isolated from Cx43 wild type (Cx43+/+) and Cx43 null (Cx43−/−) mice and analytical size exclusion studies of purified proteins identify that interactions between cyclin E and Cx43 requires Cx43 MAPK phosphorylation. We further demonstrate that Cx43 MAPK phosphorylation is required for PDGF mediated VSMC proliferation. Finally using a novel knock-in mouse containing Cx43-MK4A mutation we show in vivo that interactions between Cx43 and cyclin E are lost and VSMC proliferation does not occur following treatment of carotids with PDGF and that neointima formation is significantly reduced in carotids following injury.
Conclusions
We identify MAPK phosphorylated Cx43 as a novel interacting partner of cyclin E in VSMC and show that this interaction is critical for VSMC proliferation. This novel interaction may be important in the development of atherosclerotic lesions.
Keywords: connexin 43, phosphorylation, atherogenesis, platelet derived growth factor, vascular smooth muscle cells, proliferation, neointima
Introduction
Vascular smooth muscle cell (VSMC) proliferation is a major component of disease progression in atherosclerosis and in its treatment, e.g. following angioplasty and in restenosis.1–3 In the earliest stages of atherosclerosis (atherogenesis) VSMC de-differentiate in response to atherogenic stimuli e.g. oxidized phospholipids and growth factors, such as platelet derived growth factor-BB (PDGF).4, 5 De-differentiated VSMC increase atherosclerotic plaque size by proliferating and migrating into the plaque, followed by foam cell formation and senescence and through ongoing cellular proliferation within the plaque.3, 6, 7 Treatment of advanced plaques through angioplasty produces the unwanted side effect of stimulating further VSMC proliferation, leading to neointima formation and significantly reducing the vessel lumen.6, 8 Advanced plaques are composed of a number of different cell types including macrophages and foam cells derived from both monocytes and from VSMC originating in the medial layers of arterial vessels.9–11 Therefore, identifying the causes of VSMC proliferation becomes central in the control of atherosclerosis and its treatments.
Several lines of evidence suggest that connexins (Cx) play a key role in the regulation of atherosclerotic disease progression and VSMC proliferation in particular Cx43, which has a role in cellular proliferation.12–16 Connexins typically form gap junctions through a dodecameric association of connexin proteins which produces a functional channel between two cells that allows for coordinated cellular responses. Changes in the expression levels of Cx43 have been associated with VSMC proliferative potential in atherogenesis and in atherosclerotic plaque development and in restenosis.12, 15, 17 In addition, multiple studies have now demonstrated that Cx43 can modulate cellular proliferation in a manner that is independent of gap junctional communication.18–21 However, the exact pathways through which Cx43 modulates cellular proliferation are not known.
Activation of mitogen activated protein kinase (MAPK) pathways promotes VSMC proliferation in atherogenesis22. Many pro-atherogenic agents (e.g. PDGF) have been shown to activate MAPK pathways, yet the mechanisms through which these pathways induce VSMC proliferation are poorly defined.5 Several lines of evidence indicate that MAPK phosphorylation of Cx43 can occur at specific C-terminus (CT) residues i.e. S255/S262/S279/S282 and occurs differentially throughout the cell cycle.23–27
Recently, we identified that Cx43 becomes phosphorylated at its MAPK serines in response to atherogenic stimuli and that this is associated with VSMC proliferation both in vivo and in vitro.12 Here we demonstrate from the single protein level to whole mouse that Cx43 mediates its control over VSMC proliferation through an interaction of its C-terminus with the cell cycle control protein cyclin E in a manner that is dependent upon Cx43 MAPK phosphorylation.
Materials and Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Mice
C57/Bl6 (10–18 week), ApoE−/− (15–18 week) and novel Cx43-MK4A (10–18 week) mice were used according to the University of Virginia Animal Care and Use Committee guidelines.
Electron microscopy
Carotid arteries were imaged for VSMC morphology and immunolabeled using Cx43, cyclin E or cyclin dependant kinase 2 (CDK2) antibodies and detected with 25/ 15/ 10 nm gold beads respectively and imaged using a JEOL 6400 scanning electron microscope.
Plasmids and mutagenesis
Derivatives of pCDNA3.1 and pGEX-6P-2 for expression of full length Cx43 (Cx43, a.a.1–382, pCDNA3.1), the Cx43 C-terminus (Cx43CT, rat, a.a. 236–382, pCDNA3.1 and pGEX-6P-2), and for full length human cyclin E (pCDNA3.1) were used. Mutagenesis of Cx43 MAPK serines to either alanines or aspartates was performed by Quickchange (Strategene). Plasmids were used for in vitro transfection or analysis of protein interaction by analytical size exclusion chromatography (ANSEC).
Statistical analysis
Student T-test, 1-way or 2-way ANOVA followed by Bonferroni’s post-test were used for comparisons between treatments. A P value of <0.05 was significant.
Results
Cx43 co-localizes with cyclin E in VSMC and in vivo
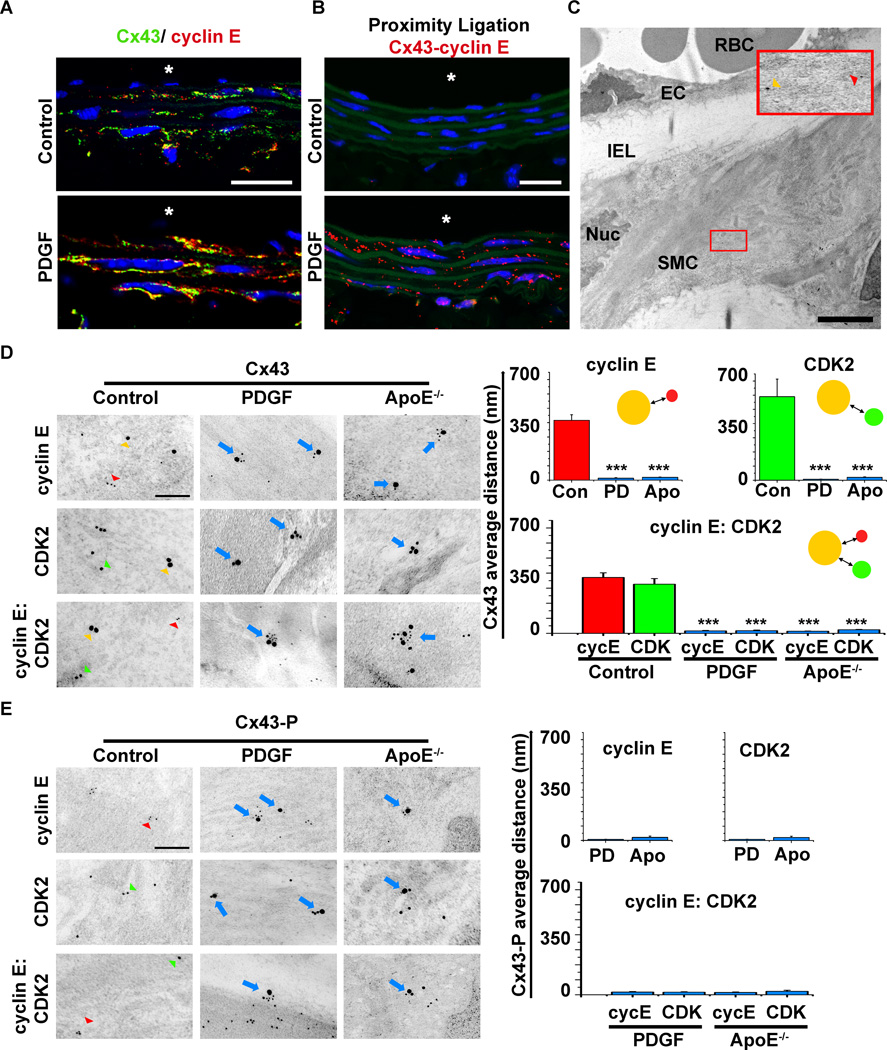
To test whether Cx43 protein expression and its post-translational modification are linked to VSMC proliferation, we initially screened potential binding partners of Cx43 that are associated with the G1 phase of cell cycle including cyclin E, cyclin D1, p21waf1cip1 and p27kip1. Co-immunoprecipitation studies identified that Cx43 associated with cyclin E but not the other proteins tested (Online Figure V). We therefore tested the ability of Cx43 to interact with cyclin E in vivo by immunofluorescence on carotid vessel sections. We identified marked increases in protein expression for both Cx43 and cyclin E within the carotid VSMC after PDGF treatment as compared to control conditions (Figure 1A). Using the proximity ligation assay we further demonstrated almost no detectable interactions between of Cx43 and cyclin E under control conditions but significant increases in interaction following PDGF treatments (Figure 1B). To determine the spatial interaction between the proteins in vivo we analyzed co-localization between Cx43, cyclin E and CDK2 by immune- transmission electron microscopy (i-TEM, Figure 1C). Under control conditions, interactions were not identifiable between Cx43 and cyclin E or CDK 2 in carotid VSMC (i.e. large average distance between beads of 490±70 nm (Cx43-cyclin E) and 379±72 nm (Cx43-CDK2, Figure 1D) were observed). In VSMC of carotids from PDGF treated mice and in ApoE−/− mice (containing proliferative VSMC)12 significant reductions in distances between Cx43-cyclin E, Cx43-CDK2 and cyclin E-Cx43-CDK2 were identified with distance averaging 10–20 nm indicative of interaction between the proteins (Figure 1D). Under control conditions Cx43 MAPK phosphorylation was not readily detectable with too few beads present as to be quantified on all sections (Figure 1E). However, treatments with PDGF and in ApoE−/− mice (as described)12 increased Cx43 MAPK phosphorylation and interactions were identified with cyclin E and CDK2 (Figure 1E).
Figure 1. In vivo co-localization of Cx43 and cell cycle proteins.
Representative immunofluorescence images show carotid vessels from control or PDGF treated C57Bl/6 mice (A–B). Immunofluorescence of Cx43 (red) and cyclin E (green) with blue indicating nuclei (DAPI), “*” indicates the luminal side of the vessels and the scale bar is 20 µm. Immunofluorescent co-localization of proteins is demonstrated by orange staining (overlay of green and red staining) in each section (A). Immunofluorescence, proximity ligation assay for Cx43 and cyclin E, where red staining indicates co-localization of the proteins within 40nm of each other (B). In B, autofluorescence of the internal elastic lamina (IEL, green) and nuclei (blue), “*” indicates the luminal side of the vessels and the scale bar is 20 µm. Immune-TEM with expanded view (red box) of a control carotid taken from a C57Bl/6 mouse (C). The nucleus (Nuc), smooth muscle cell (SMC), IEL, endothelial cell (EC) and red blood cells (RBC) are identified. In C, the red box highlights the larger 25 nm beads labeling Cx43 (yellow arrowhead) and 15 nm beads for cyclin E (red arrowhead) in VSMC layers, bar represents 2 µm. Representative higher magnification iTEM images in D and E of carotids taken from control or PDGF treated C57Bl/6 or ApoE−/− mice immuno-detected for Cx43 or Cx43-P (as labeled, 25 nm gold beads, yellow arrowhead), cyclin E (polyclonal, 8–10 nm gold beads red arrowhead) or CDK2 (monoclonal, 15 nm beads, green arrowhead). Co-localization demonstrated in representative images by the blue arrows. In the corresponding graphs average distance between beads are shown as Cx43 to cyclin E (red bars) or Cx43 and CDK2 (green bars) or Blue bars where interactions are being measured for control (Con), PDGF (PD) or ApoE−/− (Apo) mice. In E, Cx43-P expression is only shown for PDGF and ApoE−/− mice as it is not detected in controls, therefore no statistical significance was determined. In D and E, “***” indicates P<0.001 as compared to controls (n=20 interactions), the scale bar is 200 nm.
Cx43 co-precipitates with cyclin E and CDK2 in vitro
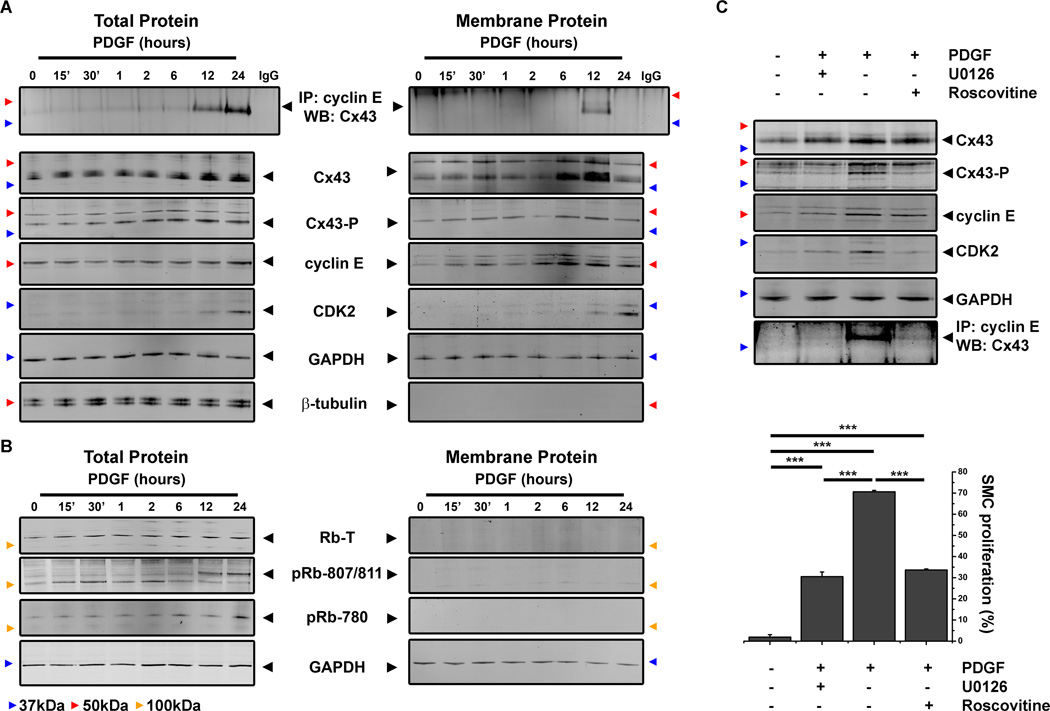
To further investigate the interaction between Cx43, cyclin E and CDK2, we isolated aortic VSMC from newborn mice (Cx43+/+, Online Figure II). Increases in Cx43, cyclin E and CDK2 protein expression and co-immunoprecipitation of Cx43 with cyclin E and CDK2 proteins were identified following 24 hour treatments with PDGF (Online Figure VI). To define the cellular compartment of protein interaction and to identify the temporal relationship between Cx43 and cyclin E interactions we investigated protein expression levels and co-immunoprecipitation by Western blot in total proteins lysates and membrane fractions of Cx43+/+ VSMC. Following PDGF treatments, expression of Cx43, MAPK phosphorylated Cx43 (Cx43-P) and cyclin E all increased following 6 hours of treatment with PDGF in total protein lysates, with increases in CDK2 observed from 12 hours (Figure 2A). In membrane fractions, expression of Cx43, Cx43-P and cyclin E increased between 6–12 hour timepoints after which marked reductions were observed (Figure 2A). Co-immunoprecipitation studies demonstrated that Cx43 and cyclin E form in complex by 12 hours in membrane fractions and were detectable at both 12 and 24 hours in total protein lysates (Figure 2A). To determine the effects on downstream targets of cyclin E and CDK2 activation, we performed Western blots of retinoblastoma (Rb) protein expression and its phosphorylation (pRB). Following PDGF treatments we identified in Rb protein expression and phosphorylation (pRb-780 and pRb-807/811) was increased between 12–24 hours following PDGF treatments (Figure 2B). In order to define whether MAPK phosphorylation of Cx43 was critical in this interaction, Cx43+/+ VSMC gown in low serum media were pre-treated with Extracellular signal-regulated protein kinase (ERK)-inhibitors U0126 or Roscovitine followed by PDGF treatment. Both U0126 and Roscovitine inhibited the ability of Cx43 to become phosphorylated at MAPK serines and inhibited interactions with cyclin E and corresponded to significant reductions in VSMC proliferation (Figure 2C). Using Cx43+/+ cells treated with PDGF in the presence of U0126 or Roscovitine, we saw significant reductions in VSMC proliferation indicating that MAPK phosphorylation of Cx43 is important in the proliferative phenotype of VSMC in response to PDGF (Figure 2C).
Figure 2. In vitro identification of Cx43 co-localization with cell cycle proteins.
Total protein and membrane fraction lysates from Cx43+/+ VSMC grown in low serum and treated with PDGF harvested were at specific timepoints over a 24 hours timecourse. Samples were analyzed by Western blot with antibodies against Cx43, Cx43-P, cyclin E, CDK2, GAPDH or β-tubulin and by co-immunoprecipitation with cyclin E coated beads and detection of Cx43 by Western Blot (A). Specificity of Western blot detection was shown using antibody coated beads treated with lysis buffer only to eliminate IgG contamination (A). Expression of Rb, phosphorylated retinoblastoma (pRb)-780, and pRb-807/811 were detected in total protein and membrane fraction lysates from PDGF timecourse experiments (B). Total protein lysates from Cx43+/+ VSMC grown in low serum, pre-treated with ERK inhibitors (U0126 and Roscovitine) or DMSO then treated with PDGF for 24 hours were analyzed by Western blot with antibodies against Cx43, Cx43-P, cyclin E or CDK2 and by co-immunoprecipitation with cyclin E coated beads and detection of Cx43 by Western Blot (C, upper panel). VSMC proliferation of Cx43+/+ VSMC grown in low serum, pre-treated with ERK inhibitors (U0126 and Roscovitine) or DMSO then treated with PDGF for 24 hours was assayed by flow cytometry (C, lower panel). In A–C, black arrowheads identify the expected molecular weight for each protein, colored arrowheads correspond to molecular weights: green is 25kDa, blue is 37 kDa, red is 50kDa, yellow is 100 kDa. In C, “***” indicates P<0.001, n=4.
Cx43 interactions with cyclin E are controlled through MAPK phosphorylation
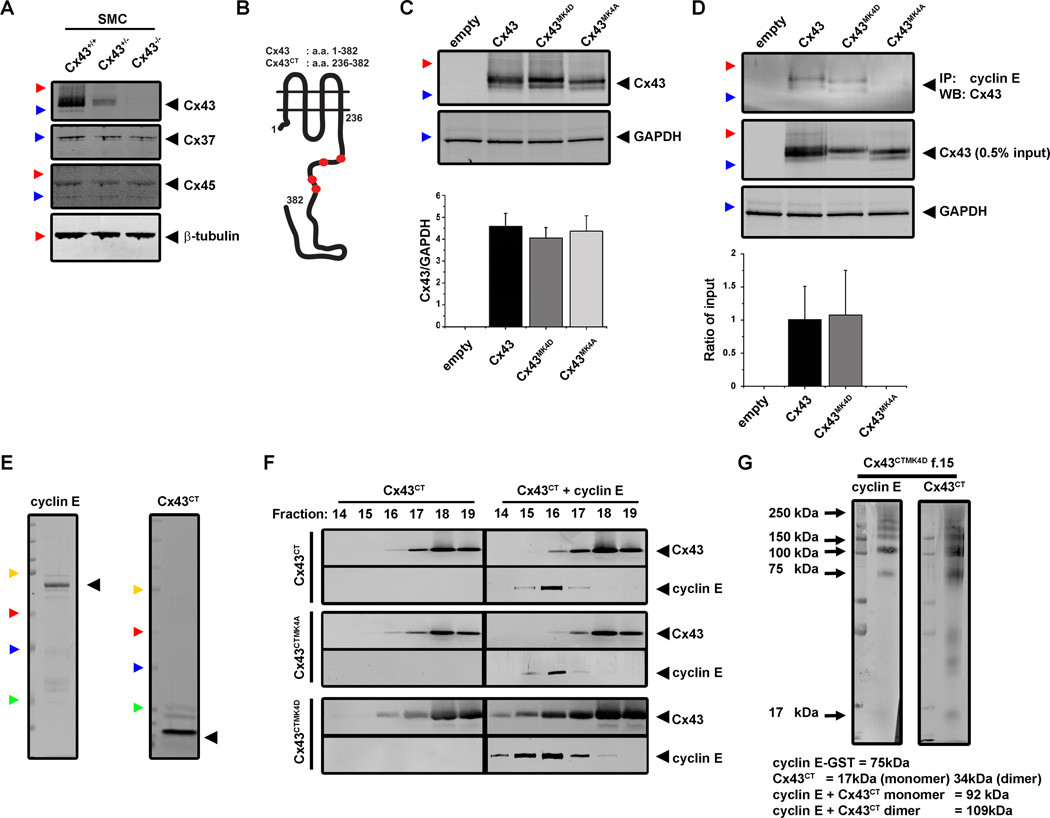
In order to further examine the role of Cx43 MAPK phosphorylation, VSMC were isolated from aortas of newborn Cx43+/+, Cx43+/− or Cx43−/− mice (Figure 3A) and we generated plasmids with mutations at the four C-terminus MAPK serines (S255/262/279/282) to mimic constitutive phosphorylation (Cx43MK4D) or null phosphorylation (Cx43MK4A) (Figure 3B, Online Figure IV). These plasmids were transfected into Cx43−/− VSMC at nearly equivalent levels (Figure 3C). Dye transfer studies in control and PDGF treated VSMC transfected with the different plasmids showed no significant differences in gap junctional communication (Online Figure VII). Pre-treatment of Cx43+/+ VSMC with the gap junction inhibitor carbenoxolone (CBX) reduced gap junctional communication but did not significantly reduce cellular proliferation in response to PDGF treatments (Online Figure VII) Co-immunoprecipitation studies from transfected Cx43−/− VSMC treated with PDGF identified that interactions with cyclin E only occurred with Cx43 and Cx43MK4D and not in Cx43MK4A (Figure 3D, Online Table I).
Figure 3. Cx43 interactions with cyclin E are dependent on MAPK phosphorylation in vitro.
The expression of vascular connexins Cx43, Cx37 and Cx45 in VSMC isolated from newborn mouse aortas was identified by Western blotting (A). Site directed mutagenesis was performed for the MAPK sites in both full length Cx43 (a.a.1–382) and Cx43CT (a.a. 236–382) for phospho-mimetic (Aspartate, Cx43MK4D, Cx43CTMK4D) and null phosphorylation (Cx43MK4A, Cx43CTMK4A) constructs (B). Primary VSMC from Cx43−/− mice were transfected with Cx43 plasmids and expression confirmed by Western blotting of Cx43 (Cx43) (C). Levels of expression were quantified against loading for GAPDH (n=3, C). Lysates from Cx43−/− VSMC transfected with each of the Cx43 plasmids and treated with PDGF were incubated with cyclin E coated beads then protein detection performed by Western blot analysis of Cx43 (n=3, D). Cx43CT-GST and cyclin E1-GST proteins were purified by GST bead purification followed by cleavage (Cx43CT proteins only) or elution (glutathione, cyclin E only). Samples were further purified to >90% purity by size exclusion chromatography (SEC) with Cx43CT detected at approximately 17 kDa and cyclin E at 75 kDa due to the addition of the 25kDa GST tag (E). Purified proteins for Cx43CT, Cx43CTMK4A, Cx43CTMK4D and cyclin E were assessed for in vitro binding via ANSEC either as solo proteins or in combination (Cx43CT + cyclin E). Eluted fractions were analyzed for the expression of Cx43 (Cx43) or cyclin E (polyclonal) by Western blotting (n=2, F). Following ANSEC analysis, samples from elution fraction 15 from the Cx43CTMK4D + cyclin E samples were cross-linked using BS3 followed by Western blot analysis (G). In C–E, black arrowheads identify the expected molecular weight for each protein, colored arrowheads correspond to molecular weights: green is 25kDa, blue is 37 kDa, red is 50kDa, Yellow is 100 kDa.
In order to show that Cx43 C-terminus specifically binds cyclin E, we generated and used purified Cx43 C-terminus and glutathione s-transferase (GST) tagged cyclin E proteins and evaluated complex formation using ANSEC (Figure 3E–F). Individually, Cx43 C-terminus proteins (≈ 17 kDa; monomer) eluted in the same fractions (16–19) and cyclin E (≈ 75 kDa) eluted in fractions 15–17 (Figure 3F). In all experiments, we did not detect significant alterations in either the Cx43CT or the Cx43CTMK4A proteins when combined with cyclin E (Figure 3F). However, following incubation of Cx43CTMK4D proteins with cyclin E, we identified a marked shift in both Cx43CTMK4D and cyclin E. Both proteins co-eluted earlier in the elution profile (fractions 14 – 17; increase in size), indicating complex formation of Cx43CTMK4D with cyclin E (Figure 3F). By cross-linking proteins in solution of fraction 15 from Cx43CTMK4D with cyclin E samples we identified high molecular weight forms of both Cx43 C-terminus and cyclin E at ≈ 110 kDa by Western Blot, corresponding to a stoichiometry of a single cyclin E and Cx43CTMK4D in dimer form (≈ 109 kDa, Figure 3G).
Based on the observations using purified proteins we aimed to determine whether expressing the free Cx43 C-terminus in VSMC would lead to binding of cyclin E and alterations in cellular proliferation. We therefore transfected Cx43−/− VSMC with plasmids to express Cx43CT, Cx43CTMK4A and Cx43CTMK4D proteins (Online Figure VIII). All 3 proteins were found to be expressed in the transfected VSMC but did not form interactions with cyclin E and did not confer increased VSMC proliferation in response to PDGF treatment (Online Figure VIII). We further determined that while all Cx43 C-terminus proteins were expressed, they did not traffic to the cellular membranes unlike full length Cx43 proteins when transfected to Cx43−/− VSMC (Online Figure VIII).
VSMC proliferation is controlled through MAPK phosphorylation of Cx43
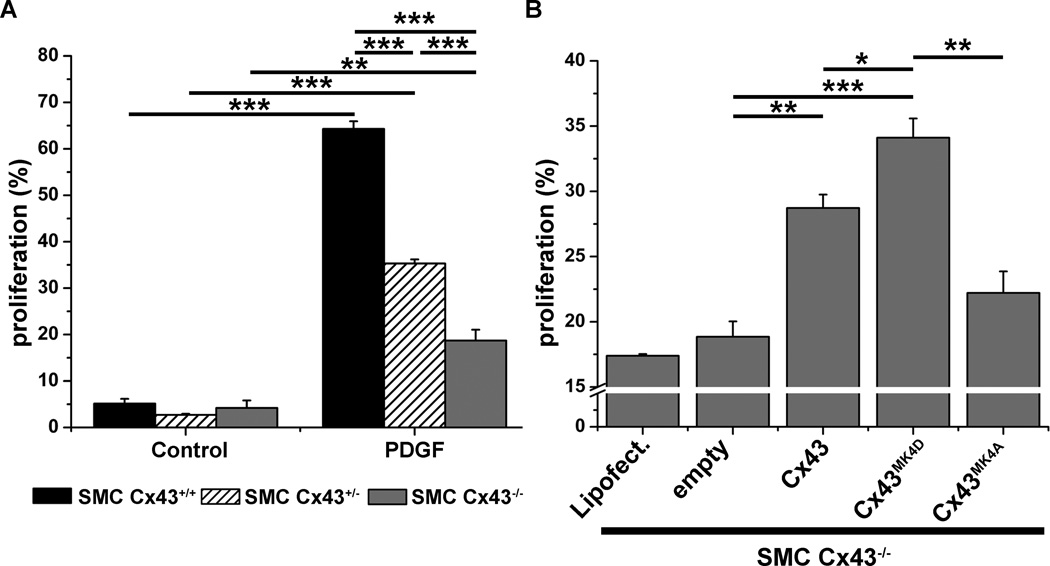
To identify a potential functional consequence of Cx43-cyclin E interaction, we compared proliferation in Cx43+/+ Cx43+/− and Cx43−/− VSMC grown in low serum media in response to PDGF stimulation. Flow cytometric analysis indicated that low serum induced a cell cycle stall (approximately 5% proliferation) in all cells. However, loss or reductions of Cx43 (Cx43−/− Cx43+/− respectively) significantly ablates PDGF induced VSMC proliferation as compared to Cx43+/+ cells (Figure 4A). Western blot analysis of cyclin E and CDK2 expression in Cx43−/− VSMC demonstrate that PDGF does not induce marked increases in cyclin E expression, however CDK2 protein expression was increased by 24 hours in the absence of Cx43 expression (Online Figure IX). Transfection of Cx43−/− VSMC with each the Cx43 plasmids followed by treatment with PDGF produced a restoration of cell proliferation in cells expressing both Cx43 and the Cx43MK4D proteins, but not the Cx43MK4A protein (Figure 4B).
Figure 4. Cx43 MAPK phosphorylation is critical in VSMC proliferation in vitro.
VSMC isolated from Cx43+/+, Cx43+/− and Cx43−/− mice were grown in low serum media for 72 hours then treated with EDU (control) or EDU + PDGF (PDGF) and VSMC proliferation measured as a percentage of total cells via flow cytometry (A). Cx43−/− VSMC were transfected with controls (lipofectamine and empty vector) or with the Cx43 plasmids (Cx43, Cx43MK4A, Cx43MK4D). Cells were then treated with EDU + PDGF and VSMC proliferation measured as a percentage of total cells via flow cytometry (B). In graphs, “*” indicates P<0.05, “**” indicates P<0.01 and “***” indicates P<0.001 (A, n=6 and B, n=3).
Loss of MAPK phosphorylation of Cx43 in vivo reduces interactions with cyclin E and VSMC proliferation
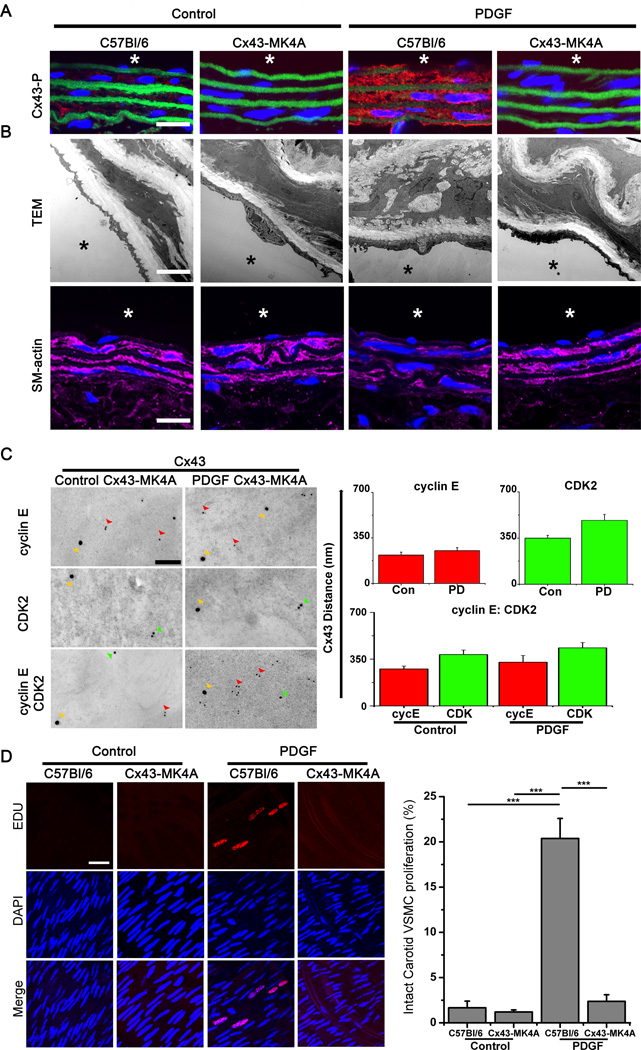
To investigate the in vivo effects of Cx43 phosphorylation on VSMC proliferation in vivo, we generated a novel knock-in mouse line containing alanine mutation for Cx43 MAPK sites (Cx43-MK4A, Online Figure I) which display similar levels of Cx43 expression to C57Bl/6 mice (Online Figure X). In the Cx43-MK4A mice, Cx43 phosphorylation was not detected either under control or PDGF treated conditions, with increases in C57Bl/6 shown (Figure 5A). Analysis by transmission electron microscopy (TEM) and through expression of SM-actin by immunofluorescence, revealed that PDGF treatment did not produce any apparent phenotypic alterations in the carotid VSMC of Cx43-MK4A mice in contrast to those which were identifiable in C57Bl/6 carotid VSMC (Figure 5B, Online Figure X). Analysis of protein co-localization in Cx43-MK4A mice in vivo using i-TEM and immunofluorescence identified a loss of interaction between Cx43 and cyclin E or CDK2 in PDGF treated carotid VSMC of Cx43-MK4A mice (Figure 5C, Online Figure XI). Treatment of C57Bl/6 mouse carotids with PDGF produced significant increases in VSMC proliferation (20.4 ± 4.4 %) but there was no evidence of VSMC proliferation in Cx43-MK4A mice treated with PDGF (Figure 5D).
Figure 5. MAPK phosphorylation of Cx43 is critical in VSMC proliferation in vivo.
Representative immunofluorescence images from control and PDGF treated carotids from C57Bl/6 and Cx43-MK4A mice (A). Phosphorylated Cx43 protein expression (Cx43-P) following PDGF treatments was analyzed by immunofluorescence (A). In each image red represents Cx43-P, green represents autofluorescence from the internal elastic lamina, blue indicates nuclei (DAPI) and “*” represents the luminal side of the vessels, scale bar is 20 µm. In B, (top panel), electron micrographs show the ultrastucture of VSMC layers in the carotids from C57Bl/6 and Cx43-MK4A mice under control or PDGF treated conditions, scale bar is 2 µm. SM-actin (SM-22α) protein expression following PDGF treatments was analyzed by immunofluorescence (B, lower panel). In each image magenta represents SM22-α, blue indicates nuclei (DAPI), “*” represents the luminal side of the vessels and scale bar is 20 µm. In C, representative i-TEM images of carotid VSMC from control or PDGF treated Cx43-MK4A mice show Cx43 (Cx43, 25 nm gold beads, yellow arrows), cyclin E (polyclonal, 8–10 nm gold beads red arrows) or CDK2 (monoclonal, 15 nm beads, green arrows). In the corresponding graphs average distance between beads are shown as Cx43 to cyclin E (red bars) or Cx43 to CDK2 (green bars). No-co-localization was demonstrated for control (Con) or PDGF vessels. In vivo cell proliferation measured EDU incorporation (Red) in the nuclei (Blue, DAPI) of C57Bl/6 and Cx43-MK4A mice under control or PDGF treated conditions. Proliferation of VSMC was measured as the number of nuclei incorporating EDU as compared to total nuclei counted (n=4, D). In D, “***” indicates P<0.001 as compared to controls.
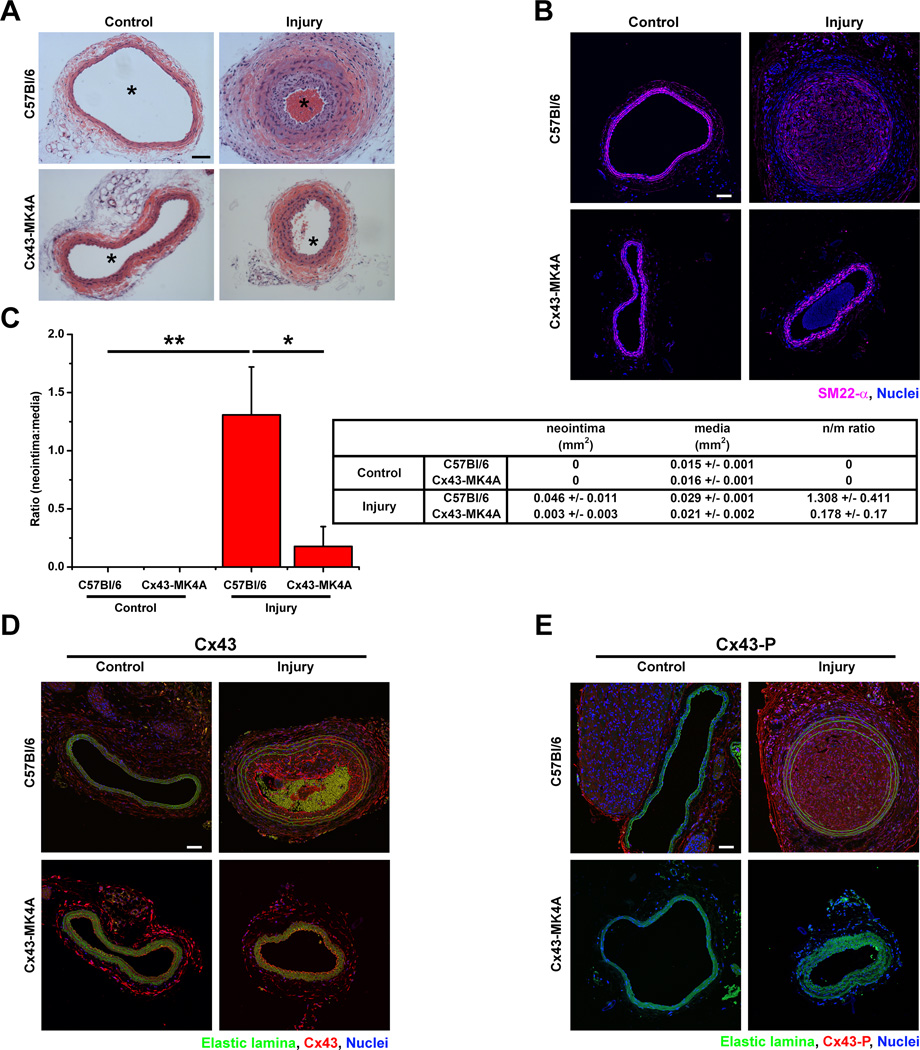
Loss of MAPK phosphorylation of Cx43 in vivo reduces neointima formation
To determine whether phosphorylation of Cx43 plays a significant role in neointimal formation we performed carotid ligation (injury) assays which have been previously demonstrated to produce neointima formation in wild type mice.28 Carotids from ligated C57Bl/6 mice demonstrated significant development of neointima, reduced luminal diameter and reductions in expression of SM-actin in media VSMC which was not identified in Cx43-MK4A mice (Figure 6A–C, Online Figure III). Increases in both Cx43 and MAPK phosphorylated Cx43 were identified in both the media and neointima layers of C57Bl/6 mice but not within in the media layers of Cx43-MK4A mice (Fig 6D–E).
Figure 6. MAPK phosphorylation is required for neointima formation following carotid injury.
Cross sections from C57Bl/6 and Cx43-MK4A mice under control or injury conditions were stained by H&E for analysis of neointima formation (A). Expression of smooth muscle actin (SM22-α, magenta) was detected in media and neointima layers by immunofluorescence (B). Measures for the areas corresponding to neointima and media layers were used to calculate the ratio in control and injured C57Bl/6 and Cx43-MK4A mice (C n=7). Immunofluorescent detection of Cx43 (D) and Cx43-P (E) in control and injured C57Bl/6 and Cx43-MK4A mice. Scale bars in panels A, B, D and E are 50 µm. In C, “*” indicates P<0.05, “**” indicates P<0.01.
Discussion
A common factor in the promotion of VSMC proliferation is phosphorylation of proteins through activation of MAPK pathways.29 We recently identified that MAPK phosphorylation of Cx43 correlates to VSMC proliferation in ApoE−/− mice.12 Here we identify that in response to atherogenic stimuli i.e. PDGF and vascular injury i.e carotid ligation, Cx43 is phosphorylated at its C-terminus MAPK residues (S255/S262/S279/S282)23 and that this is a key regulator of VSMC proliferation and neointma formation in vivo. We further demonstrate that the mechanism underlying the proliferative response depends on a direct interaction of the Cx43 C-terminus with the cell cycle control protein cyclin E. These results are summarized in Online Figure XII.
Vascular smooth muscle cell proliferation in atherogenesis has been linked with an increase in the expression of PDGF.5 It has also been shown that PDGF stimulation increases the expression of cyclin E, cyclin D1 and their associated kinases (i.e. CDK2 and CDK4) all of which are positive regulators of cellular proliferation.30–33 In addition, PDGF alters the expression levels of Cx43 which has been linked to enhanced VSMC proliferation.14 In keeping with these studies, we identified increases in Cx43, cyclin E and cyclin D1 in VSMC in response to PDGF treatments in vitro. Using proliferative VSMC (10% serum) we found that Cx43 forms interactions with cyclin E but not with cyclin D1, or the cell cycle inhibitors p21waf1/cip1 or p27kip1. Treatment of VSMC with PDGF induced MAPK phosphorylation of Cx43 and promoted formation of Cx43 complexes with cyclin E and its associated kinase CDK2. Complexes between MAPK phosphorylated Cx43 with cyclin E and CDK2 were also identified in PDGF treated C57Bl/6 and in ApoE−/− mice as demonstrated through proximity ligation and i-TEM in vivo. These data indicate that Cx43 may act to interact with specific cell cycle proteins in proliferative VSMC.
While cyclin E is ubiquitously expressed in cells, formation of active complexes with CDK2 occur at the cell membrane and is followed by removal of the complex from the cell membrane.34 Accumulation of cyclinE-CDK2 complexes in s-phase of the cell cycle can be further regulated by MAPK activation.35 In our studies we identified that PDGF temporally increases global expression of Cx43, Cx43-P, cyclin E and CDK2 proteins over a 24 hour period, and that Cx43 and cyclin E protein expression increased transiently in membrane fractions with marked reductions found between 12–24 hours. We further show that Cx43-cyclin E complexes were present at 12 hours in membrane fractions and in total protein isolates between 12–24 hours. Increases in Cx43-cyclin E binding corresponded to an increase in the expression and phosphorylation of Rb, a downstream target of the cyclin E-CDK2 complex which acts to promote cellular proliferation.36 Activation of MAPK pathways i.e. ERK1/2, following increased PDGF expression promotes a proliferative phenotype in VSMC.37, 38 Following treatments with known ERK pathway inhibitors i.e. U0126 and CDK2 inhibitors roscovitine35 we show that interactions between Cx43 and cyclin E can be inhibited and VSMC proliferation significantly reduced. Expression of free Cx43CT proteins within Cx43−/− VSMC cells failed to produce an interaction with cyclin E and failed to elicit PDGF induced proliferation as can be seen for the full length protein. In keeping with previous reports that expression of the free Cx43CT leads to a diffuse pattern of staining within cells and does not affect cellular proliferation39 we further show that the free Cx43CT does not target the cell membrane suggesting that membrane localization is a key component in the interaction between Cx43 and cyclin E. However there are previous reports that the free Cx43CT protein expressed in HeLa cells can inhibit cellular proliferation although the pathways associated with this have not been clearly demonstrated.40 These data indicate that PDGF induces proliferation in VSMC through a mechanism that involves binding of MAPK phosphorylated Cx43 to cyclin E and that formation of a complex between Cx43 and cyclin E initiates at the membrane and is followed by an internalization of the complex which promotes retinoblastoma phosphorylation and cell cycle progression.
It has previously been shown that PDGF increases Cx43 expression levels but that PDGF induced MAPK phosphorylation of Cx43 reduces gap junction permeability leading to the hypothesis that gap junctional signaling pathways may contribute regulation of VSMC proliferation.41–43 Similar studies identified that truncation of Cx43 C-terminus (a.a. 256) significantly reduces the PDGF induced response, potentially indicating that gap junctional communication is involved but also suggest that there may also be a connexin mediated (but non-gap junctional) component to PDGF associated cell cycle regulation.44, 45 In agreement with these studies, we find that a loss or reduction of Cx43 expression in Cx43−/− and Cx43+/− VSMC (respectively) correlates to significant reductions in the PDGF induced proliferative response. We also demonstrate a rescue of the proliferative response in Cx43−/− VSMC transfected to express Cx43 and Cx43MK4D. Despite this, levels of gap junction communication were not significantly altered in the Cx43−/− VSMC as compared to Cx43+/+ VSMC. A reduction in dye transfer was observed in Cx43−/− following treatment with CBX indicating that gap junctional communication does occur within Cx43−/− VSMC. The identification of dye transfer in Cx43−/− cells suggests that they are still functionally coupled potentially through the remaining Cx37 and Cx45 identified within the cells. However, treatments with CBX failed to significantly reduce VSMC proliferation suggesting that proliferation can occur in a manner that is independent of gap junctional communication. Additionally when Cx43−/− VSMC were transfected to express Cx43, the null phosphorylation (Cx43MK4A) or the phospho-mimetic (Cx43MK4D) forms, gap junctional communication was not significantly altered. However, in Cx43−/− VSMC transfected to express Cx43MK4A, the interaction between Cx43 and cyclin E was lost and there was no return to proliferation. Conversely, Cx43−/− VSMC expressing Cx43 and Cx43MK4D demonstrate formation of Cx43-cyclin E complexes and a return to proliferation in response to PDGF. Taken together these data suggests that in response to PDGF, Cx43 becomes phosphorylated at its MAPK serines and interacts with cyclin E which in turn promotes VSMC proliferation in a manner that is independent of gap junctional communication. Despite this, PDGF treatments did not significantly reduce gap junction communication in our cells and leaves the possibility that a role for gap junctions does exist in these pathways.
In order to confirm our findings, we generated a novel knock in mouse (Cx43-MK4A) that contains the Cx43 MAPK serine to alanine substitution expressed on the endogenous promoter for Cx43. In the Cx43-MK4A mice, we found levels of Cx43 expressed in carotid VSMC were similar to C57Bl/6 mice, and showed that under control and PDGF treated conditions there was no evidence for Cx43 MAPK phosphorylation in the Cx43-MK4A mice. While Cx43 was present in the VSMC as well as cyclin E and CDK2, we could not identify interactions between Cx43 and cyclin E or Cx43 and CDK2. This finding is consistent with a requirement for Cx43 MAPK phosphorylation in forming interactions with cyclin E. In addition, we demonstrated a lack of VSMC proliferation in Cx43-MK4A mice and did not observe phenotypic changes in VSMC as identified in C57Bl/6 mice in response to PDGF treatment. In response to vascular injury VSMC de-differentiate and migrate to the intima of the vessel producing the neoitima.28, 46, 47 Previous studies have demonstrated that Cx43 expression is significantly enhanced in the medial and neointimal VSMC following vascular injury.14–16, 48 Development of neointima has been associated to MAPK phosphorylation and to alterations in cyclins and CDK pathways and can be significantly inhibited through treatments with inhibitors of MAPK, pathways pathways.49–51 Following vascular injury in C57Bl/6 mice we demonstrate significant formation of neointimal lesions, derived from VSMC (SM-22α positive). In keeping with previous studies we demonstrate that Cx43 expression as well as MAPK phosphorylated Cx43 is increased in media and neointimal VSMC in C57Bl/6 mice. However, in Cx43-MK4A mice vascular injury failed to produce significant neointimal formation. Previous studies by others have demonstrated contrasting roles for Cx43 in neointimal formation, with conditional VSMC knockout of Cx43 promoting neointimal formation52 and conversely by others suggesting that reduced Cx43 expression can limit neointimal formation.17 In each of these models a number of factors could contribute to differences between results including incomplete knockdown of Cx43 in the conditional knockout mouse52 or compensation by other connexins within the Cx43+/− mice as well as differences in model systems i.e. high fat fed.17 These conflicting results however clearly demonstrate the complex nature of Cx43 in regulating VSMC proliferation in response to vascular injury. In comparison to previous models, our Cx43-MK4A mice maintain levels of Cx43 expression within the VSMC of carotids and demonstrate specifically attenuated the neointima formation suggesting that Cx43 is critical in the regulation of neointimal formation. Our data therefore demonstrates that MAPK phosphorylation of Cx43 serines critically regulate VSMC de-differentiation, proliferation and neointima formation in vivo.
Our studies demonstrate that Cx43 interacts with cyclin E in a manner that is dependent on MAPK phosphorylation of the Cx43 C-terminus. Previous studies by others have shown that the C-terminus of Cx43 can interact with a number of proteins including tubulin and zonula occludens-1 which are associated with targeting of Cx43 at the plasma membrane.53–55 Further studies have also shown the potential for interactions between the C-terminus of Cx43 and proteins that regulate cell cycle e.g. NOV and SKP2.19, 21, 56 In order to define the region on Cx43 where interactions with cyclin E occurs, we generated and purified proteins for the full length cyclin E and for the Cx43 C-terminus including the null phosphorylation and phospho-mimetic forms. We identified that only Cx43CTMK4D proteins formed interactions with cyclin E. In this in vitro system, we saw no evidence for cyclin E interactions with the native state Cx43CT or the Cx43CTMK4A. By cross-linking proteins in solution from the Cx43CTMK4D samples, we identified higher molecular weight forms of both cyclin E and Cx43 at approximately 110kDa by Western blot. This corresponds to a complex containing a single cyclin E-GST and a dimer of Cx43. This finding is consistent with previous findings that Cx43 is required to be in a dimeric form in order to interact with known binding partners.57 In addition to this, we saw no evidence for interaction at higher pH values (pH 7.4, data not shown) where Cx43 is primarily in monomeric forms and is consistent with previously published data.57 Taken together these data suggest that interactions between MAPK phosphorylated Cx43 and cyclin E occur within the C-terminus of Cx43 and requires the presence of these phosphorylation sites and that these interactions can occur outside of PDGF stimulation in VSMC.
In conclusion, our studies have demonstrated three key conclusions: 1.) That Cx43 specifically interacts with the cell cycle protein cyclin E in a manner that is dependent on MAPK phosphorylation 2.) That MAPK phosphorylated Cx43 promotes VSMC proliferation in response to PDGF treatments 3.) That following vascular injury, MAPK phosphorylated Cx43 can significantly regulate neointimal formation. Further studies on this interaction may lead to a novel target for therapeutic intervention in the development atherosclerosis and restenosis.
Supplementary Material
Novelty and Significance.
What is known
A change in smooth muscle cells from non-proliferative (e.g. normal) to proliferative (e.g. diseased) states is regulated by mitogen activated protein kinase pathways (MAPK).
Alterations in Cx43 protein expression are associated with changes in smooth muscle cells to a proliferative phenotype.
What new information does this article contribute
Cx43 directly interacts with the cell cycle control protein cyclin E
Cx43-cyclin E interactions are dependent on the MAPK phosphorylation of Cx43
Interactions between Cx43 and cyclin E are critical determinants in smooth muscle cell proliferation and neointimal formation in vivo
Summary.
Atherosclerosis has one of the highest mortality rates in the United States, with dysregulated smooth muscle cell proliferation being a hallmark of the disease. Connexin 43 (Cx43) has previously been associated with regulation of cellular proliferation, although the mechanisms have not been described. We identified direct protein interactions between Cx43 and cyclin E, a protein that regulates how cells divide. We specifically demonstrate that phosphorylation of Cx43 protein by MAPK is required for it to bind to cyclin E. Using novel mice in which Cx43 cannot be phosphorylated by MAPK, we further show that this interaction is a critical determinant of smooth muscle cell proliferation and neointimal formation such as is found in restenosis and transplant arteriopathies. This discovery could potentially lead to novel therapeutic targets for the treatment of vascular proliferative disorders.
Acknowledgements
We would like to thank Izabella Bielnicka for expertise in protein generation. Sectioning of tissue was performed by University of Virginia Research Histology Core. We gratefully acknowledge the expertise and assistance provided by the School of Medicines Flow Cytometry Core and the Electron Microscopy Core at the University of Virginia and Anita Impagliazzo for the illustration.
Source Of Funding:
This work is supported by NIH HL088554 (BEI), an American Heart Association Scientist Development Grant (BEI), Phillip Morris core facilities grant (BEI), an American Heart Association Post Doctoral Award (SRJ), NRSA IF32HL103042-1 (ACS), NIH RO1GM087828 (LC), NSF MCB0845668 (LC), Jeffress Memorial Trust Research Corporation for Science Advancement for support through a Cottrell Scholar Award (LC), NIH GM55632 (PDL), NIH R01-HL083120 (MK).
Non-Standard Abbreviations and Acronyms
- VSMC
Vascular smooth muscle cell
- Cx
Connexin
- CT
C-terminus
- PDGF
Platelet derived growth factor-BB
- MAPK
Mitogen activated protein kinase
- MK4A
Map kinase 4 alanine mutation
- MK4D
Map kinase 4 aspartate mutation
- ANSEC
Analytical size exclusion chromatography
- GST
Glutathione S-transferase
- CDK2
Cyclin dependant kinase 2
- ERK
Extracellular signal-regulated protein kinase
- TEM
Transmission electron microscopy
- i-TEM
Immune-transmission electron microscopy
- CBX
Carbenoxolone
- Rb
Retinoblastoma
- pRb
Phosphorylated retinoblastoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None.
References
- 1.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 2.Rekhter MD, Gordon D. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol. 1995;147:668–677. [PMC free article] [PubMed] [Google Scholar]
- 3.Manolakou P, Angelopoulou R, Bakoyiannis C, Psathas E, Bastounis E, Kavantzas N, Patsouris E. Cellular proliferation in complicated versus uncomplicated atherosclerotic lesions: Total cell population, foam cells and newly formed microvessels. Tissue Cell. 2009;41:408–413. doi: 10.1016/j.tice.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Blumenthal DK, Terry CM, He Y, Carlson ML, Cheung AK. Pdgf-induced proliferation in human arterial and venous smooth muscle cells: Molecular basis for differential effects of pdgf isoforms. J Cell Biochem. 112:289–298. doi: 10.1002/jcb.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keramati AR, Singh R, Lin A, Faramarzi S, Ye ZJ, Mane S, Tellides G, Lifton RP, Mani A. Wild-type lrp6 inhibits, whereas atherosclerosis-linked lrp6r611c increases pdgf-dependent vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 108:1914–1918. doi: 10.1073/pnas.1019443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuster JJ, Fernandez P, Gonzalez-Navarro H, Silvestre C, Nabah YN, Andres V. Control of cell proliferation in atherosclerosis: Insights from animal models and human studies. Cardiovasc Res. 86:254–264. doi: 10.1093/cvr/cvp363. [DOI] [PubMed] [Google Scholar]
- 7.Vukovic I, Arsenijevic N, Lackovic V, Todorovic V. The origin and differentiation potential of smooth muscle cells in coronary atherosclerosis. Exp Clin Cardiol. 2006;11:123–128. [PMC free article] [PubMed] [Google Scholar]
- 8.Bhardwaj S, Roy H, Babu M, Shibuya M, Yla-Herttuala S. Adventitial gene transfer of vegfr-2 specific vegf-e chimera induces mcp-1 expression in vascular smooth muscle cells and enhances neointimal formation. Atherosclerosis. 2011;219:84–91. doi: 10.1016/j.atherosclerosis.2011.07.103. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of whhl and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10:680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- 10.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in apoe knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 11.Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK. Origin of neointimal smooth muscle: We've come full circle. Arterioscler Thromb Vasc Biol. 2006;26:2579–2581. doi: 10.1161/01.ATV.0000249623.79871.bc. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone SR, Ross J, Rizzo MJ, Straub AC, Lampe PD, Leitinger N, Isakson BE. Oxidized phospholipid species promote in vivo differential cx43 phosphorylation and vascular smooth muscle cell proliferation. Am J Pathol. 2009;175:916–924. doi: 10.2353/ajpath.2009.090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:225–230. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
- 14.Chadjichristos CE, Morel S, Derouette JP, Sutter E, Roth I, Brisset AC, Bochaton-Piallat ML, Kwak BR. Targeting connexin 43 prevents platelet-derived growth factor-bb-induced phenotypic change in porcine coronary artery smooth muscle cells. Circ Res. 2008;102:653–660. doi: 10.1161/CIRCRESAHA.107.170472. [DOI] [PubMed] [Google Scholar]
- 15.Plenz G, Ko YS, Yeh HI, Eschert H, Sindermann JR, Dorszewski A, Hofnagel O, Robenek H, Breithardt G, Severs NJ. Upregulation of connexin43 gap junctions between neointimal smooth muscle cells. Eur J Cell Biol. 2004;83:521–530. doi: 10.1078/0171-9335-00417. [DOI] [PubMed] [Google Scholar]
- 16.Yeh HI, Lupu F, Dupont E, Severs NJ. Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler Thromb Vasc Biol. 1997;17:3174–3184. doi: 10.1161/01.atv.17.11.3174. [DOI] [PubMed] [Google Scholar]
- 17.Chadjichristos CE, Matter CM, Roth I, Sutter E, Pelli G, Luscher TF, Chanson M, Kwak BR. Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation. 2006;113:2835–2843. doi: 10.1161/CIRCULATIONAHA.106.627703. [DOI] [PubMed] [Google Scholar]
- 18.Plante I, Stewart MK, Barr K, Allan AL, Laird DW. Cx43 suppresses mammary tumor metastasis to the lung in a cx43 mutant mouse model of human disease. Oncogene. 30:1681–1692. doi: 10.1038/onc.2010.551. [DOI] [PubMed] [Google Scholar]
- 19.Gellhaus A, Dong X, Propson S, Maass K, Klein-Hitpass L, Kibschull M, Traub O, Willecke K, Perbal B, Lye SJ, Winterhager E. Connexin43 interacts with nov: A possible mechanism for negative regulation of cell growth in choriocarcinoma cells. J Biol Chem. 2004;279:36931–36942. doi: 10.1074/jbc.M404073200. [DOI] [PubMed] [Google Scholar]
- 20.Johnstone SR, Best AK, Wright CS, Isakson BE, Errington RJ, Martin PE. Enhanced connexin 43 expression delays intra-mitotic duration and cell cycle traverse independently of gap junction channel function. J Cell Biochem. 2010;110:772–782. doi: 10.1002/jcb.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YW, Kaneda M, Morita I. The gap junction-independent tumor-suppressing effect of connexin 43. J Biol Chem. 2003;278:44852–44856. doi: 10.1074/jbc.M305072200. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Zhang T, Yu X, Xin W, Lan X, Zhang D, Huang C, Du G. Asymmetric dimethylarginine confers the communication between endothelial and smooth muscle cells and leads to vsmc migration through p38 and erk1/2 signaling cascade. FEBS Lett. 585:2727–2734. doi: 10.1016/j.febslet.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes map kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: One of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solan JL, Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol. 2007;217:35–41. doi: 10.1007/s00232-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boassa D, Solan JL, Papas A, Thornton P, Lampe PD, Sosinsky GE. Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic. 11:1471–1486. doi: 10.1111/j.1600-0854.2010.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solan JL, Lampe PD. Connexin43 phosphorylation: Structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song GJ, Barrick S, Leslie KL, Bauer PM, Alonso V, Friedman PA, Fiaschi-Taesch NM, Bisello A. The scaffolding protein ebp50 promotes vascular smooth muscle cell proliferation and neointima formation by regulating skp2 and p21(cip1) Arterioscler Thromb Vasc Biol. 2011;32:33–41. doi: 10.1161/ATVBAHA.111.235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chahine MN, Blackwood DP, Dibrov E, Richard MN, Pierce GN. Oxidized ldl affects smooth muscle cell growth through mapk-mediated actions on nuclear protein import. J Mol Cell Cardiol. 2009;46:431–441. doi: 10.1016/j.yjmcc.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Findeisen HM, Gizard F, Zhao Y, Qing H, Heywood EB, Jones KL, Cohn D, Bruemmer D. Epigenetic regulation of vascular smooth muscle cell proliferation and neointima formation by histone deacetylase inhibition. Arterioscler Thromb Vasc Biol. 31:851–860. doi: 10.1161/ATVBAHA.110.221952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park ES, Yoo JM, Lim Y, Tudev M, Yoo HS, Hong JT, Yun YP. Inhibitory effects of docetaxel on platelet-derived growth factor (pdgf)-bb-induced proliferation of vascular smooth muscle cells through blocking pdgf-receptor beta phosphorylation. J Pharmacol Sci. 116:204–213. doi: 10.1254/jphs.10276fp. [DOI] [PubMed] [Google Scholar]
- 32.Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ. Wnt4/beta-catenin signaling induces vsmc proliferation and is associated with intimal thickening. Circ Res. 108:427–436. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 33.Bedel A, Negre-Salvayre A, Heeneman S, Grazide MH, Thiers JC, Salvayre R, Maupas-Schwalm F. E-cadherin/beta-catenin/t-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ Res. 2008;103:694–701. doi: 10.1161/CIRCRESAHA.107.166405. [DOI] [PubMed] [Google Scholar]
- 34.Gaulin JF, Fiset A, Fortier S, Faure RL. Characterization of cdk2-cyclin e complexes in plasma membrane and endosomes of liver parenchyma. Insulin-dependent regulation. J Biol Chem. 2000;275:16658–16665. doi: 10.1074/jbc.275.22.16658. [DOI] [PubMed] [Google Scholar]
- 35.Kisielewska J, Philipova R, Huang JY, Whitaker M. Map kinase dependent cycline/cdk2 activity promotes DNA replication in early sea urchin embryos. Dev Biol. 2009;334:383–394. doi: 10.1016/j.ydbio.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J, Sheng G, Warner BW. Epidermal growth factor-induced rapid retinoblastoma phosphorylation at ser780 and ser795 is mediated by erk1/2 in small intestine epithelial cells. J Biol Chem. 2005;280:35992–35998. doi: 10.1074/jbc.M504583200. [DOI] [PubMed] [Google Scholar]
- 37.De Donatis A, Comito G, Buricchi F, Vinci MC, Parenti A, Caselli A, Camici G, Manao G, Ramponi G, Cirri P. Proliferation versus migration in platelet-derived growth factor signaling: The key role of endocytosis. J Biol Chem. 2008;283:19948–19956. doi: 10.1074/jbc.M709428200. [DOI] [PubMed] [Google Scholar]
- 38.Lee CK, Lee HM, Kim HJ, Park HJ, Won KJ, Roh HY, Choi WS, Jeon BH, Park TK, Kim B. Syk contributes to pdgf-bb-mediated migration of rat aortic smooth muscle cells via mapk pathways. Cardiovasc Res. 2007;74:159–168. doi: 10.1016/j.cardiores.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Crespin S, Bechberger J, Mesnil M, Naus CC, Sin WC. The carboxy-terminal tail of connexin43 gap junction protein is sufficient to mediate cytoskeleton changes in human glioma cells. J Cell Biochem. 2010;110:589–597. doi: 10.1002/jcb.22554. [DOI] [PubMed] [Google Scholar]
- 40.Dang X, Jeyaraman M, Kardami E. Regulation of connexin-43-mediated growth inhibition by a phosphorylatable amino-acid is independent of gap junction-forming ability. Mol Cell Biochem. 2006;289:201–207. doi: 10.1007/s11010-006-9162-2. [DOI] [PubMed] [Google Scholar]
- 41.Hossain MZ, Jagdale AB, Ao P, Kazlauskas A, Boynton AL. Disruption of gap junctional communication by the platelet-derived growth factor is mediated via multiple signaling pathways. J Biol Chem. 1999;274:10489–10496. doi: 10.1074/jbc.274.15.10489. [DOI] [PubMed] [Google Scholar]
- 42.Hossain MZ, Jagdale AB, Ao P, Boynton AL. Mitogen-activated protein kinase and phosphorylation of connexin43 are not sufficient for the disruption of gap junctional communication by platelet-derived growth factor and tetradecanoylphorbol acetate. J Cell Physiol. 1999;179:87–96. doi: 10.1002/(SICI)1097-4652(199904)179:1<87::AID-JCP11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 43.Hossain MZ, Ao P, Boynton AL. Platelet-derived growth factor-induced disruption of gap junctional communication and phosphorylation of connexin43 involves protein kinase c and mitogen-activated protein kinase. J Cell Physiol. 1998;176:332–341. doi: 10.1002/(SICI)1097-4652(199808)176:2<332::AID-JCP11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Moorby C, Patel M. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp Cell Res. 2001;271:238–248. doi: 10.1006/excr.2001.5357. [DOI] [PubMed] [Google Scholar]
- 45.Moorby CD, Gherardi E. Expression of a cx43 deletion mutant in 3t3 a31 fibroblasts prevents pdgf-induced inhibition of cell communication and suppresses cell growth. Exp Cell Res. 1999;249:367–376. doi: 10.1006/excr.1999.4485. [DOI] [PubMed] [Google Scholar]
- 46.Horiba M, Kadomatsu K, Nakamura E, Muramatsu H, Ikematsu S, Sakuma S, Hayashi K, Yuzawa Y, Matsuo S, Kuzuya M, Kaname T, Hirai M, Saito H, Muramatsu T. Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J Clin Invest. 2000;105:489–495. doi: 10.1172/JCI7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajagopal V, Rockson SG. Coronary restenosis: A review of mechanisms and management. Am J Med. 2003;115:547–553. doi: 10.1016/s0002-9343(03)00477-7. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Chen J, Sun Y, Zhang F, Zhu J, Hu S, Wang DH. Regulation of connexin expression after balloon injury: Possible mechanisms for antiproliferative effect of statins. Am J Hypertens. 2005;18:1146–1153. doi: 10.1016/j.amjhyper.2005.03.746. [DOI] [PubMed] [Google Scholar]
- 49.Gennaro G, Menard C, Giasson E, Michaud SE, Palasis M, Meloche S, Rivard A. Role of p44/p42 map kinase in the age-dependent increase in vascular smooth muscle cell proliferation and neointimal formation. Arterioscler Thromb Vasc Biol. 2003;23:204–210. doi: 10.1161/01.atv.0000053182.58636.be. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka K, Hayashi K, Shingu T, Kuga Y, Nomura K, Kajiyama G. Probucol inhibits neointimal formation in carotid arteries of normocholesterolemic rabbits and the proliferation of cultured rabbit vascular smooth muscle cells. Cardiovasc Drugs Ther. 1998;12:19–28. doi: 10.1023/a:1007777128574. [DOI] [PubMed] [Google Scholar]
- 51.Chang MW, Barr E, Lu MM, Barton K, Leiden JM. Adenovirus-mediated over-expression of the cyclin/cyclin-dependent kinase inhibitor, p21 inhibits vascular smooth muscle cell proliferation and neointima formation in the rat carotid artery model of balloon angioplasty. J Clin Invest. 1995;96:2260–2268. doi: 10.1172/JCI118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao Y, Regan CP, Manabe I, Owens GK, Day KH, Damon DN, Duling BR. Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2007;27:1037–1042. doi: 10.1161/ATVBAHA.106.137182. [DOI] [PubMed] [Google Scholar]
- 53.Palatinus JA, Rhett JM, Gourdie RG. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giepmans BN, Verlaan I, Moolenaar WH. Connexin-43 interactions with zo-1 and alpha- and beta-tubulin. Cell Commun Adhes. 2001;8:219–223. doi: 10.3109/15419060109080727. [DOI] [PubMed] [Google Scholar]
- 55.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Wu YJ, Sala-Newby GB, Shu KT, Yeh HI, Nakayama KI, Nakayama K, Newby AC, Bond M. S-phase kinase-associated protein-2 (skp2) promotes vascular smooth muscle cell proliferation and neointima formation in vivo. J Vasc Surg. 2009;50:1135–1142. doi: 10.1016/j.jvs.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-src and zonula occludens-1. J Biol Chem. 2004;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.