Abstract
Gestational stress may have lasting deleterious effects on neuro-cognitive development of offspring. Progesterone (P), and its 5α-reduced metabolites, dihydroprogesterone (DHP) and 5α-pregnan-3α-ol-20-one (3α,5α-THP), maintain pregnancy, and can have effects on cognitive performance and/or neuronal integrity. However, whether some of the deleterious effects of gestational stress on cognitive and neural processes may be related to progestogen formation is not known. Pregnant rat dams were exposed to a regimen of variable stressors (including forced swim, restraint, fasting, social stress, and exposure to cold and light) on gestational days 17–21 or were minimally-handled controls. Male and female offspring were cross-fostered to non-manipulated dams and assessed for motor and cognitive performance between postnatal days 28 and 30. Although the motor behavior of gestationally-stressed offspring did not differ significantly from control offspring, their cognitive performance in an object recognition task was poorer. Irrespective of sex, dendritic spine density was reduced in dorsal hippocampus of stress-exposed offspring compared to control offspring. Formation of DHP was reduced in medial prefrontal cortex (mPFC) and increased in hippocampus of stressed, compared to control offspring. Notably, there were sex differences wherein estradiol in mPFC, as well as P and DHP in diencephalon, were increased with stress among females but decreased with stress among males. These data suggest that exposure to variable stress during gestation can perturb cognitive performance, concomitant with dendrite development in hippocampus, and P’s 5α-reduction in hippocampus and mPFC. Some sex differences in stress effects on progestogen formation may occur in diencephalon.
Keywords: Allopregnanolone, dihydroprogesterone, object recognition, open field, prenatal stress, pregnancy, progesterone
INTRODUCTION
Physical and psychological stressors experienced during pregnancy may contribute to poor birth outcomes. In support, physical stressors during pregnancy, such as smoking, drug use, low pre-pregnancy weight, and hospitalizations are associated with low birth-weight among infants as are psychological stressors, such as problematic family relationships, unemployment, and housing concerns [1]. Several prospective studies have demonstrated that chronic stress, anxiety, and/or depression during pregnancy are associated with preterm labor [2,3], which can put offspring at risk for neurological and behavioral aberrations [4,5], as well as impairment of cognitive skills [6]. The mechanisms that underlie these effects are not understood.
Brain regions, important for lexical decision making and performance in cognitive tasks, such as the prefrontal cortex and hippocampus, may be important targets for detrimental effects of stress. In rats, chronic exposure to unpredictable/variable stressors, increases cytokine expression in prefrontal cortex and hippocampus [7,8], which can be proinflammatory and associated with neurodegeneration. Administration of the cytokine, interleukin-1β, during gestational days 17 to 21 of pregnancy, alters development of locomotor and stress response in rats [9,10]. Notably, chronic activation of the stress axis is associated with degeneration in the hippocampus, particularly, retraction of dendritic processes in adult rats [11]. Similar degenerative effects in rats have been reported in prefrontal cortex of gestationally-stressed offspring [12,13]. Thus, exposure to variable stressors in gestation may have pervasive effects on morphology and/or function of brain regions important for cognitive performance among offspring.
One mechanism that may underlie effects of gestational stressors on offspring is perturbation of progestogen formation. Progestogens can act to dampen stress axis responding; however, exposure to gestational stress in rats has been shown to alter the homeostasis of these processes throughout life [14,15]. In support, acute perinatal stress, via maternal separation and lithium chloride injection, can alter progestogen formation in circulation and/or hippocampus, an important brain region involved in affective, cognitive, and ictal behavior, well into adulthood [14]. Regulation of progesterone’s (P) 5α-reduced metabolites, dihydroprogesterone (DHP) and 5α-pregnan-3α-ol-20-one (3α,5α-THP or allopregnanolone), may be critical, particularly throughout pregnancy. In pregnant women, plasma 3α,5α-THP levels rise throughout gestation, peak in the third trimester, and then decline to luteal levels post-partum [16]. Similar decline in circulating 3α,5α-THP is observed among rodents [17]. In rats, decline in 3α,5α-THP is observed to occur in cortex, just prior to parturition [17]. We have recently observed that exposure to restraint during gestation can reduce P’s metabolism to its 5α-reduced metabolites in medial prefrontal cortex (mPFC) of pre-pubertal offspring concomitant with deleterious effects on cognitive performance [18]. Thus, 5α-reduced progestogens may underlie important function in limbic regions of the developing brain.
In order to assess the role that gestational exposure to physical and psychological stress can have on neurodevelopment of offspring, pregnant rat dams were exposed to a variable stress regimen in late gestation. On gestational days (GDs) 17–21, pregnant dams were exposed to forced swim, restraint, fasting, light, cold exposure, and social stress. Exposure to such stressors (including restraint, unpredictable, and/or variable stress regimen) has been shown to enhance central inflammatory response to an immune challenge [8] and, in pregnant dams, to perturb cognitive and motor performance of offspring [19–21]. As such, gestationally-stressed offspring were assessed on a hippocampus- and prefrontal cortex-dependent cognitive task in pre-puberty (between post-natal days 28–30). Dendritic spine density was assessed via Golgi-Cox staining in mPFC, dorsal hippocampus, and hypothalamus (a control region important for stress response but not expected to mediate cognitive task performance). Concentrations of progestogens (P, DHP, and 3α,5α-THP) and estradiol (E2) were assessed in mPFC, hippocampus, and diencephalon. We hypothesized that gestational stress would reduce task performance, dysregulate central progestogen formation, and reduce dendritic spine density in hippocampus and/or mPFC compared to minimal handling in gestation.
METHODS
Subjects, Housing, & Procedures
Housing
All rats were housed in polycarbonate cages (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1° C) in our colony in the Laboratory Animal Care Facility at The University at Albany-SUNY, Life Sciences Research Building (Albany, NY). Rats were maintained on a 12/12 h reversed-light cycle (lights off at 08:00 h) with continuous access to Purina Rat Chow and tap water. Dams were housed 3–4 per cage until GD 21, whereupon they were single-housed. Their offspring were cross-fostered until weaning and then group-housed (4–5 per cage) after weaning.
Dams
Female, timed-pregnant, Long-Evans rats (N=8), approximately 55 days old, were obtained from Taconic Farms (Germantown, NY) and weighed daily. Dams were randomly assigned to be exposed to a variable stress regimen (n=4) or to not be further manipulated (n=4) from GDs 17 to 21. Control dams weighed 292 ± 13 g (mean ± SEM) on GD 17 and had gained an average of 23 ± 3 g by GD 21. This did not significantly differ from dam exposed to variables stressors that weighed 274 ± 28 g at GD 17 and gained an average of 20 ± 11 g by GD 21. While, not significantly different, control dams had more pups (13 ± 1) on average than did variably-stressed dams (10 ± 1).
Variable Stress Regimen
From GDs 17–21, pregnant dams were exposed to an unpredictable, variable stress paradigm. During this time, dams were exposed to (1) physical restraint in a plexiglass restrainer under a 60-watt light for 1 h, (2) 15 min of forced swim stress in room temperature water, (3) exposure to a cold environment (4 °C) for 6 h, (4) overnight food deprivation, (5) lights on for 24 h, and (6) social stress induced by overcrowded housing conditions during the dark phase of the light cycle per the schedule in Table 1.
Table 1. Variable Stressor Regimen for Dams.
Depicts course of variable stressor regimen that pregnant rat dams were exposed to. Acute stressors were administered in the morning (08:00 h), at mid-day (12:00 h), and in the evening (16:00 h).
| Time of Day | ||||
|---|---|---|---|---|
| Gestational Day | AM | Mid-day | PM | |
| 17 | Forced Swim – 15 min | Restraint – 60 min | Forced Swim – 15 min | |
| 18 | Cold Exposure – 6 h | Fast Overnight | ||
| 19 | Nothing | Forced Swim – 15 min | Light on During Dark Phase | |
| 20 | Social Stress – 12 h | |||
| 21 | Restraint – 60 min | Forced Swim – 15 min | Restraint – 60 min | |
Cross-Fostering
To minimize the potential confounds arising from differences in maternal-rearing between gestationally-stressed and control dams, litters were culled at the time of birth and pups were cross-fostered to non-manipulated dams in our colony. Among the 4 control dam litters, 3 females and 3 males were kept from each litter (with one exception wherein 4 males were kept from one litter). Among the 4 gestationally-stressed litters, 2 females and 3 males were kept from each litter (with one exceptions wherein 1 female and 4 males were kept from one litter). Although, the sex of pups was not systematically-investigated, it was notable that there were fewer females born to the gestationally-stressed dams, resulting in fewer observations in that group. We acknowledge that utilizing more than one pup per litter can yield cohort effects; however, we did not find statistically-significant differences between litters of the same experimental condition on any behavioral or endocrine measure assessed.
Experimental Subjects
Juvenile male (n=13 gestationally-stressed; n=13 control) and female (n=7 gestationally-stressed; n=12 control) offspring were weaned from foster-dams at 21 days of age and housed 4–5 per cage until post-natal day 28–30, when their performance in motor (open field) and cognitive (object recognition) tasks were assessed.
Open Field
The open field is a white, opaque chamber (76 × 57 × 35 cm) with a 48-square grid floor (6 × 8 squares, 9.5 cm/side) that is illuminated by ceiling lights from above. Per previous methods, rats were placed in the open field and the path of their exploration was recorded for five-minutes. Performance in the open field was recorded and calculated via ANY-maze (Stoelting Co., Wood Dale, IL) animal tracking software and utilized as an index of motor behavior [22,23].
Object Recognition
The object recognition task is a working memory task that is primarily dependent on an intact frontal cortex and, to a lesser extent, an intact hippocampus [24,25]. This task was used as previously described [18,26,27]. Briefly, rats are placed in a white open field (76 × 57 × 35 cm) containing two identical, spheric objects in adjacent corners (plastic toys in the shape of oranges) with which they can interact for a 3 min training phase. After a 4 h interval, one of the spheric objects is replaced with a cone-shaped, novel object (a blue plastic toy in the shape of a buoy) and rats are assessed for interaction with the novel and familiar objects in the open field again for a 3 min testing phase. A greater percentage of time spent exploring the novel object as a function of the total amount of time spent exploring both objects during testing [duration spent with novel object/(duration spent with novel object + duration spent with familiar object) × 100] is considered an index of enhanced cognitive performance in this task. All behavior in this task was collected and recorded via ANY-maze animal tracking software (Stoelting Co., Wood Dale, IL).
Tissue Collection of Experimental Subjects
Immediately following behavioral testing, between 28 and 30 days of age, offspring were euthanized via rapid decapitation. Whole brains were collected within 1 min of decapitation and were rapidly frozen on dry ice. Brains were then stored at −80 °C for later assessment. At the time of assay, brains were thawed on ice and divided in half along the midsagittal plane. For each brain, half of the tissue was placed in Golgi-Cox staining medium (described below) for later assessment of dendrite morphology. The remaining half of the brain had mPFC, hippocampus, and diencephalon grossly dissected as previously described [18,28] for radioimmunoassay of P, DHP, 3α,5α-THP, and E2.
Golgi-Cox Staining for Dendrite Morphology
Golgi-Cox staining medium consisted of 5 volumes of potassium dichromate (5% dissolved in warm dH20), 5 volumes of mercuric chloride (5% dissolved in hot dH20), and 4 volumes potassium chromate (5% dissolved in cold dH20) combined with 10 volumes of dH20. Brains were incubated in the solution and placed in the dark for 14 days. Medium was refreshed after the first 2 days. Tissues were hand-sliced at the level of the mPFC, dorsal hippocampus, and hypothalamus. Sections were immersed in the following solutions, in the order presented: dH20 for 1 min, ammonia for 5 min, increasing concentrations of ethanol (50%, 70%, 90%) for 1 min each, 100% ethanol for 5 min, xylene for 5 min [29]. Golgi-impregnated neurons that were isolated from surrounding neurons were selected for microscopic assessment. Spine density (total number of spines on dendrite / length of dendrite) was obtained from the longest dendrite at oil immersion 100× magnification. Five dendrites were characterized per tissue, for 5 animals per experimental group (yielding 25 total observations per tissue, per experimental group). The mean of the 5 dendrites per tissue, per animal, was used for statistical analysis.
Radioimmunassay for Steroid Concentrations
Concentrations of central steroids were assessed modified from previously reported methods, as described below [30–32].
Antibodies
P antibody (P#337 from Dr. G.D. Niswender, Colorado State University) was used in a 1:30,000 dilution which typically binds between 30% and 50% of [3H] P. DHP (X-947) and 3α,5α-THP antibodies (#921412-5, purchased from Dr. Robert Purdy, Veterans Medical Affairs, La Jolla, CA) were used in a 1:5,000 dilution and typically bind between 40 and 60% of [3H] 3α,5α-THP. E2 antibody (E#244, Dr. G.D. Niswender, Colorado State University, Fort Collins, CO) was used in a 1:40,000 dilution which typically binds between 40% and 60% of [3H] E2S.
Radioactive Probes
[3H] P (NET-208: specific activity=47.5 Ci/mmol), [3H] 3α,5α-THP (used for DHP and 3α,5α-THP, NET-1047: specific activity=65.0 Ci/mmol), and [3H] E2 (NET-317: specific activity=51.3 Ci/mmol) were purchased from Perkin Elmer (Boston, MA).
Extraction of Steroids from Brain Tissues
P, DHP, 3α,5α-THP, and E2 were extracted following homogenization with a glass/glass homogenizer in 50% MeOH, 1% acetic acid. Tissues were centrifuged at 3,000 × g and the supernatant was chromatographed on Sep-Pak C18 cartridges (Waters Corp., Milford, MA). Steroids were eluted with 50% MeOH followed by 100% MeOH and lyophilized using a speed dryer. Samples were reconstituted in 500 µl assay buffer.
Set-up and Incubation of Radioimmunoassays
The range of the standard curves was 0–8000 pg for P, DHP, and 3α,5α-THP, and 0–1000 pg for E2. Standards were added to assay buffer followed by addition of the appropriate antibody and [3H] steroid. Total assay volumes were and 800 µl for P, 950 µl for DHP, 1250 µl for 3α,5α-THP, and 800 µl for E2. All assays were incubated overnight at 4 °C.
Termination of Binding
Separation of bound and free steroid was accomplished by the rapid addition of dextran-coated charcoal. Following incubation with charcoal, samples were centrifuged at 3000 × g and the supernatant was decanted into a glass scintillation vial with 5 ml scintillation cocktail. Sample tube concentrations were calculated using the logit-log method of [33], interpolation of the standards, and correction for recovery with Assay Zap software (BioSoft, Cambridge, UK). The respective inter- and intra-assay reliability co-efficients were: P = 0.10 and 0.11, DHP = 0.10 and 0.09, 3α,5α-THP = 0.10 and 0.11, and E2 = 0.06 and 0.08.
Analyses
Behavioral and biochemical data were analyzed using two-way analyses of variance (sex X dam condition) and were followed up with Fisher’s protected least significant differences post-hoc tests to determine group differences. For dendritic spine density, two-way analyses of variance (sex X dam condition) were conducted on the mean of 5 dendrites per tissue, per animal among 5 animals per experimental group. When interactions were present, group differences were determined using multiple one-way analyses of variance with α level corrected for multiple comparisons. An α level of p ≤ 0.05 was used to determine statistical significance in all analyses.
RESULTS
Cognitive, but not Motor, Behavior of Pre-Pubertal Offspring is Influenced by Gestational Stress
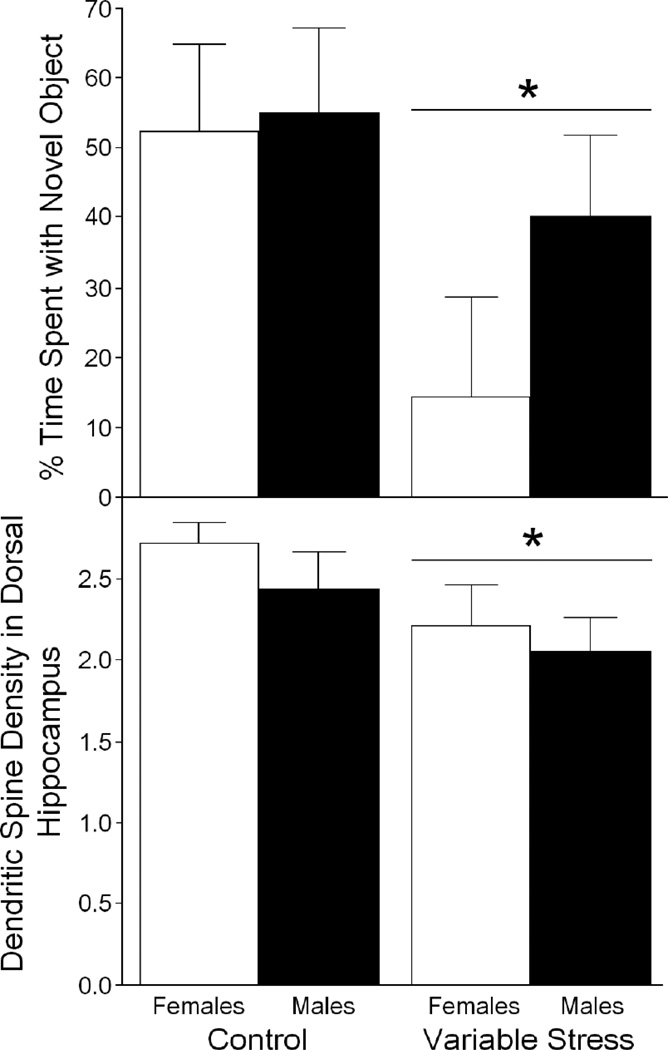
Gestational exposure to variable stressors significantly decreased cognitive performance of male and female offspring. Offspring born to dams exposed to variable stressors on GDs 17–21 spent a lower percentage [F(1,41) = 4.12, p < 0.05], Fig. (1, top) of time investigating the novel object (0.6 ± 0.3 sec with novel vs. 0.5 ± 0.2 sec with familiar object) than did offspring born to control dams (1.3 ± 0.3 sec with novel object vs. 0.6 ± 0.3 sec with familiar object). Neither a main effect of sex [F(1,41) = 1.23, n.s.], nor a sex X stressor interaction [F(1,41) = 0.80, n.s.] were observed on object recognition performance. No differences in motor behavior of offspring were observed between sexes [F(1,41) = 0.02, n.s.], gestational stress condition [F(1,41) = 0.03, n.s.], or the interaction of these factors [F(1,41) = 1.19, n.s.] to account for performance on this cognitive task (Table 2).
Fig. (1).
Juvenile offspring (28–30 days of age) exposed to a variable stress regimen on gestational days 17–21 (female n = 7, male n = 13) spent a significantly decreased percentage of time (mean ± SEM) investigating a novel object in an object recognition task compared to control offspring (female n = 12, male n = 13) that were exposed to minimal handling (top). As well, dendritic spine density in dorsal hippocampus (mean of 5 dendrites per tissue, per animal; mean ± SEM) was significantly reduced among gestationally-stressed, compared to control, offspring had significantly reduced (bottom). * indicates significant main effect of gestational stress, p ≤ 0.05. Neither, the main effect of sex, nor the stress X sex interaction, were significant.
Table 2. Exploration and Motor Behavior of Offspring.
Depicts the amount of time spent exploring objects in the training phase of the object recognition task, as well as motor behavior in an open field among juvenile rats (28–30 days of age) that were exposed to minimal handling (control) or a variable stress regimen on days 17–20 of gestation.
| Control | Variable Stress | |||
|---|---|---|---|---|
| Females (n=12) | Males (n=13) | Females (n=7) | Males (n=13) | |
| Time Spent Exploring Training Objects (sec ± SEM) | 2.3 ± 0.5 | 3.7 ± 0.5 | 4.6 ± 1.1 | 3.8 ± 0.7 |
| Total Entries in an Open Field | 220 ± 20 | 189 ± 27 | 188 ± 36 | 212 ± 19 |
indicates significant main effect of gestational stress, p < 0.05. Neither, the main effect of sex, nor the stress X sex interaction, were significant.
Dendrite Morphology in Dorsal Hippocampus is Influenced by Gestational Stress Among Pre-pubertal Offspring
Gestational exposure to variable stressors significantly decreased dendritic spine density of male and female offspring. Dendritic spine density was significantly lower in dorsal hippocampus of offspring that were gestationally-exposed to variable stressors compared to control offspring [F(1,16) = 4.32, p = 0.05], Fig. (1, bottom). Neither a main effect of sex [F(1,16) = 0.94, n.s.], nor a sex X stressor interaction [F(1,16) = 0.09, n.s.] were observed on dendritic spine density in dorsal hippocampus. In mPFC, no differences in spine density were observed between sexes [F(1,16) = 0.79, n.s.], stress conditions [F(1,16) = 0.06, n.s.], or the interaction of these factors [F(1,16) = 0.14, n.s.] (Table 3). As well, in hypothalamus, no differences in spine density were observed between sexes [F(1,16) = 1.56, n.s.], stress conditions [F(1,16) = 0.15, n.s.], or the interaction of these factors [F(1,16) = 1.38, n.s.] (Table 3).
Table 3. Dendrite Morphology of Offspring.
Depicts dendritic spine density (mean of 5 dendrites per tissue, per animal) in medial prefrontal cortex and hypothalamus of juvenile rats (28–30 days of age) that were exposed to minimal handling (control) or a variable stress regimen on days 17–21 of gestation. Significant main effects of gestational stress or sex conditions were not observed, nor was there an interaction.
| Control | Variable Stress | |||
|---|---|---|---|---|
| Females (n=5) | Males (n=5) | Females (n=5) | Males (n=5) | |
| Medial Prefrontal Cortex Dendritic Spine Density (mean ± SEM) | 2.3 ± 0.3 | 2.2 ± 0.2 | 2.5 ± 0.4 | 2.1 ± 0.3 |
| Hypothalamus Dendritic Spine Density (mean ± SEM) | 2.5 ± <0.0 | 2.1 ± 0.1 | 2.3 ± 0.2 | 2.3 ± 0.2 |
Central Formation of Progestogens and E2 are Influenced by Gestational Stress and Sex among Pre-pubertal Offspring
Gestational exposure to variable stressors significantly altered progestogen levels. Gestationally-stressed offspring had significantly lower levels of DHP in mPFC [F(1,41) = 8.71, p < 0.05], but significantly increased DHP levels in hippocampus [F(1,41) = 13.63, p < 0.05]. No main effect of stressor on P [FmPFC(1,41) = 2.23, n.s.; FHippo(1,41) = 0.05, n.s.] nor 3α,5α-THP [FmPFC(1,41) = 0.01, n.s.; FHippo(1,41) = 0.87, n.s.] were observed in these regions (Table 4).
Table 4. Central Steroid Concentrations of Offspring.
Depicts concentrations of progesterone, dihydroprogesterone, 3α,5α-THP, and estradiol in prefrontal cortex, hippocampus, and diencephalon of juvenile rats (28–30 days of age) that were exposed to minimal handling (control) or a variable stress regimen on days 17–21 of gestation.
| Control | Variable Stress | |||
|---|---|---|---|---|
| Females (n=12) | Males (n=13) | Females (n=7) | Males (n=13) | |
| Medial Prefrontal Cortex | ||||
| Progesterone (ng/g ± SEM) | 0.56 ± 0.07 | 0.57 ± 0.06 | 0.77 ± 0.06 | 0.54 ± 0.05 |
| Dihydroprogesterone (ng/g ± SEM) | 1.0 ± 0.2 | 1.2 ± 0.2 | 0.7 ± 0.2* | 0.4 ± 0.1* |
| 3α,5α-THP (ng/g ± SEM) | 0.9 ± 0.3 | 1.5 ± 0.4 | 1.3 ± 0.9 | 1.0 ± 0.4 |
| Estradiol (pg/g ± SEM)*** | 0.09 ± 0.02 | 0.08 ± 0.01 | 0.13 ± 0.01^ | 0.05 ± 0.01 |
| Hippocampus | ||||
| Progesterone (ng/g ± SEM) | 0.56 ± 0.05 | 0.53 ± 0.04 | 0.61 ± 0.05 | 0.50 ± 0.03 |
| Dihydroprogesterone (ng/g ± SEM) | 0.4 ± 0.1 | 0.6 ± 0.2 | 1.1 ± 0.3* | 1.4 ± 0.2* |
| 3α,5α-THP (ng/g ± SEM) | 1.5 ± 0.5 | 1.2 ± 0.4** | 2.9 ± 1.2 | 0.9 ± 0.3** |
| Estradiol (pg/g ± SEM) | 0.13 ± 0.01 | 0.05 ± 0.01** | 0.16 ± 0.05 | 0.03 ± 0.01** |
| Diencephalon | ||||
| Progesterone (ng/g ± SEM)*** | 0.23 ± 0.01 | 0.27 ± 0.04 | 0.33 ± 0.03^ | 0.22 ± 0.02 |
| Dihydroprogesterone (ng/g ± SEM)*** | 0.4 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.2^ | 0.6 ± 0.1^ |
| 3α,5α-THP (ng/g ± SEM) | 0.7 ± 0.2 | 0.5 ± 0.2 | 0.7 ± 0.4 | 0.4 ± 0.2 |
| Estradiol (pg/g ± SEM) | 0.04 ± 0.01 | 0.02 ± <0.00** | 0.05 ± 0.01 | 0.02 ± <0.00** |
indicates significant main effect of gestational stress.
indicates significant main effect of sex.
indicates significant interaction between gestational stress and sex conditions
(^ denotes variable stress groups that significantly differ from respective controls when interactions are present), p ≤ 0.05.
Variable stress-exposure and sex significantly interacted to influence E2 concentrations in mPFC [F(1,41) = 5.78, p < 0.05], but not in other regions examined [FHippo(1,41) = 2.61, n.s.; FDienceph(1,41) = 1.29, n.s.]. Gestationally-stressed females had a significant increase in mPFC E2 levels compared to control female offspring, whereas, gestationally-stressed males demonstrated significant decrease in E2 relative to controls. Variable stress was not observed to alter E2 in any other region examined (Table 4). No sex X stressor interactions of DHP [FmPFC (1,41) = 1.31, n.s.; FHippo(1,41) = 0.01, n.s.], P [FmPFC (1,41 = 3.49, n.s.; FHippo(1,41) = 0.89, n.s.], nor 3α,5α-THP [FmPFC(1,41) = 0.78, n.s.; FHippo(1,41) = 2.72, n.s.] were observed in mPFC or hippocampus (Table 4).
Variable stress-exposure and sex significantly interacted to influence P [F(1,41) = 5.83, p < 0.05] and DHP [F(1,41) = 6.43, p < 0.05] concentrations in diencephalon. Gestational exposure to variable stress significantly increased P and DHP in females, but decreased P and DHP in males compared to control offspring. No effects of sex [F(1,41) = 0.80, n.s.], stressor [F(1,41) = 0.20, n.s.], or their interaction [F(1,41) = 0.12, n.s.] were observed for 3α,5α-THP in diencephalon (Table 4).
As expected, there were sex differences in central concentrations of some steroids. 3α,5α-THP was significantly greater in hippocampus of females compared to males [F(1,41) = 4.65, p < 0.05]. As well, females had significantly greater concentrations of E2 in all observed regions, including hippocampus [F(1,41) = 42.57, p < 0.05] and diencephalon [F(1,41) = 24.76, p < 0.05] (Table 4).
DISCUSSION
The hypothesis that gestational stress would reduce performance in the object recognition task, dysregulate central progestogen formation, and reduce dendritic spine density was upheld, albeit, some of these effects were brain region-specific. Irrespective of sex, offspring that were gestationally-exposed to variable stressors performed more poorly on the object recognition task, had dysregulated DHP formation in mPFC and hippocampus, and had reduced dendritic spine density in dorsal hippocampus (but not mPFC or control hypothalamus). Some sex-specific effects were observed wherein stress-exposure increased E2 in mPFC, as well as P and DHP in diencephalon, among females, but decreased these substrates in mPFC and hypothalamus among males. Thus, gestational exposure to variable stressors produces decrements in cognitive performance, hippocampal integrity, and progestogen regulation of hippocampus and prefrontal cortex among juvenile offspring.
The time at which stressors occurred in the present study, (GDs) 17–21, may be a particularly vulnerable neuro-developmental period for offspring. Placental expression of the 11β-hydroxysteroid dehydrogenase type 2 enzyme, which inactivates maternal glucocorticoid, is reduced in this interval given that physical development of the fetus is dependent on some glucocorticoid exposure [34]. Inhibition of 11β-hydroxysteroid dehydrogenase increases early exposure of the gestating fetus to glucocorticoids and can perturb anti-anxiety and cognitive performance of offspring [35,36]. Chronic stress during this time may be detrimental [37–41] and the present investigation supports the notion that unpredictable physical and psychological stressors during this period can have pervasive effects on hippocampal morphology that last at least until pre-puberty. These effects may persist throughout life, given previous findings that adult rat females (prenatal day 75) that were gestationally-exposed to restraint stress have reduced granule cells in hippocampus compared to non-stressed rats [42]. Notably, the aforementioned study yielded no evidence for neurodegenerative effects in gestationally-stressed adult males which may suggest a role for activational steroid effects associated with puberty and development. Indeed, the present investigation reveals aberrations in 5α-reduction of P in mPFC and hippocampus. Females may be more susceptible to sequelae associated with progestogen dysregulation given the importance of progestogens for sexual and physical maturation throughout the female life-cycle.
In females and males, progestogens have important trophic effects throughout development as evidenced by poor in vitro fertilization outcomes when progestogen milieu is low or aberrant [43–45] and improved pregnancy rates when P is administered [46]. Perinatal stress, achieved by maternally-isolating pups within the first three weeks of life, is associated with increased levels of glucocorticoid in plasma, and reduced levels of 3α,5α-THP in hippocampus, at baseline or in response to stress [14,47]. As well, cognitive deficits in water maze performance are observed in perinatally-stressed offspring compared to non-stressed controls [48]. We have recently demonstrated that P turnover to its 5α-reduced metabolites is reduced in mPFC of pre-pubertal females and males that were born to gestationally-restrained dams compared to those born to non-stressed dams [18]. Notably, dams utilized in the current study were young adults and had not had prior pregnancies. This may be considered an additional insult given that multiple prior pregnancies are associated with reduced circulatory corticosterone during pregnancy and greater object recognition performance among dams [26]. Herein, we find that DHP levels are reduced in mPFC of gestationally-stressed offspring, whereas hippocampal DHP is increased compared to controls. The object recognition task is dependent on both, an intact prefrontal cortex and hippocampus and it is of interest that task performance was decreased while hippocampal P turnover was increased. In sheep, central 5α-reduction has been found to be acutely increased in response to a hypoxic insult [49] and pharmacologically blocking this enhancement is associated with greater hippocampal degeneration in the asphyxiated brain [50]. As such, acute elevations of P conversion to its metabolites may be a compensatory mechanism in response to central insult and may explain the greater DHP formation observed in the present study, concomitant with reduced dendritic spine density in hippocampus.
It is notable, that there were no sex differences in functional effects or neural outcomes of gestational stress. While, E2 in mPFC and P/DHP formation in diencephalon were elevated among stress-exposed females compared to stress-exposed males, functional effects on cognition were not readily observed. However, the present observations occurred in juvenile rats, approaching the time of pubertal onset. Some of these effects may be organizing differences or early activational effects of hormones that are sex-specific. Indeed, the hypothalamus is contains sexually-dimorphic nuclei that are hormone sensitive. Given the pre-pubertal ages of the subjects, greater activational influences of steroid hormones can be expected to be minimized which may account for the lack of sex differences in behavior. Among adult rats, exposure to 45 mins of thrice daily restraint from GDs 14–21 were found to eliminate the male-typical advantage in the object recognition task, but, effects of stress impairment on task performance were not globally-observed [51]. As such, the lack of sex differences observed in the present study may reflect the lack of activational hormone variation among the sexes at this age. It would be of interest to assess whether there are fetal programming effects on litter size and sex ratio that influence later hormonal milieu. Future investigations will aim to assess these endpoints.
The present investigation demonstrates that exposure to physical/psychological stressors in late gestation (GDs 17–21) can perturb cognitive development of pre-pubertal offspring, irrespective of sex. Exposure to variable and unpredictable stressors was associated with decreased dendrite spine density in dorsal hippocampus and dysregulated progestogen formation in mPFC and hippocampus. Commensurate with prior investigations, increased 5α-reduction in hippocampus may be indicative of fetal insult which may explain the reduced dendritic spine density in hippocampus concomitant with poorer cognitive performance. These effects may involve neurodegenerative actions of glucocorticoids on hippocampus given that 3α,5α-THP can dampen stress responsivity. Progestogens have been used as tocolytic therapeutics in pregnant women when preterm birth is a risk factor [52–55]. In animal models, gestational 3α,5α-THP administration may ameliorate some anxiety-related aberrations associated with prenatal stress [56]. Whether prenatal administration of P’s 5α-reduced metabolites can ameliorate some of the cognitive and/or neural degenerative sequelae of prenatal stress should be the subject of future investigation.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Science Foundation (IBN03-16083) and the National Institute of Mental Health (MH06769801). We appreciate technical assistance of Kassandra Edinger in collection of behavioral data.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Orr ST, James SA, Miller CA, Barakat B, Daikoku N, Pupkin M, Engstrom K, Huggins G. Psychosocial stressors and low birthweight in an urban population. Am. J. Prev. Med. 1996;12:459–466. [PubMed] [Google Scholar]
- 2.Dayan J, Creveuil C, Herlicoviez M, Herbel C, Baranger E, Savoye C, Thouin A. Role of anxiety and depression in the onset of spontaneous preterm labor. Am. J. Epidemiol. 2002;155:293–301. doi: 10.1093/aje/155.4.293. [DOI] [PubMed] [Google Scholar]
- 3.Lobel M, DeVincent CJ, Kaminer A, Meyer BA. The impact of prenatal maternal stress and optimistic disposition on birth outcomes in medically high-risk women. Health Psychol. 2000;19:544–553. doi: 10.1037//0278-6133.19.6.544. [DOI] [PubMed] [Google Scholar]
- 4.Hadders-Algra M, Huisjes HJ, Touwen BC. Perinatal correlates of major and minor neurological dysfunction at school age: a multivariate analysis. Dev. Med. Child. Neurol. 1988;30:472–481. doi: 10.1111/j.1469-8749.1988.tb04774.x. [DOI] [PubMed] [Google Scholar]
- 5.Holst K, Andersen E, Philip J, Henningsen I. Antenatal and perinatal conditions correlated to handicap among 4-year-old children. Am. J. Perinatol. 1989;6:258–267. doi: 10.1055/s-2007-999588. [DOI] [PubMed] [Google Scholar]
- 6.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.De Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J. Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J. Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Götz F, Dörner G, Malz U, Rohde W, Stahl F, Poppe I, Schulze M, Plagemann A. Short- and long-term effects of perinatal interleukin-1β-application in rats. Neuroendocrinology. 1993;58:344–351. doi: 10.1159/000126560. [DOI] [PubMed] [Google Scholar]
- 10.Uehara A, Gottschall PE, Dahl RR, Arimura A. Stimulation of ACTH release by human interleukin-1β, but not by interleukin-1α, in conscious, freely-moving rats. Biochem. Biophys. Res. Commun. 1987;146:1286–1290. doi: 10.1016/0006-291x(87)90788-1. [DOI] [PubMed] [Google Scholar]
- 11.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutapakdeegul N, Afadlal S, Polaboon N, Phansuwan-Pujito P, Govitrapong P. Repeated restraint stress and corticosterone injections during late pregnancy alter GAP-43 expression in the hippocampus and prefrontal cortex of rat pups. Int. J. Dev. Neurosci. 2010;28:83–90. doi: 10.1016/j.ijdevneu.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur. J. Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Rhodes ME, Raol YH, Brooks-Kayal AR. Early postnatal stimulation alters pregnane neurosteroids in the hippocampus. Psychopharmacology (Berl) 2006;186:343–350. doi: 10.1007/s00213-005-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews SG. Foetal experience: lifelong consequences. J. Neuroendocrinol. 2007;19:73–74. doi: 10.1111/j.1365-2826.2006.01504.x. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert-Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3α-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol. Endocrinol. 2005;21:268–279. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- 17.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl. Acad. Sci. USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paris JJ, Frye CA. Juvenile offspring of rats exposed to restraint stress in late gestation have impaired cognitive performance and dysregulated progestogen formation. Stress. 2010;14(1):23–32. doi: 10.3109/10253890.2010.512375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gué M, Bravard A, Meunier J, Veyrier R, Gaillet S, Recasens M, Maurice T. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behav. Brain Res. 2004;150:149–157. doi: 10.1016/S0166-4328(03)00250-X. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Zhang L, Fang Z, Lin L, Wu C, Huang Q. Behavioral and neurobiological studies on the male progeny of maternal rats exposed to chronic unpredictable stress before pregnancy. Neurosci. Lett. 2010;469:278–282. doi: 10.1016/j.neulet.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Song TB, Li YJ, He KS, Ge L, Wang LR. Prenatal restraint stress impairs learning and memory and hippocampal PKCβ1 expression and translocation in offspring rats. Brain Res. 2007;1141:205–213. doi: 10.1016/j.brainres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol. Behav. 1975;14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- 23.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol. Biochem. Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 24.Broadbent N, Squire L, Clark R. Spatial memory, recognition memory, and the hippocampus. Neuroscience. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain. Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 26.Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurbiol. Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson WJ, Felten DL. A modified Golgi-Cox technique for morphological characterization of serotonergic neurons. J. Histochem. Cytochem. 1982;30:750–755. doi: 10.1177/30.8.6181119. [DOI] [PubMed] [Google Scholar]
- 30.Choi S, Dallman MF. Hypothalamic obesity: multiple routes mediated by loss of function in medial cell groups. Endocrinology. 1999;140:4081–4088. doi: 10.1210/endo.140.9.6964. [DOI] [PubMed] [Google Scholar]
- 31.Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. J. Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- 32.Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain. Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- 33.Rodbard D, Hutt DM. Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. In: International Atomic Energy Agency, editor. Symposium on Radioimmunoassay and Related Procedures in Medicine. New York: Uniput; 1974. pp. 209–233. [Google Scholar]
- 34.Seckl JR. Glucocorticoids, feto-placental 11β-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids. 1997;62:89–94. doi: 10.1016/s0039-128x(96)00165-1. [DOI] [PubMed] [Google Scholar]
- 35.Seckl JR, Walker BR. 11β-hydroxysteroid dehydrogenase type 1 as a modulator of glucocorticoid action: from metabolism to memory. Trends Endocrinol. Metab. 2004;15:418–424. doi: 10.1016/j.tem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Welberg LA, Seckl JR, Holmes MC. Inhibition of 11β-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur. J. Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 37.Frye CA, Orecki ZA. Prenatal stress produces deficits in socio-sexual behavior of cycling, but not hormone-primed, Long-Evans rats. Pharmacol. Biochem. Behav. 2002;73:53–60. doi: 10.1016/s0091-3057(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 38.Frye CA, Orecki ZA. Prenatal stress alters reproductive responses of rats in behavioral estrus and paced mating of hormone-primed rats. Horm. Behav. 2002;42:472–483. doi: 10.1006/hbeh.2002.1834. [DOI] [PubMed] [Google Scholar]
- 39.Kapoor A, Leen J, Matthews SG. Molecular regulation of the hypothalamic-pituitary-adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J. Physiol. 2008;586:4317–4326. doi: 10.1113/jphysiol.2008.153684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapoor A, Kostaki A, Janus C, Matthews SG. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav. Brain. Res. 2009;197:144–149. doi: 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Setiawan E, Jackson MF, MacDonald JF, Matthews SG. Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus. J. Physiol. 2007;581:1033–1042. doi: 10.1113/jphysiol.2006.127381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol. Psychiatry. 2002;7:810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- 43.Albano C, Grimbizis G, Smitz J, Riethmüller-Winzen H, Reissmann T, Van Steirteghem A, Devroey P. The luteal phase of nonsupplemented cycles after ovarian superovulation with human menopausal gonadotropin and the gonadotropin-releasing hormone antagonist Cetrorelix. Fertil. Steril. 1998;70:357–359. doi: 10.1016/s0015-0282(98)00135-6. [DOI] [PubMed] [Google Scholar]
- 44.Edwards RG, Steptoe PC, Purdy JM. Establishing full-term pregnancies using cleaving embryos grown in vitro. Br. J. Obstet. Gynaecol. 1980;87:737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- 45.Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, Van Waesberghe L, Wisanto A, Van Steirteghem AC. The luteal phase and early pregnancy after combined GnRH-agonist/HMG treatment for superovulation in IVF or GIFT. Hum. Reprod. 1988;3:585–590. doi: 10.1093/oxfordjournals.humrep.a136750. [DOI] [PubMed] [Google Scholar]
- 46.Soliman SS, Daya S, Collins J, Hughes EG. The role of luteal phase support in infertility treatment: a meta-analysis of randomized trials. Fertil. Steril. 1994;61:1068–1076. doi: 10.1016/s0015-0282(16)56758-2. [DOI] [PubMed] [Google Scholar]
- 47.McCormick CM, Kehoe P, Mallinson K, Cecchi L, Frye CA. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol. Biochem. Behav. 2002;73:77–85. doi: 10.1016/s0091-3057(02)00758-x. [DOI] [PubMed] [Google Scholar]
- 48.Frisone DF, Frye CA, Zimmerberg B. Social isolation stress during the third week of life has age-dependent effects on spatial learning in rats. Behav. Brain. Res. 2002;128:153–160. doi: 10.1016/s0166-4328(01)00315-1. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen PN, Ross Young I, Walker DW, Hirst JJ. Allopregnanolone in the brain and blood after disruption of the hypothalamic-pituitary-adrenal axis in fetal sheep. J. Endocrinol. 2004;182:81–88. doi: 10.1677/joe.0.1820081. [DOI] [PubMed] [Google Scholar]
- 50.Yawno T, Yan EB, Walker DW, Hirst JJ. Inhibition of neurosteroid synthesis increases asphyxia-induced brain injury in the late gestation fetal sheep. Neurosci. 2007;146:1726–1733. doi: 10.1016/j.neuroscience.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 52.Christian MS, Brent RL, Calda P. Embryo-fetal toxicity signals for 17α-hydroxyprogesterone caproate in high-risk pregnancies: a review of the non-clinical literature for embryo-fetal toxicity with progestins. J. Matern. Fetal Neonatal Med. 2007;20:89–112. doi: 10.1080/14767050601178758. [DOI] [PubMed] [Google Scholar]
- 53.Di Renzo GC, Rosati A, Mattei A, Gojnic M, Gerli S. The changing role of progesterone in preterm labour. BJOG. 2005;112:57–60. doi: 10.1111/j.1471-0528.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 54.Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br. J. Obstet. Gynaecol. 1990;97:149–154. doi: 10.1111/j.1471-0528.1990.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 55.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O'Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17α-hydroxyprogesterone caproate. N. Engl. J. Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 56.Zimmerberg B, Blaskey LG. Prenatal stress effects are partially ameliorated by prenatal administration of the neurosteroid allopregnanolone. Pharmacol. Biochem. Behav. 1998;59:819–827. doi: 10.1016/s0091-3057(97)00540-6. [DOI] [PubMed] [Google Scholar]