Abstract
Our aim was to investigate whether high-risk HPV (hrHPV) mRNA detection by PreTect HPV-Proofer can be used to stratify hrHPV DNA-positive women of different cytology classes for risk of high-grade cervical intraepithelial neoplasia or worse (cervical precancer or cancer, i.e., cervical intraepithelial neoplasia grade 2 or higher [≥CIN2]). A total of 375 women participating in population-based screening, with a GP5+/6+-PCR hrHPV DNA-positive cervical scrape with normal cytology (n = 202), borderline or mild dyskaryosis (BMD) (n = 88), or moderate dyskaryosis or worse (>BMD) (n = 85), were enrolled. Cervical scrapes were additionally subjected to HPV16/18/31/33/45 E6/E7 mRNA analysis by PreTect HPV-Proofer (mRNA test). Referral and follow-up policies were based on cytology, hrHPV DNA, and mRNA testing. The primary study endpoint was the number of ≥CIN2 detected within 3 years of follow-up. The mRNA positivity increased with the severity of cytological abnormality, ranging from 32% (64/202) in hrHPV DNA-positive women with normal cytology to 47% (41/88) in BMD and 68% (58/85) in >BMD groups (P < 0.01). Women with ≥CIN2 were more likely to test positive by mRNA test (63%) than women without evidence of ≥CIN2 (32%; P < 0.01). A positive mRNA test result conferred an increased ≥CIN2 risk in hrHPV DNA-positive women with normal cytology, i.e., 0.55 (95% confidence interval [95% CI], 0.34 to 0.76) in mRNA-positive versus 0.20 (95% CI, 0.07 to 0.33) in mRNA-negative women. In hrHPV DNA-positive women with BMD or >BMD, the result of the mRNA test did not influence the ≥CIN2 risk. In conclusion, mRNA testing by PreTect HPV-Proofer might be of value to select hrHPV DNA-positive women with normal cytology in need of immediate referral for colposcopy.
INTRODUCTION
To reduce the mortality and morbidity of cervical cancer, most developed countries have adopted some form of cervical screening using cytological examination of cervical smears. However, cervical cytology is known to display only a modest sensitivity for cervical precancer or cancer (i.e., cervical intraepithelial neoplasia grade 2 or higher [≥CIN2]) (3, 15).
Infection with high-risk human papillomavirus (hrHPV) has been recognized as the necessary cause of cervical cancer (27, 44). Recently, evidence has accumulated that a considerable improvement of the effectiveness of cervical cancer screening can be achieved by using hrHPV DNA testing as a primary screening tool. hrHPV DNA testing has a higher sensitivity for ≥CIN2 and consequently better protects against high-grade CIN and cervical cancer in the subsequent screening round than cytology (2, 8, 21, 29, 33, 35). This permits extension of the screening interval. Nonetheless, hrHPV infections are rather common in a screened population and most are transient. In fact, the positive predictive value (PPV) of an hrHPV test for ≥CIN2 is rather low (i.e., only a small proportion of the women who test positive for hrHPV DNA will have or develop ≥CIN2 lesions). It is therefore important to consider alternative or supplementary testing methods in order to limit unnecessary follow-up procedures for women with clinically irrelevant, transient hrHPV infections.
In this context, reflex cytology has been advocated as a valuable triage tool for hrHPV DNA-positive women. In population-based screening programs, hrHPV-positive women with abnormal cytology have a high risk of underlying ≥CIN2, but this does not account for the up to 8% of the hrHPV-positive women with normal cytology who also have or will develop ≥CIN2 (16, 34). Thus, there is a need for biomarkers that allow stratification of hrHPV-positive women with normal cytology for risk of ≥CIN2 or, alternatively, for biomarkers that can replace cytology as a triage tool. As activity of the hrHPV oncogenes E6 and E7 is pivotal not only for the initiation but also for maintenance of the malignant phenotype (48), demonstration of hrHPV E6/E7 transcripts might be more specific than hrHPV DNA testing for detection of ≥CIN2. Transcript analysis is nowadays feasible on cervical scrapings because the introduction of liquid-based cytology has resulted in collection media that preserve RNA sufficiently to allow in vitro amplification and detection (13). The PreTect HPV-Proofer assay (NorChip AS, Klokkarstua, Norway) has been developed for hrHPV E6/E7 transcript analysis. This is a nucleic acid sequence-based amplification (NASBA) (13)-based, isothermal, RNA amplification method in a real-time format that detects E6/E7 mRNA of the five hrHPV types (i.e., HPV16, -18, -31, -33, and -45) that are the major types associated with cervical squamous cell carcinomas and adenocarcinomas (17). Several studies have investigated the PreTect HPV-Proofer assay on biopsy and cervical scraping samples (14, 22, 24–26, 43) and showed that the ratio of hrHPV E6/E7 mRNA positivity to hrHPV DNA positivity increased along with the histological severity of dysplasia. This suggests a higher specificity of this mRNA assay for high-grade cervical lesions compared to HPV DNA assays. The clinical performance of the PreTect HPV-Proofer test as a triage test for cytology and/or HPV DNA testing has been determined in various studies (4, 5, 10, 31, 32, 38, 39), but it has not yet been extensively evaluated in women participating in population-based cervical screening.
In the current study, we investigated whether hrHPV mRNA detection by PreTect HPV-Proofer can be used as a reflex test to stratify hrHPV DNA-positive women of different cytology classes from a screening population for risk of ≥CIN2. Risk estimates of mRNA testing per cytology class (i.e., normal cytology, borderline or mild dyskaryosis [BMD], and severe dyskaryosis or worse [>BMD]) were assessed and compared to those of (repeated) HPV DNA testing and genotyping.
MATERIALS AND METHODS
Study subjects.
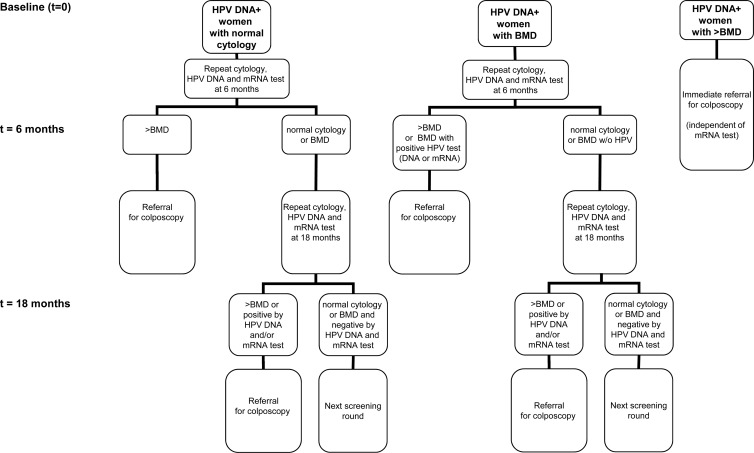
Women were recruited among those who visited general practitioner practices for a cervical smear in the greater Amsterdam area in The Netherlands from January 2005 till March 2006. The target group participated in a population-based cervical screening program in which women between 30 and 60 years of age are invited for screening every 5 years. Exclusion criteria included a history of ≥CIN2 or abnormal cytology in the preceding 2 years or hysterectomy. The resulting 13,401 women were tested both by conventional cytology and by GP5+/6+-PCR for hrHPV DNA presence. For the purpose of this study, we enrolled 238 hrHPV DNA-positive women among 6,555 consecutive women who had normal cytology (Fig. 1). In addition, out of 266 consecutive women with borderline or mild dyskaryosis (BMD), the 101 with a positive hrHPV DNA test result were enrolled as well. Finally, we enrolled 91 hrHPV DNA-positive women from 108 consecutive women with a cytological outcome of >BMD (moderate dyskaryosis or worse, equaling high-grade squamous intraepithelial lesion [HSIL]). The 430 hrHPV DNA-positive women were subsequently subjected to hrHPV mRNA analysis by PreTect HPV-Proofer (mRNA test). An invalid mRNA test result (i.e., negative for both the housekeeping gene U1A and E6/E7 mRNA) was obtained for 36 enrolled women with normal cytology, 13 with BMD, and 6 with >BMD. These 55 women were excluded, leaving a total of 375 hrHPV DNA-positive women (median age, 35 years; range, 19 to 68 years), including 202 with normal cytology, 88 with BMD, and 85 with>BMD. Referral strategy is depicted in Fig. 2. hrHPV DNA-positive women with >BMD were immediately referred for colposcopy, and those with hrHPV DNA-positive normal cytology and BMD at baseline were advised to have repeat cytology, HPV DNA, and mRNA analyses at 6 and 18 months. At 6 months, women were referred for colposcopy if cytology was >BMD. For women with BMD at baseline, colposcopy referral at 6 months was also advised in case of repeat BMD cytology and a positive result of either HPV test, i.e., HPV DNA positive or mRNA positive. At 18 months, women were referred in case of >BMD cytology or a positive HPV DNA and/or mRNA test result. Otherwise, women were recalled at the subsequent screening round. Informed consent was obtained from all study participants, and this study followed the ethical guidelines of the medical center.
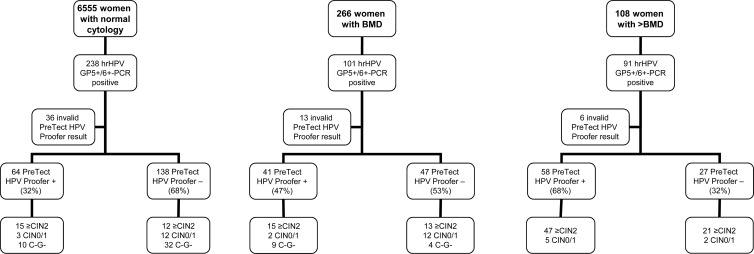
Fig 1.
Flowchart study population. Flowchart of the GP5+/6+-PCR hrHPV-positive women who were enrolled in this study and baseline HPV mRNA results. C−G−, a double-negative cytology (C−) and hrHPV DNA GP5+/6+-PCR (G−) test result at the last follow-up visit.
Fig 2.
Referral strategy. Flowchart of the referral strategy used in this study. hrHPV DNA-positive women with >BMD were immediately referred for colposcopy, and those with hrHPV DNA-positive normal cytology and BMD at baseline were advised to have repeat cytology, HPV DNA, and mRNA analyses at 6 and 18 months. At 6 months, women were referred for colposcopy if cytology was >BMD. For women with BMD at baseline, colposcopy referral at 6 months was also advised in the event of repeat BMD cytology and a positive result of either HPV test, i.e., HPV DNA positive or mRNA positive. At 18 months, women were referred in case of >BMD cytology or a positive HPV DNA and/or mRNA test result. Otherwise, women were recalled at the subsequent screening round.
Specimen collection, preparation, and nucleic acid testing.
Conventional cytological smears were taken with a Cervex-Brush (Rovers, Oss, The Netherlands) or a cytobrush. After the smear was made on a glass slide, cervical scrapes were collected for hrHPV testing by placing the brush in 5 ml universal collection medium (UCM) (Digene) (40). Pilot studies suggested that this collection medium preserves RNA sufficiently for successful NASBA analysis (data not shown). For GP5+/6+-PCR enzyme immunoassay analysis and subsequent genotyping for 14 high-risk types (i.e., HPV16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66, and -68), samples were processed and analyzed as described previously (19, 41). For hrHPV DNA-positive samples, 1/10th of the specimen was centrifuged at 3,000 × g for 10 min, and pellets were resuspended in 1 ml NucliSENS lysis buffer (bioMérieux, Boxtel, The Netherlands). Total RNA was extracted by the NucliSENS easyMAG procedure according to the recommendations of the manufacturer (bioMérieux, Boxtel, The Netherlands). Subsequently, PreTect HPV-Proofer analysis for HPV16/18/31/33/45 E6/E7 mRNA was performed according to the manufacturer's instructions (NorChip AS, Klokkarstua, Norway). The assay includes detection of mRNA from the human U1 small nuclear ribonucleoprotein (snRNP)-specific protein A (U1A) to monitor sample mRNA integrity. The PreTect HPV-Proofer assay uses molecular beacon probes in three real-time multiplex NASBA assays that run in parallel.
Cytology reading.
Cervical smears were classified blinded for the HPV testing results, according to the CISOE-A (national pro forma reporting on composition, inflammation, squamous, other and endometrium, and endocervical cylindrical epithelium, and adequacy) classification used in The Netherlands. The results can be translated easily into the Bethesda 2001 classification (7). In the Bethesda classification, 2001 BMD equals ASC-US/ASC-H/LSIL, and >BMD is equal to high-grade squamous intraepithelial lesion (HSIL).
Histology.
Histological follow-up data were obtained from the countrywide automated pathology registry (PALGA) within 3 years after baseline analysis. Histological examination was done locally, and specimens were classified as normal (CIN0), CIN1, CIN2, CIN3, or invasive cancer, according to international criteria (1, 46).
Statistical analysis.
The proportions of mRNA-positive samples at baseline in hrHPV DNA-positive women with normal cytology, BMD, and >BMD were compared using the chi-square test. The primary study endpoint in this study was the number of histologically confirmed ≥CIN2 lesions detected within 3 years of follow-up. We used two outcome measures for absence of ≥CIN2. The first was the presence of histologically confirmed CIN0/1. The second added a double-negative cytology and hrHPV DNA test at the last follow-up visit in case no histology was available, since the risk of ≥CIN2 in this circumstance is extremely low (8, 9, 29, 36). Both outcome measures were used to calculate separately the 3-year risks (PPV) of ≥CIN2 after an mRNA result at baseline for hrHPV DNA-positive women with normal cytology, BMD, and >BMD. Adjusted ≥CIN2 risks, calculated using Kaplan-Meier analysis, were obtained by adjusting for women who did not attend or only partially attended repeat follow-up testing and women whose result had no defined endpoint. The study focused on the calculation of PPV (≥CIN2 risks) because of the aim to study the potential application of the test as a positive triage tool to select women for immediate referral for colposcopy. Given the skewed study population, the denominators for sensitivity and specificity calculations are essentially unknown and therefore were not calculated.
≥CIN2 risks of hrHPV DNA-positive women per cytology class were calculated for mRNA testing and genotyping. The persistence of viral nucleic acids was determined in the subset of women who had a second test result 6 months later. ≥CIN2 risks were also calculated for 6-month persistence of hrHPV DNA and RNA, defined as a positive test result at the 6-month visit for the same hrHPV type detected at baseline.
RESULTS
A flowchart of the hrHPV DNA-positive study population and the baseline mRNA test results per cytology class are given in Fig. 1. Among all enrolled women with normal cytology, 69.8% (141/202) tested positive for DNA of at least one of the five HPV types (i.e., HPV16, -18, -31, -33, and -45) included in the mRNA test. This percentage was 64.8% (57/88) among enrolled women with BMD and 78.8% (67/85) among enrolled women with >BMD. The mRNA positivity increased with severity of cytological abnormality and ranged from 32% (64/202) in normal cytology to 47% (41/88) in BMD and 68% (58/85) in >BMD. This increase was statistically significant (P < 0.01).
Among the 163 women testing positive by mRNA test at baseline, HPV16 predominated and accounted for 74% (120/163), followed by HPV18 (9%), HPV45 (7%), HPV33 (7%), and HPV31 (5%). At baseline, the mRNA test showed a 92% (150/163) type concordance with HPV DNA. Of the 13 discordant samples, 7 samples were found to have different HPV types at DNA and mRNA levels among types HPV16, -18, -31, -33, and -45, and 6 samples positive with the mRNA test were found positive with non-HPV16/18/31/33/45 type(s) with the DNA test.
Histology results were obtained from 159 women within 3 years of follow-up (Table 1). The median follow-up time for women with histology-proven CIN0/1 was 10.8 months (range, 0.7 to 39.3 months) and 4.3 months (range, 0.2 to 37.6 months) for women with ≥CIN2. The mRNA positivity increased with severity of histological abnormality and ranged from 28% (10/36) in CIN0/1 to 59% (24/41) in CIN2, 63% in CIN3 (50/79), and 100% (3/3) in squamous cell carcinoma (SCC). Women with ≥CIN2 were more likely to test positive for mRNA at baseline than those with CIN0/1 (63% versus 28%; P < 0.01). Among HPV16 DNA-positive women, 88% (56/64) of ≥CIN2 and 42% (5/12) of CIN0/1 were mRNA test positive, and 50% (19/38) of ≥CIN2 and 40% (4/10) of CIN0/1 were mRNA test positive among HPV18, -31, -33, and/or -45 DNA-positive women.
Table 1.
mRNA test results at baseline stratified by cytology class and histological outcome
| Cytology | Histology | No. (%) with mRNA status |
||
|---|---|---|---|---|
| mRNA+ | mRNA− | Total | ||
| Normal | CIN0/1 | 3 (20) | 12 (80) | 15 (100) |
| CIN2 | 8 (62) | 5 (38) | 13 (100) | |
| CIN3 | 7 (50) | 7 (50) | 14 (100) | |
| SCC | ||||
| BMD | CIN0/1 | 2 (14) | 12 (86) | 14 (100) |
| CIN2 | 5 (42) | 7 (58) | 12 (100) | |
| CIN3 | 10 (63) | 6 (37) | 16 (100) | |
| SCC | ||||
| >BMD | CIN0/1 | 5 (71) | 2 (29) | 7 (100) |
| CIN2 | 11 (69) | 5 (31) | 16 (100) | |
| CIN3 | 33 (67) | 16 (33) | 49 (100) | |
| SCC | 3 (100) | 0 (0) | 3 (100) | |
| All classes | CIN0/1 | 10 (28) | 26 (72) | 36 (100) |
| CIN2 | 24 (59) | 17 (41) | 41 (100) | |
| CIN3 | 50 (63) | 29 (37) | 79 (100) | |
| SCC | 3 (100) | 0 (0) | 3 (100) | |
Of 216 women with no histological follow-up, 55 had normal cytology and a negative hrHPV DNA test result at the last follow-up visit. These HPV DNA-negative women with normal cytology were considered as having no ≥CIN2 and were added to those with a histological outcome in the calculations as shown in Table 2. The median follow-up time for the total group of 91 women without evidence of ≥CIN2 was 17.2 months (range, 0.7 to 39.3 months). Twenty-nine (32%) of them had a positive baseline mRNA test, a result which was also significantly lower than the 63% of women with ≥CIN2 who had a positive mRNA test (P < 0.01).
Table 2.
mRNA results at baseline stratified by cytology class and clinical outcome
| Cytology | Histology | No. (%) with mRNA status |
||
|---|---|---|---|---|
| mRNA+ | mRNA− | Total | ||
| Normal | ≥CIN2 | 15 (56) | 12 (44) | 27 (100) |
| CIN0/1 or C−G−a | 13 (23) | 44 (77) | 57 (100) | |
| Total | 28 (33) | 56 (67) | 84 (100) | |
| BMD | ≥CIN2 | 15 (54) | 13 (46) | 28 (100) |
| CIN0/1 or C−G− | 11 (41) | 16 (59) | 27 (100) | |
| Total | 26 (47) | 29 (53) | 55 (100) | |
| >BMD | ≥CIN2 | 47 (69) | 21 (31) | 68 (100) |
| CIN0/1 or C−G− | 5 (71) | 2 (29) | 7 (100) | |
| Total | 52 (69) | 23 (31) | 75 (100) | |
| All classes | ≥CIN2 | 77 (63) | 46 (37) | 123 (100) |
| CIN0/1 or C−G− | 29 (32) | 62 (68) | 91 (100) | |
| Total | 106 (50) | 108 (50) | 214 (100) | |
Either histologically confirmed CIN0/1 or a double-negative cytology (C−) and hrHPV DNA GP5+/6+-PCR (G−) test result at the last follow-up visit.
≥CIN2 risk estimates of mRNA testing and genotyping are depicted in Table 3. A positive mRNA test result in HPV DNA-positive women with normal cytology revealed a 55% (95% CI, 34 to 76%) risk of ≥CIN2 in contrast to a risk of 20% (95% CI, 7 to 33%) among those testing mRNA negative. The ≥CIN2 risk estimate of a positive mRNA test among HPV DNA-positive women with normal cytology conferred a marginally, though not significantly, increased risk over both the HPV16 DNA-positive test (42% [95% CI, 22 to 63]) and the HPV16, -18, -31, -33, and/or -45 DNA-positive test (43% [95% CI, 28 to 58]). In hrHPV DNA-positive women with BMD or >BMD, the result of the mRNA test did not influence the ≥CIN2 risk.
Table 3.
Adjusted 3-year risk (PPV) of ≥CIN2 stratified by cytology, mRNA testing, and HPV genotyping
| Baseline cytology class | Risk ≥CIN2 (95% CI)a by single test group at baseline for: |
|||||
|---|---|---|---|---|---|---|
| mRNA− | mRNA+ | HPV16/18/31/33/45 DNA− | HPV16/18/31/33/45 DNA+ | HPV16 DNA− | HPV16 DNA+ | |
| Normal | 0.20 (0.07–0.33) | 0.55 (0.34–0.76) | 0.08 (−0.07–0.22) | 0.43 (0.28–0.58) | 0.27 (0.12–0.43) | 0.42 (0.22–0.63) |
| BMD | 0.43 (0.25–0.62) | 0.54 (0.32–0.75) | 0.38 (0.17–0.59) | 0.55 (0.36–0.73) | 0.39 (0.24–0.55) | 0.75 (0.48–1.02) |
| >BMD | 0.91 (0.80–1.03) | 0.90 (0.82–0.98) | 0.80 (0.60–1.00) | 0.93 (0.87–1.00) | 0.88 (0.77–0.99) | 0.93 (0.85–1.01) |
Risk estimates were corrected for lost to follow-up by means of Kaplan-Meier analysis. For these calculations, the absence of ≥CIN2 was defined as histology-proven CIN0/1 or a double-negative cytology and hrHPV DNA test result at the last follow-up visit. Similar data were obtained when only women with histology-confirmed CIN0/1 were considered to have ≥CIN2 (data not shown).
DISCUSSION
In this study, we evaluated PreTect HPV-Proofer E6/E7 mRNA assessment as a candidate triage tool to stratify hrHPV DNA-positive women of different cytology classes for the risk of high-grade CIN or worse within 3 years of follow-up. Assessment per cytology class revealed that PreTect HPV-Proofer is particularly of value for hrHPV DNA-positive women with normal cytology given their markedly increased risk of ≥CIN2 in case of a positive mRNA test result.
Triage and/or follow-up strategies for hrHPV DNA-positive women are important topics of current research given that referring all hrHPV DNA-positive women to colposcopy will result in overdiagnosis, overtreatment, and high costs. Several triage suggestions have been published, including (repeat) cytology, hrHPV genotyping, hrHPV type-specific persistence analysis, viral load, and p16 staining (6, 11, 18, 20, 28, 30, 34, 37, 42, 45, 47). This study adds E6/E7 mRNA assessment to those suggestions.
In this study, a slightly higher risk of ≥CIN2 with mRNA assay than with HPV genotyping for HPV16 or HPV16, -18, -31, -33, and -45 was seen among HPV-DNA-positive women with normal cytology. This suggests that the value of PreTect HPV-Proofer mRNA analysis is not solely based on HPV type restriction but to some extent is also related to mRNA detection, although the numbers in this study are too small to make fair conclusions. The ≥CIN2 risk of a single positive mRNA test result in women with normal cytology was similar to that for for women with normal cytology having a positive hrHPV DNA test result at two consecutive visits 6 months apart. Although this estimate is based on a very small sample size (n = 11 women), the crude ≥CIN2 risk after a 6-month persistently positive hrHPV DNA test was 45% (95% CI, 30 to 60) for women with normal cytology (data not shown). This finding might suggest that the ≥CIN2 risk associated with a single positive mRNA test result (i.e., 0.55 [range, 0.34 to 0.76]) (Table 3) is in the same range as the risk conferred by a 6-month persistently positive hrHPV DNA test. A 6-month persistently positive mRNA test, observed in 8 women with normal cytology, conferred the highest risk of ≥CIN2 in women with normal cytology, i.e., 63% (95% CI, 30 to 86).
A limitation of the mRNA test is its sensitivity for ≥CIN2 of 63% (77/123) among those with a positive DNA test. Our data suggest that that PreTect HPV-Proofer assay misses slightly more HPV18, -31, -33, and -45-associated ≥CIN2 than HPV16-associated ≥CIN2. This finding may be related to differences in mRNA expression level of the different genotypes and/or differences in the sensitivities of the assay for detecting mRNA of the different genotypes.
Considering a ≥10% risk of CIN3 within the subsequent 2 or 3 years to warrant immediate colposcopic evaluation, and a 2% to 10% CIN3 risk to warrant repeat testing in a year, as exemplified by Castle et al. (12), the results of the current study would indicate that mRNA test-positive, HPV DNA-positive, cytologically normal women should be directly referred for colposcopy. The above is based on the estimate that the CIN3 risk would be higher than 10% (11%; 7/64) even under the assumption that mRNA test-positive women who were lost to follow-up in our study had no CIN3. On the other hand, HPV DNA-positive, cytologically normal women who are mRNA test negative would still need to undergo follow-up examinations. Even when assuming that none of the mRNA test-negative, cytologically normal women who were lost to follow-up had a CIN3, the risk of CIN3 among them would be 5% (7/138). As ≥CIN3 risk for hrHPV DNA-positive women with ≥BMD is high (39% [68/173]), direct referral for colposcopy for this group remains warranted irrespective of the mRNA test outcome (34). Nonetheless, in settings where cytology is lacking, any mRNA test-positive women could be considered for direct referral for colposcopy given the high risk of ≥CIN2, while those hrHPV DNA-positive women who test negative with an mRNA test should undergo follow-up examinations.
Expression of HPV E6/E7 mRNA was demonstrated in 43% of hrHPV DNA-positive smears, and 92% of the double DNA/mRNA-positive smears showed HPV type concordance. mRNA test positivity increased with the severity of cytomorphological abnormalities as well as the severity of CIN disease. These findings are in line with those of previous studies (25, 43) and suggest that infections with HPV16, -18, -31, -33, and -45 coinciding with substantial E6/E7 mRNA levels as detected by PreTect HPV-Proofer are preferentially associated with high-grade CIN disease and relatively rare under conditions (i.e., transient hrHPV infections) with a low likelihood of progression to premalignant disease.
This study has some limitations. Women were recruited at general practitioner practices in The Netherlands. Although the target group was women invited for population-based screening, in practice a number of women outside the age range for population-based cervical screening in The Netherlands (i.e., <30 or >60 years) who underwent opportunistic screening in these practices had also been enrolled. However, data were similar when we restricted our analyses to women within the screening age (data not shown). During follow-up, exceptions to the protocol were also encountered, resulting in a loss of samples for clinical evaluation. Furthermore, as cytology was the selection criterion for inclusion in the study, baseline mRNA data cannot be compared to baseline cytological results. The statistical outcomes will therefore be biased toward cytology, which is further emphasized by the fact that cytology was the main decisive factor for colposcopy and biopsy referral. Furthermore, it should be realized that the number of women with histologically confirmed absence of ≥CIN2 was relatively low. We therefore extended this category by including women who had a double-negative cytology and hrHPV DNA test result at the last follow-up visit. These women have a negligible risk of ≥CIN2 (8, 9, 23). Hence, it is unlikely that extension of the absence of ≥CIN2 group with “double-negative” women would have a major influence on the risk evaluation.
The collection medium used in this study has, as far as we are aware of, not been validated for mRNA analysis by PreTect HPV-Proofer, and only 1/10th of the specimens was available for mRNA analysis. As a consequence, the input amount was less than what is recommended for HPV E6/E7 mRNA analysis by PreTect HPV-Proofer. Indeed, the observed U1A failure rate in the current study (12.8%) was higher than what is usually seen (i.e., 3 to 5%), and therefore, it cannot be ruled out that some of the HPV mRNA results in this study are false negatives.
In conclusion, this study showed that a positive PreTect HPV-Proofer reflex test confers an increased risk of ≥CIN2 in hrHPV DNA-positive women, a finding that is of particular significance for those with normal cytology. Hence, the PreTect HPV-Proofer test may be used to select those hrHPV DNA-positive women who are in direct need of colposcopy examination. This approach would save these women from extra follow-up visits. However, HPV DNA-positive women who test negative with PreTect HPV-Proofer should still undergo follow-up examinations, since their risk of CIN3 is too high to leave these women without follow-up exams or treatment.
ACKNOWLEDGMENTS
We thank F. Topal for excellent technical assistance. The PreTect HPV-Proofer reagents were kindly provided by NorChip AS.
This work was supported by a grant from the Dutch Cancer Society (KWF VU2006-3570). The sources of funding did not have any influence on the design and the analysis of the results.
H.S., F.K., and E.M. are shareholders of NorChip AS. C.J.L.M.M., P.J.F.S., and D.A.M.H. are shareholders of Self-screen BV, a recent spin-off company of VU University Medical Center. All other authors declare that they have no conflict of interest.
Footnotes
Published ahead of print 2 May 2012
REFERENCES
- 1. Anderson MC. 1995. Premalignant and malignant squamous lesions of the cervix, p 292–297 In Fox H, Wells M. (ed), Haines and Taylor obstetrical and gynaecological pathology. Churchill Livingstone, New York, NY [Google Scholar]
- 2. Anttila A, et al. 2010. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 340:c1804 doi:10.1136/bmj.c1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arbyn M, et al. 2006. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine 24(Suppl 3):S78–S89 [DOI] [PubMed] [Google Scholar]
- 4. Benevolo M, et al. 2011. Diagnostic and prognostic validity of the human papillomavirus E6/E7 mRNA test in cervical cytological samples of HC2-positive patients. Cancer Causes Control 22:869–875 [DOI] [PubMed] [Google Scholar]
- 5. Benevolo M, et al. 2011. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J. Clin. Microbiol. 49:2643–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berkhof J, et al. 2006. HPV type-specific 18-month risk of high-grade cervical intraepithelial neoplasia in women with a normal or borderline/mildly dyskaryotic smear. Cancer Epidemiol. Biomarkers Prev. 15:1268–1273 [DOI] [PubMed] [Google Scholar]
- 7. Bulk S, van Kemenade FJ, Rozendaal L, Meijer CJ. 2004. The Dutch CISOE-A framework for cytology reporting increases efficacy of screening upon standardisation since 1996. J. Clin. Pathol. 57:388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bulkmans NW, et al. 2007. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 370:1764–1772 [DOI] [PubMed] [Google Scholar]
- 9. Bulkmans NW, Rozendaal L, Voorhorst FJ, Snijders PJ, Meijer CJ. 2005. Long-term protective effect of high-risk human papillomavirus testing in population-based cervical screening. Br. J. Cancer 92:1800–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burger EA, Kornor H, Klemp M, Lauvrak V, Kristiansen IS. 2011. HPV mRNA tests for the detection of cervical intraepithelial neoplasia: a systematic review. Gynecol. Oncol. 120:430–438 [DOI] [PubMed] [Google Scholar]
- 11. Carozzi F, et al. 2008. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 9:937–945 [DOI] [PubMed] [Google Scholar]
- 12. Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. 2007. Risk assessment to guide the prevention of cervical cancer. Am. J. Obstet. Gynecol. 197:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuschieri KS, Beattie G, Hassan S, Robertson K, Cubie H. 2005. Assessment of human papillomavirus mRNA detection over time in cervical specimens collected in liquid based cytology medium. J. Virol. Methods 124:211–215 [DOI] [PubMed] [Google Scholar]
- 14. Cuschieri KS, Whitley MJ, Cubie HA. 2004. Human papillomavirus type specific DNA and RNA persistence—implications for cervical disease progression and monitoring. J. Med. Virol. 73:65–70 [DOI] [PubMed] [Google Scholar]
- 15. Cuzick J, et al. 2006. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int. J. Cancer 119:1095–1101 [DOI] [PubMed] [Google Scholar]
- 16. Cuzick J, et al. 2008. Long-term follow-up of cervical abnormalities among women screened by HPV testing and cytology—results from the Hammersmith study. Int. J. Cancer 122:2294–2300 [DOI] [PubMed] [Google Scholar]
- 17. Guan P, et al. 2012. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int. J. Cancer doi:10.1002/ijc.27485 [DOI] [PubMed] [Google Scholar]
- 18. Hesselink AT, et al. 2011. Combined promoter methylation analysis of CADM1 and MAL: an objective triage tool for high-risk human papillomavirus DNA-positive women. Clin. Cancer Res. 17:2459–2465 [DOI] [PubMed] [Google Scholar]
- 19. Jacobs MV, et al. 1997. A general primer GP5+/GP6+-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J. Clin. Microbiol. 35:791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan MJ, et al. 2005. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J. Natl. Cancer Inst. 97:1072–1079 [DOI] [PubMed] [Google Scholar]
- 21. Kitchener HC, et al. 2009. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 10:672–682 [DOI] [PubMed] [Google Scholar]
- 22. Kraus I, et al. 2004. Human papillomavirus oncogenic expression in the dysplastic portio; an investigation of biopsies from 190 cervical cones. Br. J. Cancer 90:1407–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayrand MH, et al. 2007. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N. Engl. J. Med. 357:1579–1588 [DOI] [PubMed] [Google Scholar]
- 24. Molden T, Kraus I, Karlsen F, Skomedal H, Hagmar B. 2006. Human papillomavirus E6/E7 mRNA expression in women younger than 30 years of age. Gynecol. Oncol. 100:95–100 [DOI] [PubMed] [Google Scholar]
- 25. Molden T, et al. 2005. Comparison of human papillomavirus messenger RNA and DNA detection: a cross-sectional study of 4,136 women >30 years of age with a 2-year follow-up of high-grade squamous intraepithelial lesion. Cancer Epidemiol. Biomarkers Prev. 14:367–372 [DOI] [PubMed] [Google Scholar]
- 26. Molden T, et al. 2005. Predicting CIN2+ when detecting HPV mRNA and DNA by PreTect HPV-Proofer and consensus PCR: a 2-year follow-up of women with ASCUS or LSIL pap smear. Int. J. Cancer 114:973–976 [DOI] [PubMed] [Google Scholar]
- 27. Munoz N, et al. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518–527 [DOI] [PubMed] [Google Scholar]
- 28. Naucler P, et al. 2009. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J. Natl. Cancer Inst. 101:88–99 [DOI] [PubMed] [Google Scholar]
- 29. Naucler P, et al. 2007. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N. Engl. J. Med. 357:1589–1597 [DOI] [PubMed] [Google Scholar]
- 30. Petry KU, et al. 2011. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol. Oncol. 121:505–509 [DOI] [PubMed] [Google Scholar]
- 31. Ratnam S, et al. 2010. Clinical performance of the PreTect HPV-Proofer E6/E7 mRNA assay in comparison with that of the Hybrid Capture 2 test for identification of women at risk of cervical cancer. J. Clin. Microbiol. 48:2779–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ratnam S, et al. 2011. Aptima HPV E6/E7 mRNA test is as sensitive as Hybrid Capture 2 Assay but more specific at detecting cervical precancer and cancer. J. Clin. Microbiol. 49:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rijkaart DC, et al. 2012. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: results over two screening rounds of the POBASCAM randomized controlled trial. Lancet Oncol. 13:78–88 [DOI] [PubMed] [Google Scholar]
- 34. Rijkaart DC, et al. 2012. Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int. J. Cancer 130:602–610 [DOI] [PubMed] [Google Scholar]
- 35. Ronco G, et al. 2010. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 11:249–257 [DOI] [PubMed] [Google Scholar]
- 36. Ronco G, et al. 2008. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J. Natl. Cancer Inst. 100:492–501 [DOI] [PubMed] [Google Scholar]
- 37. Snijders PJ, et al. 2006. Determination of viral load thresholds in cervical scrapings to rule out CIN 3 in HPV 16, 18, 31 and 33-positive women with normal cytology. Int. J. Cancer 119:1102–1107 [DOI] [PubMed] [Google Scholar]
- 38. Sorbye SW, Fismen S, Gutteberg T, Mortensen ES. 2010. Triage of women with minor cervical lesions: data suggesting a “test and treat” approach for HPV E6/E7 mRNA testing. PLoS One 5:e12724 doi:10.1371/journal.pone.0012724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorbye SW, Fismen S, Gutteberg TJ, Mortensen ES. 2010. HPV mRNA test in women with minor cervical lesions: experience of the University Hospital of North Norway. J. Virol. Methods 169:219–222 [DOI] [PubMed] [Google Scholar]
- 40. Taha NS, et al. 2006. Universal Collection Medium (UCM) is as suitable as the Standard Transport Medium (STM) for Hybrid Capture II (HC-2) assay. J. Clin. Virol. 36:32–35 [DOI] [PubMed] [Google Scholar]
- 41. van den Brule AJ, et al. 2002. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J. Clin. Microbiol. 40:779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Duin M, et al. 2002. Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int. J. Cancer 98:590–595 [DOI] [PubMed] [Google Scholar]
- 43. Varnai AD, et al. 2008. Predictive testing of early cervical pre-cancer by detecting human papillomavirus E6/E7 mRNA in cervical cytologies up to high-grade squamous intraepithelial lesions: diagnostic and prognostic implications. Oncol. Rep. 19:457–465 [PubMed] [Google Scholar]
- 44. Walboomers JM, et al. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12–19 [DOI] [PubMed] [Google Scholar]
- 45. Wentzensen N, et al. 2006. Identification of high-grade cervical dysplasia by the detection of p16(INK4a) in cell lysates obtained from cervical samples. Cancer 107:2307–2313 [DOI] [PubMed] [Google Scholar]
- 46. Wright TC. 2009. Precancerous lesions of the cervix, p 248–257 In Kurman RJ. (ed), Blaustein's pathology of the female genital tract, 4th ed Springer Verlag, New York, NY [Google Scholar]
- 47. Ylitalo N, et al. 2000. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet 355:2194–2198 [DOI] [PubMed] [Google Scholar]
- 48. zur Hausen H. 2000. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 92:690–698 [DOI] [PubMed] [Google Scholar]