Abstract
Enteropathogenic Escherichia coli (EPEC) is the most important cause of persistent diarrhea in children, particularly in developing countries. Animals serve as pathogenic E. coli reservoirs, and compelling evidence for cross-species EPEC transmission exists. In this report, enzootic EPEC infection associated with up to 10.5% diarrhea-associated morbidity in a large laboratory Dutch Belted rabbit colony was investigated. These rabbits were obtained from a commercial vendor and had acute diarrhea following shipment. Fecal culture of 20 rabbits yielded 48 E. coli isolates, 83% of which were eae positive. Repetitive sequence-based PCR (REP-PCR) and serologic analysis identified a single disease-associated EPEC O145:H2 strain. In sampled rabbits, EPEC-positive culture and the presence of diarrhea were significantly associated. This strain displayed a localized adherence-like HEp-2 cell adherence pattern, as seen in diarrheic human infant EPEC isolates. Treatment was instituted with the fluoroquinolone antibiotic enrofloxacin, to which all isolates were susceptible. Preshipment parenteral enrofloxacin administration reduced diarrhea-associated morbidity 22-fold and mortality 12-fold in subsequent deliveries. This report emphasizes the zoonotic potential of animal EPEC strains and the need for virulence determinant-based screening of E. coli isolates from diarrheic animals.
INTRODUCTION
In 2011, diarrheal diseases caused 15% of deaths in children under age 5, second only to pneumonia as a cause of child death (49). Enteropathogenic Escherichia coli (EPEC) is the most important cause of diarrhea of extended duration (1, 21, 31). While often mild and self-limiting, diarrhea persists in a subset of patients, particularly in developing countries, causing malabsorption, dehydration, failure to thrive, malnutrition, and other life-threatening complications (21, 47). Diverse EPEC strains have been isolated from the feces of children with diarrhea (2, 4, 17, 40). However, the abundance of human intestinal E. coli, EPEC strain heterogeneity, the need for virulence determinant-based testing to assess isolate pathogenicity, and the presence of EPEC in individuals without clinical illness confound diagnosis and complicate epidemiologic investigations (3, 24, 32, 38).
Current evidence suggests that EPEC is readily cross-transmitted between humans and animals in a manner similar to that of enterohemorrhagic E. coli (EHEC) (13). Animal and human origin EPEC strains possess similar virulence factors and are clonally related based on multilocus sequence typing and pulsed-field gel electrophoresis (28). Studies have confirmed that human and rabbit origin EPEC strains cause characteristic attaching and effacing (A/E) lesions in infant pigs, regardless of the isolate's original host species, indicating that EPEC colonization is not host dependent (27). Human and bovine origin EHEC isolates cause disease in infant pigs and rabbits in a similar manner (42). EHEC transmission also occurs between cattle and wild rabbits (36, 39).
Rabbits experimentally infected with rabbit origin E. coli strains have been used as experimental models of human disease. For example, infection with strain RDEC-1, a well-described model of human EPEC infection (6), mimics a disease process seen in children with infectious gastroenteritis (45). Other recent studies have shown that Dutch Belted rabbits naturally or experimentally infected with EHEC strains exhibit bloody diarrhea and typhlocolitis, as well as nephropathy and renal thrombosis characteristic of the hemolytic-uremic syndrome (11, 12, 14).
In rabbits, natural EPEC infection causes profuse watery diarrhea that can be mucoid or bloody, accompanied by anorexia, dehydration, and lethargy (19, 34). Many factors modulate infection in rabbits, including intestinal segmented filamentous bacteria (20), concurrent infection with Lawsonia intracellularis (41) or rotavirus (44), dietary fiber intake (15), and stress level (10). The similarity of E. coli-associated enteritis in rabbits and humans, both in clinical presentation and pathological mechanism, make the rabbit an ideal experimental model and, due to its susceptibility to natural infection, a likely EPEC reservoir. Indeed, a survey of rabbits from laboratory vendors and a zoo identified both EPEC and EHEC in fecal cultures, though their ability to infect humans remains unknown (12). Because rabbits are used for research and food production and compose large wildlife populations, numerous opportunities exist for interspecies pathogen transmission.
In this study, the diarrhea etiology in 145 laboratory Dutch Belted rabbits was investigated. These rabbits were obtained from a single source and presented with acute diarrhea following shipment. Diarrhea abated or ceased upon parenteral enrofloxacin administration. Fecal culture and molecular characterization identified a single serotype O145:H2 EPEC strain. This serotype has been previously identified in wastewater (9), the feces of healthy sheep (46), and diarrheic infant feces (17). This report highlights the zoonotic potential of animal EPEC strains, as well the need for routine virulence determinant screening of animal E. coli isolates.
MATERIALS AND METHODS
Rabbits.
Dutch Belted rabbits (9 to 15 weeks old; male; n = 2,224) were received from two commercial vendors. Rabbits received from vendor 1 were free of Pasteurella spp., Treponema cuniculi, Clostridium piliforme, cilium-associated respiratory bacillus, Salmonella spp., Encephalitozoon cuniculi, and Toxoplasma gondii. Rabbits received from vendor 2 were free from all of the above pathogens except Encephalitozoon cuniculi. Rabbits from both vendors have been sporadically positive for Eimeria magna, Eimeria perforans, or Eimeria media. All rabbits were evaluated by veterinary staff upon receipt. Rabbits with mild diarrhea, characterized by a soft but formed stool, were monitored but not given antibiotics. Those with moderate to severe diarrhea were given subcutaneous fluid replacement therapy and 10 mg of enrofloxacin per kg of body weight intramuscularly (i.m.) daily for 10 days (Table 1). Due to the scale and severity of diarrhea, rabbits in subsequent rabbit deliveries were given a single prophylactic 10 mg/kg i.m. enrofloxacin dose prior to shipment (Table 1, cohort 3). Prophylactically treated rabbits that presented with moderate to severe diarrhea were given the remainder of the enrofloxacin course. No diarrhea was noted in rabbits from vendor 2 (Table 1, cohort 2).
Table 1.
Diarrhea-associated morbidity and mortality in rabbit cohorts
| Parameter | Value or result fora: |
||
|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | |
| Rabbit population size | 1,385 | 123 | 839 |
| Source | Vendor 1 | Vendor 2 | Vendor 1 |
| Preshipment enrofloxacin prophylaxis | − | − | + |
| No. (%) of rabbits | |||
| Total diarrhea-associated morbidity | 145 (10.5) | 0 | 4 (0.477) |
| Full-course enrofloxacin-treated morbidity | 131 (9.46) | 0 | 3 (0.357) |
| Enrofloxacin-treated mortality | 13 (0.939) | 0 | 1 (0.119) |
| Total diarrhea-associated mortality | 20 (1.44) | 0 | 1 (0.119) |
Diarrhea-associated morbidity and mortality in three cohorts of Dutch Belted rabbits are shown. Cohort size, source, the use of preshipment enrofloxacin prophylaxis (−, not used; +, used), and total morbidity and mortality are shown. Diarrheic rabbits treated with 10 mg/kg enrofloxacin i.m. daily for 10 days are indicated in a separate row. Subsequent mortality following this treatment regimen is likewise separately shown. Percentage values are relative to rabbit population size.
Bacterial culture.
Fecal samples were collected from a shipment of 20 cohort 1 rabbits containing both diarrheic and clinically normal animals. Feces were collected in tryptic soy broth, homogenized, and streaked on blood and MacConkey agar plates for aerobic culture and were also inoculated into thioglycolate broth for anaerobic culture. Lactose-positive colonies were subcultured based on morphology and identified to the species level using API 20E identification test strips (bioMérieux, Marcy l'Etoile, France). Antibiotic susceptibility was evaluated by the Kirby-Bauer method using Mueller-Hinton agar and antibiotic-impregnated disks containing 10 μg ampicillin, 20 μg amoxicillin plus 10 μg clavulanic acid, 30 μg cephalothin, 5 μg enrofloxacin, 10 μg gentamicin, and 1.25 μg trimethoprim plus 23.75 μg sulfamethoxazole.
Molecular characterization.
DNA was obtained from all E. coli isolates using the High Pure PCR template preparation kit (Roche Applied Science, Indianapolis, IN). Using previously published primer sequences and methods, all isolates were tested for the presence of eae (14), bfpA (18), stx1 (33), and stx2 (14). PCRs were performed using either the Expand high-fidelity PCR system (Roche Applied Science, Indianapolis, IN) or Illustra PuReTaq Ready-To-Go PCR beads (GE Healthcare, Piscataway, NJ). EHEC strain EDL933 and EPEC strain JPN15(pMAR7) (22) were used as controls for PCR testing. Three representative eae-positive PCR products were purified and sequenced in triplicate using an ABI PRISM 3500 genetic analyzer (Life Technologies, Carlsbad, CA). The resulting products shared 100% identity, and a representative sequence was submitted to GenBank. Repetitive sequence-based PCR (REP-PCR) genotyping was performed using REP1R-I and REP2-I as previously described (12). Serotyping and pulsed-field gel electrophoresis (PFGE) were performed on representative isolates of each REP-PCR genotype and API code by the Pennsylvania State University E. coli Reference Center (University Park, PA).
Statistical analysis.
The association between eae-positive E. coli isolation and clinical diarrhea was evaluated using a contingency table and Fisher's exact test (α = 0.05) (GraphPad Prism 5; GraphPad Software, La Jolla, CA).
HEp-2 cell adherence.
E. coli isolates were grown overnight in tryptic soy broth (TSB) containing 1% d-mannose to an optical density at 600 nm (OD600) of 0.72 to 0.93. HEp-2 cell culture lines were maintained as recommended by the manufacturer (American Type Culture Collection, Manassas, VA). Cells were prepared by rinsing with phosphate-buffered saline (PBS) and applying 0.05% trypsin EDTA until cell rounding and lifting were noted. This solution was poured off, and cells were diluted to 1.5 × 104 to 2.0 × 104 cells/ml with minimal essential medium containing Earle's balanced salts (MEM/EBSS) and 2.0 mM l-glutamine (HyClone Laboratories, Logan, UT) supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO). Six-well culture plates containing sterile coverslips were inoculated with 9 × 107 cells per well. Culture plates were then incubated for 2 h at 37°C, 5% CO2, and 95% humidity. TSB bacterial culture was then added (1:5) to the HEp-2 cell-containing chambers in the presence of 1% d-mannose. Mixtures were incubated for 3 h at 37°C, washed twice with PBS, and air dried. Slides were fixed and stained using a three-step stain kit (Richard-Allan Scientific, Kalamazoo, MI) and visualized by light microscopy.
Histopathology.
The duodenum, jejunum, ileum, cecum, colon, rectum, liver, gallbladder, kidneys, adrenal glands, stomach, trachea, lung, heart, thyroid, skeletal muscle, eyes, brain, pancreas, urinary bladder, thymus, spleen, mesenteric and bronchial lymph nodes, thymus, testes, and epididymis were collected from three symptomatic rabbits following sodium pentobarbital euthanasia. Tissues were fixed in 10% buffered formalin, paraffin embedded, cut into 5-μm sections, and mounted on glass slides. Hematoxylin and eosin (H&E)-stained sections of all organs and Gram-stained sections of the intestinal tract were evaluated by a board-certified veterinary pathologist (Nicola M. A. Parry).
Nucleotide sequence accession number.
A representative sequence of the eae-positive PCR products determined in this study was submitted to GenBank (accession no. JQ700206).
RESULTS
Initial morbidity and mortality.
Initially, rabbits from cohort 1 (n = 1,385) had a 10.5% incidence of diarrhea upon receipt (Table 1). Most of these (9.46% of cohort 1) had diarrhea severe enough to merit treatment with a 10-day course of enrofloxacin, a second-generation fluoroquinolone similar to ciprofloxacin commonly used in veterinary practice. The antibiotic-responsive nature of these symptoms suggested a bacterial etiology. Despite treatment, 1.44% total mortality was observed. Enrofloxacin-treated rabbits accounted for 13 (65.0%) of the 20 deaths encountered in this cohort, resulting in a treatment failure rate of 9.92%. The high incidence of diarrhea, the large number of rabbits requiring treatment due to severe clinical signs, and the high mortality rate required substantial intervention by veterinary staff, prompting both a search for alternate Dutch Belted rabbit sources and a laboratory investigation to ascertain the disease's etiology.
Following the initial outbreak, another rabbit vendor was utilized, and these rabbits never presented with diarrhea (Table 1, cohort 2). However, they were deemed unacceptable due to infection with other rabbit pathogens, particularly the microsporidian Encephalitozoon cuniculi, which can cause background ocular pathology (16). As a result, the initial vendor was again utilized, with all animals receiving 10 mg/kg enrofloxacin i.m. prior to shipment to prevent diarrhea (Table 1, cohort 3). Following prophylaxis initiation, diarrhea incidence was reduced 22-fold compared to cohort 1. Cohort 3 rabbits presenting with diarrhea were given the remainder of the 10-day enrofloxacin course, which was successful in two of three cases. Mortality was thus reduced 12-fold relative to that of cohort 1.
Bacterial isolation and characterization.
Fecal samples were obtained from 20 cohort 1 rabbits immediately following shipment. Aerobic culture yielded bacteria of several genera, including Escherichia, Bacillus, Pseudomonas, Enterobacter, Klebsiella, Proteus, Staphylococcus, and Streptococcus, which were identified based on API 20E strips and colony morphology. E. coli isolates (n = 48) were obtained from 12 cohort 1 rabbits, and 3 of these animals had diarrhea. All E. coli isolates were subjected to Kirby-Bauer disk diffusion testing for antibiotic susceptibility (Fig. 1). No isolates were fully susceptible to cephalothin, while all isolates were susceptible to enrofloxacin. Isolates displayed variable sensitivity to all other antibiotics tested. As a result of these findings, treatment with enrofloxacin was continued and the prophylactic treatment described above was instituted.
Fig 1.
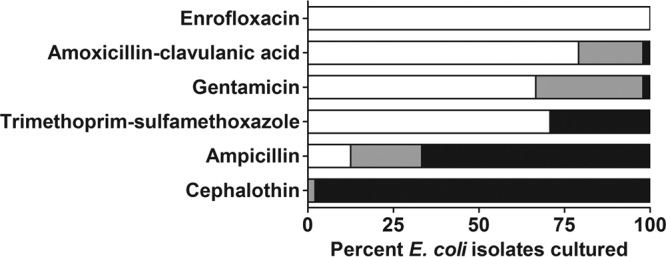
Percentages of E. coli isolates (n = 48) obtained from fecal culture that were susceptible (white), of intermediate susceptibility (gray), and resistant (black) to commonly used antibiotics by the Kirby-Bauer disk diffusion method.
E. coli isolates (n = 48) were tested by PCR for virulence factors and genotyped by REP-PCR to determine their relatedness. Five different REP-PCR genotypes were identified, and 83.0% (n = 40) of isolates represented a single genotype (genotype 1) (Fig. 2). Four other genotypes were identified, composed of the eight remaining isolates. PFGE of selected isolates confirmed the REP-PCR genotyping results, including the 100% similarity of genotype 1 isolates. PCR-based virulence factor tests were also performed (Table 2). Surprisingly, all eae-positive isolates were classified as the same REP-PCR genotype. Isolates of all other genotypes tested eae negative. All isolates tested negative for Shiga-like toxin genes stx1 and stx2, as well as the bundle-forming pilus gene bfpA. The eae+ stx mutant bfpA mutant genetic profile classifies these isolates as atypical EPEC.
Fig 2.
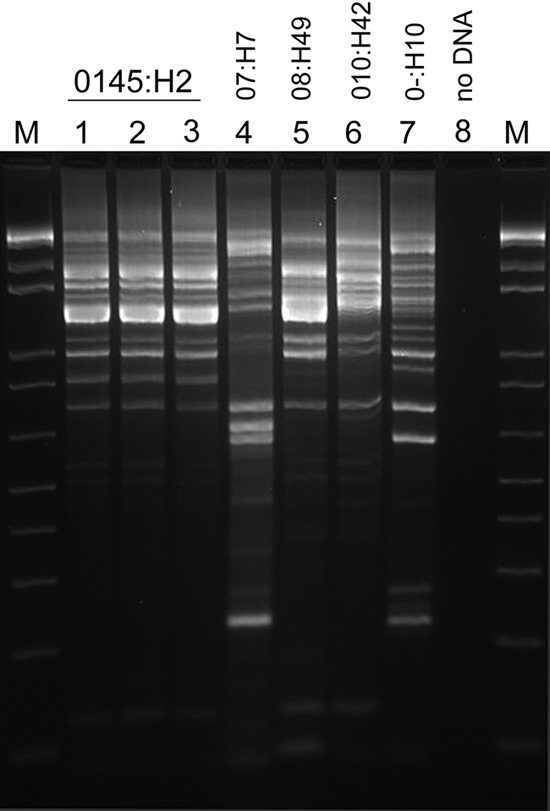
Representative repetitive element sequence-based PCR (REP-PCR) patterns obtained from fecal E. coli isolates are shown. Five REP-PCR patterns were obtained from the 48 isolates characterized (lanes 1 to 7), and each represented a distinct serotype as indicated. M, marker; lane 8, negative-control reaction mixture containing no DNA template.
Table 2.
Virulence factor PCR results
| Parameter | Result for REP-PCR genotype: |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Serotype | O145:H2 | O7:H7 | O8:H49 | O10:H42 | O−:H10 |
| Isolates tested (n) | 40 | 5 | 1 | 1 | 1 |
| Resulta for: | |||||
| eae | + | − | − | − | − |
| stx1 | − | − | − | − | − |
| stx2 | − | − | − | − | − |
| bfpA | − | − | − | − | − |
Positive and negative tests were 100% consistent among all isolates in each column.
The clonality of these isolates, determined by their 100% relatedness in REP-PCR and PFGE analyses, indicated that a single EPEC isolate was associated with the clinical signs seen. Representative isolates of each genotype and API code were serotyped (Table 2). Genotype 1 EPEC isolates were all serotype O145:H2, while genotypes 2 to 5 were of serotypes O7:H7, O8:H49, O10:H42, and O−:H10, respectively. All 40 EPEC O145:H2 isolates were obtained from 7 of the 20 rabbits sampled from cohort 1. Three of these rabbits had severe diarrhea, prompting enrofloxacin treatment, while four others were subclinically infected. Only one culture-negative rabbit in cohort 1 developed diarrhea, but it did not do so until 9 days after arrival. In sampled rabbits, a statistically significant association was present between EPEC-positive culture and the presence of diarrhea (P = 0.0307).
Cytoadherence.
Previous studies utilized cytoadherence as an adjunct assay for human origin EPEC virulence (4, 17, 29, 40). To correlate this study with human surveys, three representative EPEC O145:H2 isolates were added to HEp-2 cell monolayers and the resulting adherence patterns visualized. All EPEC O145:H2 isolates tested displayed a localized adherence-like pattern identical to that seen in human EPEC strains associated with diarrhea (Fig. 3F). One O7:H7 isolate was also tested and did not display adherence properties.
Fig 3.
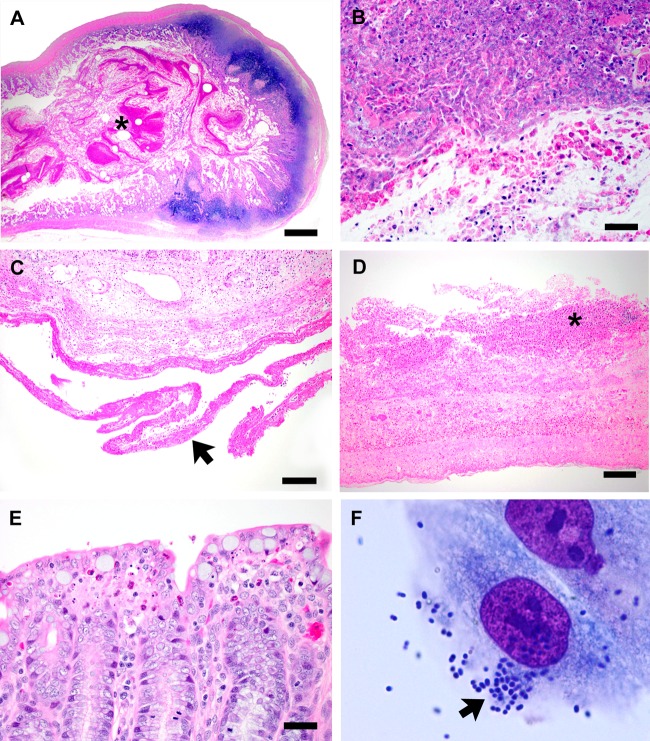
(A and B) Ileum from a rabbit with severe diarrhea. The lumen contains large amounts of fibrinonecrotic debris (asterisk), numerous heterophils, and pyknotic nuclei. The sections were stained with H&E. Bars, 500 μm (A) and 25 μm (B). (C and D) Duodenum from a rabbit with severe diarrhea. A fibrin tag is adherent to the serosal surface (arrow) and a fibrinonecrotic cap containing heterophils is adherent to the luminal mucosa (asterisk). H&E stain. Bars, 100 μm (C) and 50 μm (D). (E) Cecum from a rabbit with severe diarrhea. Clusters of heterophils and pyknotic nuclei are present at crypt tips. H&E stain. Bar, 25 μm. (F) Adherence of EPEC O145:H2 (REP-PCR genotype 1) isolates to HEp-2 cells. These isolates displayed a localized adherence-like (LAL) pattern (arrow). Magnification, ×1,000.
Histopathology.
Lesions were noted only in the gastrointestinal tract, and while autolysis was apparent, the presence of heterophils (the rabbit functional equivalent of neutrophils) indicated an antemortem disease process. Severe lesions were noted in the ileum, where the lumen contained large amounts of fibrinonecrotic debris, sloughed epithelial cells and their pyknotic nuclei, and heterophils (Fig. 3A and B). Similar changes were also noted in the lumen of the duodenum, where fibrinonecrotic debris containing heterophils adhered to the mucosa (Fig. 3C and D). Additionally, a fibrin tag adhered to the serosal surface, indicating fibrinous peritonitis. Less-severe change was noted in the cecum, where clusters of heterophils and pyknotic nuclei were noted at crypt tips (Fig. 3E).
DISCUSSION
This study's objective was to identify the etiologic agent responsible for diarrhea causing 10.5% morbidity and 1.44% mortality in a Dutch Belted rabbit cohort. Responsiveness to empirical enrofloxacin treatment suggested a bacterial etiology. An aerobic and anaerobic fecal culture-based screen was performed on 20 rabbits obtained from a vendor whose rabbits had consistently presented with diarrhea. Vendor health reports also indicated a positive intestinal Eimeria spp. test history. While coccidia were considered a possible diarrhea cause in this case, their presence was variable among symptomatic animals and persisted in the colony following enrofloxacin treatment and diarrhea resolution.
Culture yielded several bacterial genera, but E. coli was the only potential pathogen identified from all diarrheic animals. In addition, PCR analysis showed that 83% of isolates recovered were eae positive, further suggesting pathogenicity. The identification of this diarrhea-associated EPEC strain depended upon PCR-based eae gene identification, arguing for the importance of molecular virulence determinant screening when diagnosing bacterial enteritis. Diagnostic challenges remain an important EPEC research issue, and increased utilization of virulence determinant-based molecular diagnostic tests would facilitate specific E. coli isolate classification and thus aid outbreak investigations. EPEC identification was not surprising given its historical importance in rabbits. Strain RDEC-1 was previously used to model human diarrheal disease, where infection with as few as 150 bacteria produced diarrhea in 77% of rabbits, causing mortality in excess of 12% (6, 7). Interestingly, RDEC-1 was also acquired from recently vendor-delivered diarrheic rabbits (6). Surveys conducted in diarrheic and healthy rabbits from commercial sources, laboratories, and zoos have also identified many diverse, potentially pathogenic EPEC strains (12, 34, 35).
The E. coli isolates obtained were enrofloxacin susceptible, and treatment successfully reduced morbidity and mortality without promoting detrimental disease sequelae or dysbiosis. The rabbits in subsequent shipments were given a single enrofloxacin dose, which reduced morbidity 22-fold and mortality 12-fold in subsequent deliveries. However, several factors surrounding antibiotic use require consideration. Fluoroquinolones increase stx2 production via phage induction (23, 26), worsening disease in experimentally infected piglets (50) and predisposing human patients to hemolytic-uremic syndrome development (43, 48). Phage induction also promotes horizontal virulence factor transfer, a mechanism implicated in a recent O104:H4 outbreak (37).
Definitive solutions to enzootic EPEC should be considered, particularly systematic eradication of EPEC from vendor breeding stock. Tandem cesarean section rederivation and intensive E. coli virulence determinant-based surveillance could accomplish this. Pathogenic E. coli strains have been associated with Crohn's disease in humans (8) and colitis in cotton top tamarins (25). Infection with the similar pathogen Citrobacter rodentium promotes colitis (5) and colonic adenoma formation (30) in susceptible mice. These studies show that enteric bacterial infection profoundly impacts laboratory animal health and animal models of inflammation and cancer. EPEC eradication by cesarean section or antimicrobial treatment would also mitigate the zoonotic infection risk suggested by prior studies (13, 27, 28, 36, 39, 42) and the HEp-2 cytoadherence seen with this EPEC O145:H2 strain. That 33% of isolates were recovered from asymptomatic animals suggests that apparently healthy rabbits may act as EPEC reservoirs, presenting a zoonotic risk to laboratory personnel, pet owners, and others who have rabbit contact.
ACKNOWLEDGMENTS
This work was supported by NIH T32 RR070036.
We thank Yehia Wafa for collecting the fecal samples, Alexandra Booth for assistance compiling the clinical data, Alison Darby for providing the HEp2 cells, and Elaine Robbins for preparing the final figures.
Footnotes
Published ahead of print 9 May 2012
REFERENCES
- 1. Abba K, Sinfield R, Hart CA, Garner P. 2009. Pathogens associated with persistent diarrhoea in children in low and middle income countries: systematic review. BMC Infect. Dis. 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Afset JE, Bergh K, Bevanger L. 2003. High prevalence of atypical enteropathogenic Escherichia coli (EPEC) in Norwegian children with diarrhoea. J. Med. Microbiol. 52:1015–1019 [DOI] [PubMed] [Google Scholar]
- 3. Afset JE, Bevanger L, Romundstad P, Bergh K. 2004. Association of atypical enteropathogenic Escherichia coli (EPEC) with prolonged diarrhoea. J. Med. Microbiol. 53:1137–1144 [DOI] [PubMed] [Google Scholar]
- 4. Bokete TN, et al. 1997. Genetic and phenotypic analysis of Escherichia coli with enteropathogenic characteristics isolated from Seattle children. J. Infect. Dis. 175:1382–1389 [DOI] [PubMed] [Google Scholar]
- 5. Borenshtein D, Nambiar PR, Groff EB, Fox JG, Schauer DB. 2007. Development of fatal colitis in FVB mice infected with Citrobacter rodentium. Infect. Immun. 75:3271–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cantey JR, Blake RK. 1977. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J. Infect. Dis. 135:454–462 [DOI] [PubMed] [Google Scholar]
- 7. Cantey JR, Hosterman DS. 1979. Characterization of colonization of the rabbit gastrointestinal tract by Escherichia coli RDEC-1. Infect. Immun. 26:1099–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Darfeuille-Michaud A, et al. 2004. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 127:412–421 [DOI] [PubMed] [Google Scholar]
- 9. Doughari HJ, Ndakidemi PA, Human IS, Benade S. 2011. Virulence factors and antibiotic susceptibility among verotoxic non O157:H7 Escherichia coli isolates obtained from water and wastewater samples in Cape Town, South Africa. Afr. J. Biotechnol. 10:14160–14168 [Google Scholar]
- 10. Everest P. 2007. Stress and bacteria: microbial endocrinology. Gut 56:1037–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia A, et al. 2006. Renal injury is a consistent finding in Dutch Belted rabbits experimentally infected with enterohemorrhagic Escherichia coli. J. Infect. Dis. 193:1125–1134 [DOI] [PubMed] [Google Scholar]
- 12. Garcia A, Fox JG. 2003. The rabbit as a new reservoir host of enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 9:1592–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia A, Fox JG, Besser TE. 2010. Zoonotic enterohemorrhagic Escherichia coli: a One Health perspective. ILAR J. 51:221–232 [DOI] [PubMed] [Google Scholar]
- 14. Garcia A, et al. 2002. A naturally occurring rabbit model of enterohemorrhagic Escherichia coli-induced disease. J. Infect. Dis. 186:1682–1686 [DOI] [PubMed] [Google Scholar]
- 15. Gidenne T, Licois D. 2005. Effect of a high fibre intake on the resistance of the growing rabbit to an experimental inoculation with an enteropathogenic strain of Escherichia coli. Anim. Sci. 80:281–288 [Google Scholar]
- 16. Giordano C, et al. 2005. Immunohistochemical identification of Encephalitozoon cuniculi in phacoclastic uveitis in four rabbits. Vet. Ophthalmol. 8:271–275 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez R, et al. 1997. Age-specific prevalence of Escherichia coli with localized and aggregative adherence in Venezuelan infants with acute diarrhea. J. Clin. Microbiol. 35:1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gunzburg ST, Tornieporth NG, Riley LW. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heczko U, Abe A, Finlay BB. 2000. In vivo interactions of rabbit enteropathogenic Escherichia coli O103 with its host: an electron microscopic and histopathologic study. Microbes Infect. 2:5–16 [DOI] [PubMed] [Google Scholar]
- 20. Heczko U, Abe A, Finlay BB. 2000. Segmented filamentous bacteria prevent colonization of enteropathogenic Escherichia coli O103 in rabbits. J. Infect. Dis. 181:1027–1033 [DOI] [PubMed] [Google Scholar]
- 21. Hill SM, Phillips AD, Walker-Smith JA. 1991. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut 32:154–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jerse AE, Yu J, Tall BD, Kaper JB. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 87:7839–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimmitt PT, Harwood CR, Barer MR. 2000. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knutton S, et al. 2001. Phenotypic and genetic analysis of diarrhea-associated Escherichia coli isolated from children in the United Kingdom. J. Pediatr. Gastroenterol. Nutr. 33:32–40 [DOI] [PubMed] [Google Scholar]
- 25. Mansfield KG, et al. 2001. Enteropathogenic Escherichia coli and ulcerative colitis in cotton-top tamarins (Saguinus oedipus). J. Infect. Dis. 184:803–807 [DOI] [PubMed] [Google Scholar]
- 26. McGannon CM, Fuller CA, Weiss AA. 2010. Different classes of antibiotics differentially influence Shiga toxin production. Antimicrob. Agents Chemother. 54:3790–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moura RA, et al. 2009. Clonal relationship among atypical enteropathogenic Escherichia coli strains isolated from different animal species and humans. Appl. Environ. Microbiol. 75:7399–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moyenuddin M, Wachsmuth IK, Moseley SL, Bopp CA, Blake PA. 1989. Serotype, antimicrobial resistance, and adherence properties of Escherichia coli strains associated with outbreaks of diarrheal illness in children in the United States. J. Clin. Microbiol. 27:2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newman JV, Kosaka T, Sheppard BJ, Fox JG, Schauer DB. 2001. Bacterial infection promotes colon tumorigenesis in Apc(Min/+) mice. J. Infect. Dis. 184:227–230 [DOI] [PubMed] [Google Scholar]
- 31. Nguyen RN, Taylor LS, Tauschek M, Robins-Browne RM. 2006. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg. Infect. Dis. 12:597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ochoa TJ, Contreras CA. 2011. Enteropathogenic Escherichia coli infection in children. Curr. Opin. Infect. Dis. 24:478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paton AW, Beutin L, Paton JC. 1995. Heterogeneity of the amino-acid sequences of Escherichia coli Shiga-like toxin type-I operons. Gene 153:71–74 [DOI] [PubMed] [Google Scholar]
- 34. Peeters JE, Pohl P, Charlier G. 1984. Infectious agents associated with diarrhoea in commercial rabbits: a field study. Ann. Rech. Vet. 15:335–340 [PubMed] [Google Scholar]
- 35. Peeters JE, Pohl P, Okerman L, Devriese LA. 1984. Pathogenic properties of Escherichia coli strains isolated from diarrheic commercial rabbits. J. Clin. Microbiol. 20:34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pritchard GC, et al. 2001. Wild rabbits—a novel vector for verocytotoxigenic Escherichia coli O157. Vet. Rec. 149:567. [PubMed] [Google Scholar]
- 37. Rasko DA, et al. 2011. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 365:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robins-Browne RM, et al. 2004. Escherichia coli and community-acquired gastroenteritis, Melbourne, Australia. Emerg. Infect. Dis. 10:1797–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scaife HR, Cowan D, Finney J, Kinghorn-Perry SF, Crook B. 2006. Wild rabbits (Oryctolagus cuniculus) as potential carriers of verocytotoxin-producing Escherichia coli. Vet. Rec. 159:175–178 [DOI] [PubMed] [Google Scholar]
- 40. Scaletsky IC, et al. 1999. A localized adherence-like pattern as a second pattern of adherence of classic enteropathogenic Escherichia coli to HEp-2 cells that is associated with infantile diarrhea. Infect. Immun. 67:3410–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schauer DB, et al. 1998. Proliferative enterocolitis associated with dual infection with enteropathogenic Escherichia coli and Lawsonia intracellularis in rabbits. J. Clin. Microbiol. 36:1700–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shringi S, et al. 2012. Differential virulence of clinical and bovine-biased enterohemorrhagic Escherichia coli O157:H7 genotypes in piglet and Dutch Belted rabbit models. Infect. Immun. 80:369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slutsker L, et al. 1998. A nationwide case-control study of Escherichia coli O157:H7 infection in the United States. J. Infect. Dis. 177:962–966 [DOI] [PubMed] [Google Scholar]
- 44. Thouless ME, DiGiacomo RF, Deeb BJ. 1996. The effect of combined rotavirus and Escherichia coli infections in rabbits. Lab. Anim. Sci. 46:381–385 [PubMed] [Google Scholar]
- 45. Ulshen MH, Rollo JL. 1980. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N. Engl. J. Med. 302:99–101 [DOI] [PubMed] [Google Scholar]
- 46. Vettorato MP, et al. 2009. Shiga toxin-producing Escherichia coli and atypical enteropathogenic Escherichia coli strains isolated from healthy sheep of different populations in Sao Paulo, Brazil. Lett. Appl. Microbiol. 49:53–59 [DOI] [PubMed] [Google Scholar]
- 47. Walker-Smith JA. 2001. Post-infective diarrhoea. Curr. Opin. Infect. Dis. 14:567–571 [DOI] [PubMed] [Google Scholar]
- 48. Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. World Health Organization 2011. World health statistics 2011. World Health Organization, Geneva, Switzerland [Google Scholar]
- 50. Zhang Q, et al. 2009. Gnotobiotic piglet infection model for evaluating the safe use of antibiotics against Escherichia coli O157:H7 infection. J. Infect. Dis. 199:486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]