Abstract
Resistance to antimonial drugs has been documented in Leishmania isolates transmitted in South America, Europe, and Asia. The frequency and distribution of resistance to these and other antileishmanial drugs are unknown. Technical constraints have limited the assessment of drug susceptibility of clinical strains of Leishmania. Susceptibility of experimentally selected lines and 130 clinical strains of Leishmania panamensis, L. braziliensis, and L. guyanensis to meglumine antimoniate and miltefosine was determined on the basis of parasite burden and percentage of infected U-937 human macrophages. Reductions of infection at single predefined concentrations of meglumine antimoniate and miltefosine and 50% effective doses (ED50s) were measured and correlated. The effects of 34°C and 37°C incubation temperatures and different parasite-to-host cell ratios on drug susceptibility were evaluated at 5, 10, and 20 parasites/cell. Reduction of the intracellular burden of Leishmania amastigotes in U-937 cells exposed to the predefined concentrations of meglumine antimoniate or miltefosine discriminated sensitive and experimentally derived resistant Leishmania populations and was significantly correlated with ED50 values of clinical strains (for meglumine antimoniate, ρ = −0.926 and P < 0.001; for miltefosine, ρ = −0.906 and P < 0.001). Incubation at 37°C significantly inhibited parasite growth compared to that at 34°C in the absence of antileishmanial drugs and resulted in a significantly lower ED50 in the presence of drugs. Susceptibility assessment was not altered by the parasite-to-cell ratio over the range evaluated. In conclusion, measurement of the reduction of parasite burden at a single predetermined drug concentration under standardized conditions provides an efficient and reliable strategy for susceptibility evaluation and monitoring of clinical strains of Leishmania.
INTRODUCTION
Long-term use of antimonial drugs as monotherapy for leishmaniasis and nonadherence to treatment have contributed to the increase in treatment failures (3, 5). In addition to intrinsic differences in the susceptibility of different Leishmania populations (18, 23), acquired resistance has also been reported (12, 15). Consequent variations in drug efficacy impact clinical management and leishmaniasis control programs, yet little is known about the prevalence of tolerant and/or resistant populations (11) and the impact of treatment policies on drug susceptibility.
Evaluation of drug susceptibility of clinical strains of Leishmania has been constrained by the unavailability of an efficient method that yields consistent results. Intracellular amastigotes are the target of treatment, are strikingly more susceptible to antimony than promastigotes (2, 11, 21), and importantly, are killed in vitro at clinically achievable drug concentrations. However, drug susceptibility evaluation of clinical strains using intracellular amastigotes is a slow, low-throughput biological assay that is influenced by numerous poorly understood variables. Individual clinical strains are heterogeneous; even strains having a low 50% effective dose (ED50) often contain subpopulations that are effectively resistant (15). Hence, although cloned parasites transfected with reporter genes have proven useful for drug screening (17), the protracted, intensive in vitro antibiotic selection required to recover and maintain the transfected population may alter the original composition of clinical strains and, consequently, the susceptibility phenotype.
Conventional susceptibility assays are based on the determination of the ED50 using murine peritoneal macrophages or macrophage cell lines under diverse conditions of infection with different numbers and intervals of drug concentrations, periods of drug exposure, and readout parameters. Since these factors influence the outcome of the evaluation (20), results of drug susceptibility testing have been neither consistent nor generalizable.
We report a novel approach to drug susceptibility assessment of clinical strains of Leishmania based on quantification of parasite burden after exposure to single discriminatory concentrations of two representative antileishmanial drugs, meglumine antimoniate (SbV) and miltefosine (HePC).
MATERIALS AND METHODS
Experimental strategy.
Susceptibility of clinical strains of Leishmania (Viannia) species to SbV and HePC was determined in parallel by conventional dose-response assay and reduction of intracellular parasite burden at a single drug concentration. Discriminatory drug concentrations for susceptibility were defined as follows: the antimony concentration of 32 μg SbV/ml was based on the estimated maximum concentration (Cmax) in plasma (6), and the miltefosine concentration of 16 μM was chosen because it clearly and consistently distinguished the susceptible wild-type (WT) control strain and an experimentally selected resistant line without cytotoxicity for host macrophages. Parasite burden based on enumeration of intracellular amastigotes and the percentage of cells infected was determined microscopically and by luminometry (Lucy 1; Anthos-Labtec Instruments, Lagerhausstrasse, Austria) using luc-transfected WT and drug-resistant control populations.
Leishmania strains and lines.
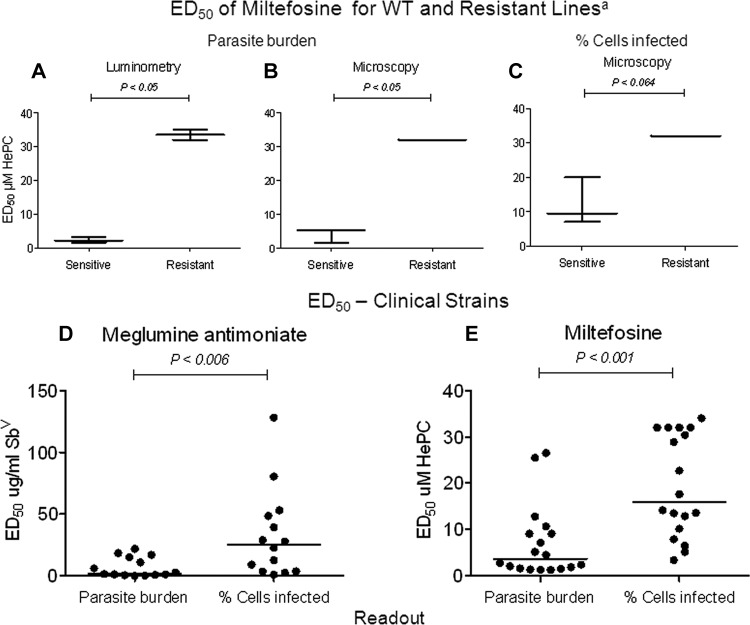
One hundred thirty clinical strains originating from patients diagnosed with dermal leishmaniasis were obtained from institutional biobanks, and their use for this study was approved by the Ethical Review Committees of the Centro Internacional de Entrenamiento en Investigaciones Médicas (CIDEIM) and the Centro Dermatológico Federico Lleras Acosta (CDFLLA). The control WT strain of L. (V.) panamensis (MHOM/COL/86/1166LUC) is sensitive to both antimony and miltefosine (14, 22). Drug-resistant lines derived from this strain were employed as controls: MHOM/COL/86/1166-1000.1, resistant to antimony (10, 22), and MHOM/COL/86/1166-LUC056, resistant to miltefosine. The latter line was selected from MHOM/COL/86/1166LUC by culturing of promastigotes in the presence of increasing concentrations of miltefosine of from 20 to 60 μM. The promastigote form of this line is propagated in 60 μM miltefosine, and the amastigote form has an ED50 exceeding 32 μM, the maximum concentration of miltefosine used for in vitro intracellular assays. The WT line has an ED50 approximating 4 μM (see Fig. 1A and B).
Fig 1.
Comparison of readout as parasite burden and percentage of cells infected in evaluation of Leishmania susceptibility to meglumine antimoniate and miltefosine. The ED50 of miltefosine determined as parasite burden using luciferase activity (A) and microscopy (B) consistently distinguished sensitive and experimentally selected resistant populations. Statistically significant differences substantiate discrimination of sensitive and resistant populations based on parasite burden but not by readout as the percentage of cells infected (C). The ED50s of meglumine antimoniate (n = 14) (D) and miltefosine (n = 18) (E) for clinical strains of Leishmania were significantly higher when determined as the percentage of cells infected than as parasite burden. ED50 values are presented as medians. a, based on three independent assays. Significant differences were determined by the Mann-Whitney U test.
Susceptibility assessment.
Susceptibility assays were conducted with human promonocytic U-937 cells (ATCC CRL-159.3) as previously described (15) but using 1.2 × 105 cells/well of 24-well cell culture plates containing sterile round 12-mm glass coverslips (Fisher Scientific) for microscopic evaluation. Phorbol 12-myristate 13-acetate (PMA; 100 ng/ml; Sigma) was added together with the cell suspension to induce differentiation of U-937 cells during 5 days of culture (9, 13). To evaluate the effect of temperature on susceptibility assessment, promastigotes opsonized for 1 h with 10% heat-inactivated type AB-positive human serum in RPMI medium were used to infect macrophages at a ratio of 5:1, 10:1, or 20:1 (parasite/cell) for 2 h at 34°C or 37°C in 5% CO2. Infected cells were washed twice with phosphate-buffered saline to remove free parasites and then incubated for 24 h at 34°C or 37°C in 5% CO2 in RPMI 1640 medium (R6504; Sigma) containing 10% heat-inactivated fetal bovine serum (FBS; 10082; Gibco). For evaluation of susceptibility at the defined discriminatory drug concentration, medium was replaced with complete RPMI medium containing 32 μg SbV/ml as additive-free meglumine antimoniate (Glucantime; Walter Reed 214975AK; lot no. BLO918690-278-1A1W601; antimony analysis, 25% to 26.5%, by weight) or 16 μM hexadecylphosphocholine (miltefosine; Cayman Chemical Co., Ann Arbor, MI). ED50 assays were performed with 2, 4, 8, 16, 32, 64, and 128 SbV/ml and 0.5, 1, 2, 4, 8, 16, and 32 μM HePC. Complete RPMI medium containing the discriminatory drug concentration or serial dilutions of meglumine antimoniate was replenished 48 h later, and incubation was continued for 24 h. Incubation with miltefosine was conducted over 48 h without replenishment. Infected macrophages in complete medium served as controls.
Glass coverslips with infected cells were fixed with methanol, stained with 3% Giemsa (Sigma), and then evaluated in a blinded manner by two experienced microscopists. One hundred macrophages per well were examined microscopically to determine the percentage of cells infected and the number of morphologically intact amastigotes in 100 cells. Four replicate wells were evaluated for susceptibility at the predefined discriminatory drug concentration, and three replicate wells were evaluated for each drug concentration in ED50 assays. For assays using the single discriminatory drug concentration, drug susceptibility was expressed as percent reduction of infection in cells exposed to the drug versus cells cultured without drug. Luciferase activity of WT and drug-resistant lines was expressed as relative light units (RLU).
Statistical methods.
The ED50 value was determined using the probit procedure of the SPSS program (version 7.5 for Windows, 1996; SPSS). The receiver operating characteristic (ROC) curve was constructed to define the cutoff to classify clinical strains as sensitive or of low susceptibility on the basis of assessment at the discriminatory drug concentration. ED50 values of ≤32 μg SbV/ml and ≤16 μM HePC were adopted as reference standards to define sensitive strains, and ED50s of >32 μg SbV/ml and >16 μM HePC were adopted as reference standards to define strains of low susceptibility. Comparison of groups was conducted using the Mann-Whitney U test. Correlation analysis between ED50 and percent reduction of parasite burden was performed using the Spearman test (nonparametric methods allow the inclusion of assays in which an actual value of the ED50 was not found because it exceeded the highest drug concentration evaluated), and concordance analysis of ED50 was performed using different readouts (percentage of cells infected and number of amastigotes in 100 cells) and the intraclass correlation coefficient. A two-sided significance level of 5% was used.
RESULTS
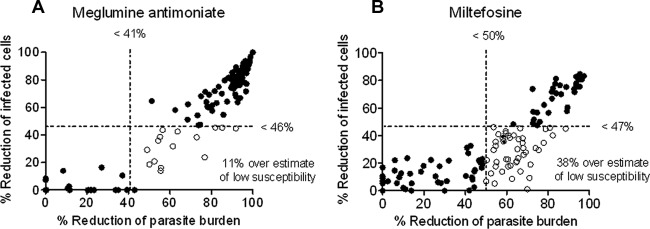
In vitro drug susceptibility testing of clinical strains of Leishmania has been hindered by the inconsistency of results obtained using diverse experimental conditions. The results of this study substantiated the greater accuracy of a readout based on reduction of intracellular parasite burden than an evaluation based on the percentage of cells infected. Assessment of parasite burden by microscopy clearly distinguished experimentally selected resistant and susceptible populations with a resolution comparable to that of luminometry (Fig. 1A and B), whereas readouts based on the percentage of cells infected did not consistently discern the susceptible WT but did distinguish experimentally selected resistant populations (Fig. 1C). Concordance between the ED50 for miltefosine determined microscopically as parasite burden and the ED50 based on luciferase activity was 99.2%. In contrast, concordance between the ED50 based on microscopic determination of the percentage of cells infected and luciferase activity was 81% (data not shown), reflecting the inability of the readout based on the percentage of infected cells to discriminate between cells with one or a few parasites and cells harboring abundant parasites. This limitation leads to significantly higher ED50 values (Fig. 1D and E) and overestimation of low susceptibility to meglumine antimoniate and miltefosine compared to those from the readout as the parasite burden for clinical strains (Fig. 2A and B). Reduction of infection by exposure to discriminatory concentrations of miltefosine (n = 125) and meglumine antimoniate (n = 130) was significantly lower (P < 0.001) for readout as the percentage of cells infected (median reductions, 69% for SbV and 27% for HePC) than for readout as the parasite burden (median reductions, 86% for SbV and 60% for HePC). Hence, evaluation of the reduction of infection by a single drug concentration corroborated the overestimate of low drug susceptibility when evaluation was based on the percentage of cells infected.
Fig 2.
Comparison of the susceptibility of clinical strains of Leishmania (Viannia) to meglumine antimoniate and miltefosine evaluated as the percentage of cells infected and parasite burden. Using thresholds of susceptibility defined by ROC curve analyses, low susceptibility (tolerance/resistance) was overestimated in clinical strains by readout as the percentage of cells infected cells compared to readout as the parasite burden at discriminatory concentrations of meglumine antimoniate (n = 130) (A) and miltefosine (n = 125) (B). Discontinuous lines correspond to cutoff thresholds. Empty circles represent strains erroneously classified as having low drug susceptibility by readout as the percentage of cells infected.
A high and significant correlation (for meglumine antimoniate, ρ = −0.926 and P < 0.001; for miltefosine, ρ = −0.906 and P < 0.001) between susceptibility, expressed as percent reduction of parasite burden by a predefined single drug concentration, and ED50 values was shown for both meglumine antimoniate and miltefosine (Fig. 3A and B). Strains with high ED50 values displayed a low percent reduction of parasite burden at the discriminatory concentration of each drug. Conversely, strains with low ED50 values showed a high percent reduction of parasite burden. Low susceptibility to discriminatory concentrations of meglumine antimoniate and miltefosine was predicted using the ROC curve at cutoffs of a <41% (having 100% sensitivity and 100% specificity) and a <50% (having 93% sensitivity and 100% specificity) reduction of parasite burden, respectively (Fig. 3C and D).
Fig 3.
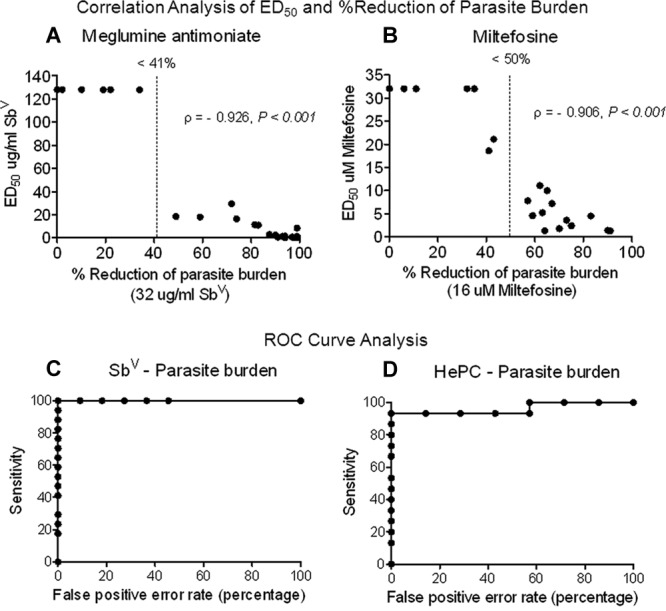
Correlation of drug susceptibility evaluation based on discriminatory drug concentration and ED50 values of clinical strains of Leishmania (Viannia) and determination of cutoff threshold for screening test. Susceptibility determination using the discriminatory drug concentration was significantly correlated (using the Spearman coefficient) with the ED50 of meglumine antimoniate (A) and miltefosine (B). Both the ED50 and susceptibility to discriminatory drug concentrations were determined by readout as parasite burden. The cutoff thresholds for low susceptibility defined by ROC curve analysis was a <41% reduction of parasite burden by meglumine antimoniate (n = 28) (C) and a <50% reduction of parasite burden by miltefosine (n = 20) (D).
Standardization of the susceptibility evaluation included evaluation of the effects of the parasite-to-host cell ratio and incubation temperature on the outcome of susceptibility assessment. The infective dose of parasites and resulting level of in vitro infection of host macrophages did not significantly alter the outcome of susceptibility assessment based on parasite burden. Infection with 5, 10, and 20 luc-transfected parasites per cell resulted in proportionately higher parasite burdens of 5,869, 10,497, and 30,144 RLU, respectively; however, ED50 values for miltefosine were similar (medians, 1.7, 4.2, and 3.3 μM, respectively). Likewise, reduction of parasite burden at the discriminatory drug concentration yielded comparable results (medians, 96, 89, and 91%, respectively; n = 3), irrespective of the parasite-to-host cell ratio. The susceptibility readout was not affected by parasite ratios of between 5 and 20 parasites per cell. Nevertheless, the lower ratio facilitates microscopic evaluation. An incubation temperature above 34°C during infection with L. (V.) panamensis resulted in lower rates and burdens of infection and altered host and amastigote morphology, impairing microscopic evaluation. Furthermore, the percent reduction of parasites after incubation at 37°C in the presence or absence of antimony was significantly higher than that obtained at 34°C with the same strains (Table 1).
Table 1.
Effect of incubation temperature above 34°C on susceptibility assessment of clinical strains of L. (V.) panamensisa
| Experimental condition | No. of parasites in 100 cells at incubation temp during infection |
% reduction of parasite burden at 37°C | P valueb | |
|---|---|---|---|---|
| 34°C | 37°C | |||
| Without drug | 371 | 83 | 91 | 0.016 |
| With SbV | 264 | 3 | 98 | 0.008 |
| % reduction of parasite burden by addition of drug | 21 | 91 | 0.021 | |
Data correspond to the median (n = 5).
Mann-Whitney U test.
DISCUSSION
In vitro assessment of drug susceptibility of clinical strains of Leishmania based on reduction of parasite burden was feasible. Determination of the reduction of parasite burden at drug concentrations that distinguish experimentally selected resistant lines from the corresponding WT population allowed classification of clinical strains as sensitive or of low susceptibility. The wide spectrum of the proportional reduction of parasite burden in clinical strains (Fig. 3A and B) demonstrates the resolution of evaluation based on the discriminatory drug concentrations in discerning populations with different levels of susceptibility to the test drugs.
Definition of cutoffs based on ROC curve analyses of ED50 and percent reduction of parasite burden at the discriminatory drug concentration provided a reference threshold for emerging insusceptibility whether it is due to acquired resistance or expansion of populations having low susceptibility to specific drugs. The strains classified as having low susceptibility to meglumine antimoniate in this study presented ED50 values above the reported Cmax in plasma (32 μg/ml) (6) that could signal an increased risk of treatment failure. Although the Cmax of miltefosine in plasma is well above the concentration used for in vitro evaluation, the recognized toxicity of miltefosine for host macrophages in vitro limits the maximum concentration that can be evaluated. However, the interaction of miltefosine with blood components, which bind approximately 95% of the drug (7), effectively reduces the amount of unbound drug to which intracellular parasites may be exposed in vivo. This fact and the ability of 16 μM to discriminate between an experimentally selected line and corresponding WT support the biological relevance of insusceptibility to this concentration and its use in surveillance for early detection of tolerance or resistance to miltefosine.
The results of this study document the impact of the incubation temperature on the interpretation of susceptibility assessment. The temperature sensitivity of intracellular amastigotes has long been recognized. Though not systematically examined, evidence has generally shown that dermatotropic species have significantly lower growth at temperatures above 35°C, while vicerotropic species grow equally well at 37°C and 35°C (1, 19). We found that parasite burden was significantly lower after 4 days of culture at 37°C than at 34°C in the absence or presence of antileishmanial drug (Table 1). Hence, assessment of drug susceptibility at 34°C avoids the potentially confounding effect of temperature on the reduction of infection and accommodates the temperature-dependent growth of all Leishmania species.
Microscopic readout is influenced by the level of infection: a very high level of infection encumbers the visual enumeration of parasites to determine burden. Furthermore, drug-mediated reduction of the number of parasites may not comparably diminish the proportion of cells that harbor one or a few parasites, leading to a perception of lower susceptibility when readout is based on the percentage of cells infected. Infectivity varies among Leishmania strains. Consequently, a fixed parasite-to-host cell ratio results in an intensity of infection that varies among different clinical strains. Over the range of 5 to 20 parasites per cell, we found that the ratio did not alter the outcome of susceptibility assessment, although high ratios led to levels of infection that can be difficult to visually evaluate.
In this study, significantly higher ED50 values were obtained when drug-mediated reduction of infection was based on the percentage of cells infected than on reduction of parasite burden. Hence readout based on the percentage of cells infected is vulnerable to overestimation of low susceptibility (drug tolerance or resistance). These findings and ROC curve analyses substantiate the greater sensitivity and specificity of quantitative evaluation of the parasite burden compared to those achieved by estimation of the percentage of cells infected. Notably, most published reports of drug susceptibility evaluation of intracellular Leishmania have been based on the assessment of the percentage of cells infected (4, 8, 23, 24). Evaluation of percentage of cells infected is more rapid and convenient yet may underestimate drug cytotoxicity for intracellular amastigotes, since one or a few intracellular parasites are not discriminated from large numbers of parasites in a single cell, which effectively leads to overestimation of low susceptibility.
The accuracy of ED50 values and reliability of their interpretation depend upon the number and range of drug concentrations evaluated, since informative drug concentration intervals vary in relation to the susceptibility of the population being tested. Therefore, use of a standard range of drug concentrations, such as is generally used for ED50 determination, is not uniformly suited to the spectrum of susceptibility phenotypes found among clinical strains. Moreover, strains are often heterogeneous with respect to drug sensitivity; isolates of a strain presenting a low ED50 may nevertheless include a substantial population that is tolerant or resistant to high drug concentrations. The assessment of drug susceptibility based on the absolute reduction of parasites at a drug concentration defined in relation to in vivo pharmacokinetics and tolerance thresholds of experimentally selected lines obviates these important and underappreciated sources of error in ED50 determination and interpretation.
The reduced complexity and reduced cost of evaluation based on a single discriminatory drug concentration facilitate the monitoring of drug susceptibility. This strategy is applicable to any Leishmania species/strain and can be standardized for other drugs, just as the conventional ED50 assay. However, dependence on microscopic reading remains a limitation. The development of methods amenable to medium throughput for the determination of viable parasites is needed. Methods based on detection of parasite RNA combined with this novel approach to susceptibility evaluation may allow monitoring of drug susceptibility to be routinely used in defining treatment policy (16).
In summary, evaluation of susceptibility using a single drug concentration under standardized conditions provides an efficient and reliable strategy for susceptibility evaluation and monitoring of clinical strains of Leishmania.
ACKNOWLEDGMENTS
We thank Neal Alexander and Laura González for their comments and critical review of the manuscript, Alejandra Arcos for technical assistance in the propagation of Leishmania strains and lines used in this study, and Ricardo Obonaga for his technical support in the development of susceptibility assays.
This work was supported by COLCIENCIAS grant (222940820418) and a U.S. National Institute of Allergy and Infectious Diseases grant (U19-AI0658669).
Footnotes
Published ahead of print 18 April 2012
REFERENCES
- 1. Berman JD, Neva FA. 1981. Effect of temperature on multiplication of Leishmania amastigotes within human monocyte-derived macrophages in vitro. Am. J. Trop. Med. Hyg. 30:318–321 [DOI] [PubMed] [Google Scholar]
- 2. Berman JD, Wyler DJ. 1980. An in vitro model for investigation of chemotherapeutic agents in leishmaniasis. J. Infect. Dis. 142:83–86 [DOI] [PubMed] [Google Scholar]
- 3. Bryceson A. 2001. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop. Med. Int. Health 6:928–934 [DOI] [PubMed] [Google Scholar]
- 4. Carrio J, Portus M. 2002. In vitro susceptibility to pentavalent antimony in Leishmania infantum strains is not modified during in vitro or in vivo passages but is modified after host treatment with meglumine antimoniate. BMC Pharmacol. 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz A, et al. 2007. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J. Infect. Dis. 195:602–608 [DOI] [PubMed] [Google Scholar]
- 7. Dorlo TP, et al. 2008. Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients. Antimicrob. Agents Chemother. 52:2855–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Escobar P, Matu S, Marques C, Croft SL. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 81:151–157 [DOI] [PubMed] [Google Scholar]
- 9. Gidlund M, et al. 1981. Natural killer cells kill tumour cells at a given stage of differentiation. Nature 292:848–850 [DOI] [PubMed] [Google Scholar]
- 10. Goyeneche-Patino DA, Valderrama L, Walker J, Saravia NG. 2008. Antimony resistance and trypanothione in experimentally selected and clinical strains of Leishmania panamensis. Antimicrob. Agents Chemother. 52:4503–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar D, Kulshrestha A, Singh R, Salotra P. 2009. In vitro susceptibility of field isolates of Leishmania donovani to miltefosine and amphotericin B: correlation with sodium antimony gluconate susceptibility and implications for treatment in areas of endemicity. Antimicrob. Agents Chemother. 53:835–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laurent T, et al. 2007. Epidemiological dynamics of antimonial resistance in Leishmania donovani: genotyping reveals a polyclonal population structure among naturally-resistant clinical isolates from Nepal. Infect. Genet. Evol. 7:206–212 [DOI] [PubMed] [Google Scholar]
- 13. Liu MY, Wu MC. 1992. Induction of human monocyte cell line U937 differentiation and CSF-1 production by phorbol ester. Exp. Hematol. 20:974–979 [PubMed] [Google Scholar]
- 14. Lucumi A, Robledo S, Gama V, Saravia NG. 1998. Sensitivity of Leishmania viannia panamensis to pentavalent antimony is correlated with the formation of cleavable DNA-protein complexes. Antimicrob. Agents Chemother. 42:1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rojas R, et al. 2006. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J. Infect. Dis. 193:1375–1383 [DOI] [PubMed] [Google Scholar]
- 16. Romero I, et al. 2010. Viability and burden of Leishmania in extralesional sites during human dermal leishmaniasis. PLoS Negl. Trop. Dis. 4(9):pii=e819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy G, et al. 2000. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol. Biochem. Parasitol. 110:195–206 [DOI] [PubMed] [Google Scholar]
- 18. Sanchez-Canete MP, Carvalho L, Perez-Victoria FJ, Gamarro F, Castanys S. 2009. Low plasma membrane expression of the miltefosine transport complex renders Leishmania braziliensis refractory to the drug. Antimicrob. Agents Chemother. 53:1305–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scott P, Sacks D, Sher A. 1983. Resistance to macrophage-mediated killing as a factor influencing the pathogenesis of chronic cutaneous leishmaniasis. J. Immunol. 131:966–971 [PubMed] [Google Scholar]
- 20. Seifert K, Escobar P, Croft SL. 2010. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J. Antimicrob. Chemother. 65:508–511 [DOI] [PubMed] [Google Scholar]
- 21. Vermeersch M, et al. 2009. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 53:3855–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walker J, et al. 2012. Discovery of factors linked to antimony resistance in Leishmania panamensis through differential proteome analysis. Mol. Biochem. Parasitol. 183:166–176 [DOI] [PubMed] [Google Scholar]
- 23. Yardley V, et al. 2005. The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am. J. Trop. Med. Hyg. 73:272–275 [PubMed] [Google Scholar]
- 24. Yardley V, et al. 2006. American tegumentary leishmaniasis: is antimonial treatment outcome related to parasite drug susceptibility? J. Infect. Dis. 194:1168–1175 [DOI] [PubMed] [Google Scholar]