Abstract
The detection of outbreaks of methicillin-resistant Staphylococcus aureus (MRSA) infections and a rapid and accurate identification of sources and routes of transmission should be conducted in hospital settings as early and swiftly as possible. In this study, we investigated the application potential of a new approach based on multiple-locus variable-number tandem-repeat fingerprinting (MLVF) and microfluidics technology for a rapid discrimination of MRSA lineages in outbreak settings. A total of 206 nonrepetitive MRSA isolates recovered from infected patients at the University Medical Center Groningen between 2000 and 2010 were tested. The results obtained by MLVF using microcapillary electrophoresis with newly designed primers were compared to those obtained by spa typing and multiple-locus variable-number tandem-repeat analysis (MLVA). The discriminatory power was 0.980 (107 patterns), 0.969 (85 allelic profiles), and 0.959 (66 types) for MLVF, MLVA, and spa typing, respectively. All methods tested showed a good concordance of results calculated by the adjusted Rand's coefficient method. Comparisons of data obtained by the three approaches allowed us to propose an 88% cutoff value for the similarity between any two MLVF patterns, which can be used in S. aureus epidemiological studies, including analyses of outbreaks and strain transmission events. Of the three tested methods, MLVF is the cheapest, fastest, and easiest to perform. MLVF applied to microfluidic polymer chips is a rapid, cheap, reproducible, and highly discriminating tool to determine the clonality of MRSA isolates and to trace the spread of MRSA strains over periods of many years. Although spa typing should be used due to its portability of data, MLVF has a high added value because it is more discriminatory.
INTRODUCTION
In the past decade, various DNA typing methods have been developed to study the epidemiology of Staphylococcus aureus. Of particular concern is tracking the spread of methicillin-resistant S. aureus (MRSA) in the community and in hospital settings. When typing is applied at the hospital level, it can be used to trace clonal outbreaks of MRSA and chains of transmission within institutions. Molecular typing systems for continuous surveillance must not only be able to accurately separate the most prevalent MRSA types but also have adequate stability to provide a report in a timely manner in order to implement optimal infection control measures. Moreover, typing methods that allow interlaboratory data exchange via easily accessible databases have a clear advantage. Therefore, an optimal typing approach must be endowed with high discriminatory power and produce portable data and at the same time must be rapid, highly reproducible, easy to perform, and inexpensive.
Pulsed-field gel electrophoresis (PFGE) has been considered to be the gold standard in typing of a variety of bacteria, including S. aureus. It is a highly discriminatory approach. However, PFGE is currently regarded as too labor intensive and costly for further use as a primary typing tool in the hospital setting. Moreover, the interlaboratory comparison of data produced by PFGE is challenging (3).
Multilocus sequence typing (MLST) is based on the sequence polymorphism of internal fragments of seven housekeeping genes. This is an excellent tool to investigate the core genetic population structure of S. aureus (5). During a study, isolates are grouped into clonal complexes and singletons by using the program BURST (Based Upon Related Sequence Types) and further analysis of the data can be conducted in conjunction with the entire S. aureus MLST database (http://saureus.mlst.net). However, MLST is labor intensive and costly and has only a moderate discriminatory power. Therefore, it is not suitable for use in an outbreak setting.
In recent years, spa sequence typing has become the most popular typing method for S. aureus. Genetic diversity in the spa locus arises from both a variable number of tandem repeats (VNTRs) and point mutations in the gene encoding cell surface protein A. Although spa typing showed less discriminatory power than PFGE (21, 22), its low cost, high reproducibility, appropriate stability, high throughput due to the StaphType software, and full data portability via the Ridom database (Ridom GmbH, Würzburg, Germany) made this method the primary tool for characterization of MRSA isolates at the local, national, and international level (4, 9, 11, 13, 14, 20). Moreover, implementation of the BURP (based upon repeat patterns) algorithm with the StaphType software greatly simplified classification of the isolates into clonal complexes and singletons. The BURP algorithm calculates the costs for clustering spa types. In the BURP analysis, the costs stand for the steps of evolution between the spa types; the more genetic changes between two different spa types, the higher the cost. However, spa typing also has certain limitations. The method can misclassify particular types due to recombination/homoplasy, and there are no clear rules as to which cost of the BURP algorithm should be used during a study. Therefore, criteria for the clustering of spa types need to be determined and validated in different studies in relation to the size of investigated isolate collections and the levels of the laboratories conducting the typing analyses (i.e., local, regional, or national levels).
More recently, the multilocus VNTR analysis (MLVA) method has been developed to overcome the inherent limitations of PFGE, MLST, and spa typing (25). Schouls and colleagues (25) showed that MLVA was at least as discriminatory as PFGE and at the same time produced portable data with ease of interpretation comparable to that of MLST and spa typing. Moreover, significant congruence of the results produced by MLVA, PFGE, MLST, and spa typing was observed (25).
The multilocus VNTR studies for bacterial typing were first used in the second half of the 1990s (10, 26). Since then, a number of MLVA methods for the typing of different bacterial species were published before they were adopted for S. aureus (6, 7, 18, 19). Multilocus VNTR fingerprinting (MLVF), formerly called MLVA, was the first multiplex PCR-based assay targeting various VNTRs for typing of S. aureus isolates (23). MLVF analyzes polymorphism of the VNTR regions located in seven individual genes (sspA, spa, sdrC, sdrD, sdrE, clfA, and clfB). The discriminatory power of MLVF was found to be comparable with that of PFGE and higher than that of MLST, MLVA, spa typing, and other PCR-based methods (15, 17, 21, 22, 24). MLVF is much cheaper, faster, and easier to use than PFGE and, therefore, more useful in an outbreak setting than the gold standard method. However, like PFGE, MLVF produces subjective results, which cannot be easily compared between different laboratories via the Internet. The present study was designed to improve the resolution of MLVF by (i) designing a new set of primers for the same VNTR regions; (ii) combining the method with microfluidics technology; and (iii) testing more-stringent criteria for the generation of dendrograms. Moreover, criteria for clustering of MLVF patterns were proposed based on comparisons with the results produced by the MLVA and spa typing methods.
MATERIALS AND METHODS
Bacterial isolates.
A total of 206 nonreplicate MRSA isolates (see Table S1 in the supplemental material), recovered from patients at the University Medical Center Groningen between 2000 and 2010, were analyzed in the current study. Among them, 19 MRSA isolates were considered to belong to a nosocomial cluster based on their identical antibiotic resistance profiles and isolation dates (18 isolates recovered within a 2-month window and the remaining isolate 4 months later).
Extraction of total DNA for PCR.
Total DNA was prepared from 10 to 15 colonies lifted from blood agar plates incubated for 24 h at 37°C and suspended in 500 μl of Tris-EDTA (TE) buffer (pH 8.0). The cell suspension was transferred to 2-ml bead-beating tubes with screw caps containing zirconia/silica beads with a diameter of 100 μm and 500 μl of phenol-chloroform solution. The tubes were fixed in a Precellys bead beater, which was operated 3 times for 30 s per pulse at a speed of 5,000 rpm, with 30-s intervals between pulses. Subsequently, the samples were sequentially extracted with phenol and then chloroform. The DNA was precipitated with isopropanol, washed with 70% ethanol, air dried, and then dissolved in 50 μl of DNase- and RNase-free water. The DNA concentration was quantified using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE) at 260 nm.
MLVA.
MLVA was performed according to the protocol developed by Schouls et al. (25). Isolates that differed by one or more alleles were considered distinct types. Minimum-spanning-tree analysis of MLVA was performed by using BioNumerics software (Applied Maths) to classify related types into clonal complexes (CCs). Such CCs were assigned when two or more neighboring types differed only at a single locus. A singleton was defined as a type that was not grouped into a clonal complex.
spa typing.
Amplification of the variable X region of the spa gene was performed as described by Aires-de-Sousa et al. (1). The spa types were assigned through the use of Ridom StaphType software version 1.4.6 (Ridom GmbH, Würzburg, Germany) and the Ridom SpaServer (http://www.spaserver.ridom.de) (14). spa clonal complexes (spa-CCs) were composed of 2 or more related spa types and were identified based upon the repeat pattern (BURP) algorithm implemented in the Ridom StaphType software. A spa type which was not grouped into any spa-CC was regarded as a singleton.
MLVF.
MLVF typing was performed with a new set of primers (Table 1) designed for flanking sequences of the VNTR regions selected previously (23). Amplification of the DNA fragments was performed using the following cycling conditions: a predenaturation at 98°C for 30 s followed by 20 cycles of 98°C for 10 s, 60°C for 30 s, and 72°C for 30 s, with a final extension at 72°C for 5 min. Each PCR mixture had a final volume of 5 μl, containing 0.25 U of Phire Hot Start II DNA polymerase (Finnzymes, Espoo, Finland), 0.2 mM deoxynucleoside triphosphates, 1× Phire reaction buffer, 0.5 μM each ClfB1-F, ClfB1-R, SpaI-F, and SpaI-R, 1 μM each ClfA1-F, ClfA1-R, SdrCDE-F, and SdrCDE1-R, 2 μM each SspA1-F and SspA1-R, and 5 ng of template DNA. After PCR amplification, 1 μl of each PCR product was loaded on a DNA 7500 chip and amplicon detection was automated with the microfluidics-based Agilent 2100 BioAnalyzer (Agilent Technologies, Palo Alto, CA) according to the manufacturer's protocol. Any two MLVF fingerprints differing by one or more bands were considered distinct patterns. The MLVF patterns were imported as comma-separated-values (CSV) files into GelCompar software (Applied Maths, Kortrijk, Belgium) for further analysis. The position tolerance and optimization were set to 0.6% and 0.5%, respectively. Pairwise similarity coefficients were calculated using the Dice formula, and dendrograms were created using the unweighted-pair group method using average linkages (UPGMA). Only identical patterns were regarded as representing the same subtype.
Table 1.
Primers used in this study
| Marker locus | Primer designation | Primer sequence | Reference |
|---|---|---|---|
| clfA | ClfA1-F | 5′-CAGATTCTGACCCAGGTTCAG | This study |
| ClfA1-R | 5′-TTCAGAACCTGTATCTGGTAATGG | This study | |
| clfB | ClfB1-F | 5′-TGATGGTGATTCAGCAGTAAATCC | This study |
| ClfB1-R | 5′-ATTATTTGGTGGTGTAACTCTTGAATC | This study | |
| sdr | SdrCDE-F | 5′-GTAACAATTACGGATCATGATG | 23 |
| SdrCDE1-R | 5′-TTCAYTACCWGTTTCTGGTAATGCTTT | This study | |
| spa | SpaI-F | 5′-CTAAACGATGCTCAAGCACCAAAA | This study |
| SpaI-R | 5′-GTATCACCAGGTTTAACGACATGT | This study | |
| ssp | SspA1-R | 5′-TTGTCWGAATTATTGKTATCGCCATTRTC | This study |
| SspA1-F | 5′-GAAGATATCMATTTYGCMAAYGATGACC | This study |
Data analysis.
Ridom EpiCompare software version 1.0 (http://www3.ridom.de/epicompare/) was used to calculate the discriminatory power and concordance of the typing methods. The discriminatory power was estimated by Simpson's index of diversity, expressing the probability that two unrelated and different isolates sampled from the test population would be grouped as different subtypes by a specific typing method (16). The 95% confidence intervals (CI) were calculated according to the method previously described by Grundmann et al. (12). Nonoverlapping confidence intervals were regarded as representing statistically significant differences in discriminatory power (12). The concordance between typing methods was assessed by the adjusted Rand's (AR) and Wallace's (W) coefficients (2). The AR coefficient indicates the global agreement between two methods, whereas the W coefficient shows the probability that two isolates classified as the same type by one method would also be classified as the same type by another method.
RESULTS
MLVA.
The 206 MRSA isolates produced 85 MLVA types (see Table S1 in the supplemental material). Thirty types were shared by 2 or more isolates (151 isolates in total), whereas 55 types were represented by a single isolate. Fifty-six types comprising 143 isolates were classified into 11 CCs, while the remaining 29 types were singletons (63 isolates in total) (Fig. 1).
Fig 1.
Minimum spanning tree produced using 206 MRSA isolates by MLVA. A categorical coefficient and the priority rule using the highest number of single-locus changes were used for the clustering of MLVA profiles. Each circle represents a single MLVA profile, and the circle size indicates the number of isolates with that profile. Different clonal complexes are indicated by the different colors. These clonal complexes were assigned if 2 neighboring MLVA types did not differ in more than 1 VNTR locus. MLVA types and complexes are also indicated in characters, e.g., 5 denotes MLVA type 5 and MC5 represents MLVA clonal complex 5.
spa typing.
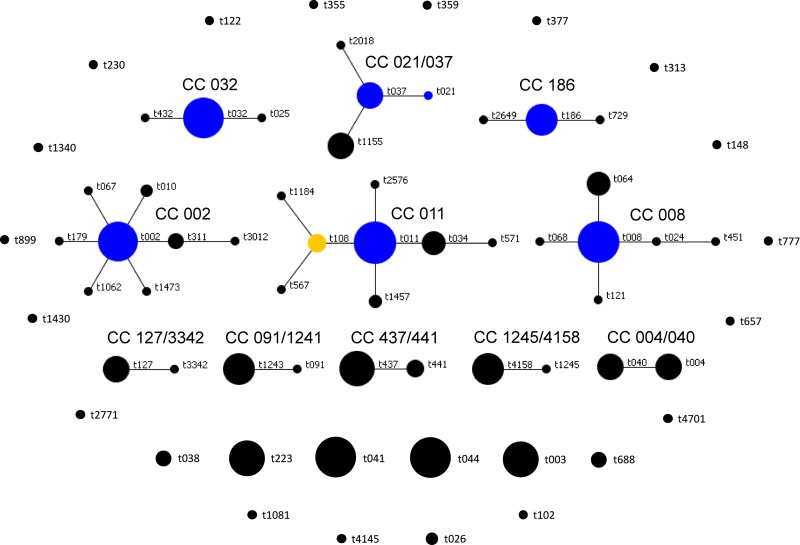
Among all examined isolates, our analysis yielded 66 spa types, ranging in length between 3 (t026 and t777) and 16 (t032) repeats (see Table S1 in the supplemental material). Twenty-seven types were represented by 2 or more isolates (167 isolates in total), while 39 types contained a single isolate. To assign the isolates into spa-CCs, we tested different cost values of BURP, and the spa typing results were compared to those produced by the MLVA method. For the comparisons, we used all spa types, even including those that were shorter than 5 repeats in length (spa types t026 and t777). Initially, spa types were clustered if the cost of the BURP algorithm was less than or equal to 4 (default value in Ridom StaphType software version 2.2.1). Further, the costs of BURP at values lower than 4 were tested. The concordance (AR) value between MLVA and spa clonal complexes was 0.779 when a BURP cost of ≤4 was applied. The concordance (AR) between clonal complexes of the two typing methods increased to a value of 0.938 and subsequently decreased to the initial level, 0.767, when the costs of BURP were ≤3 and ≤2, respectively. We used for further comparisons a BURP cost of ≤3, as that was the value at which MLVA and spa clonal clustering showed the highest concordance, calculated by adjusted Rand's coefficients. spa typing (with a BURP cost of ≤3) produced 11 spa-CCs (135 isolates in total) and 24 singletons (71 isolates in total) (Fig. 2).
Fig 2.
Population structure of the UMCG MRSA isolates after BURP analysis with a cost of ≤3. Clusters of linked spa types correspond to spa-CCs. The spa types that were defined as founders of particular clusters are indicated in blue, and subfounders are indicated in yellow.
New MLVF primers to improve efficiency and specificity of the method.
To improve the amplification efficiency as well as the specificity of MLVF, a new set of primers was designed based on the whole genome sequences of 24 S. aureus strains deposited in the NCBI database for complete microbial genomes (www.ncbi.nlm.nih.gov/genomes/lproks.cgi) (accessed July 2011). Analysis of the reliability of MLVF using previously and newly design primers was further performed on 4 genetically unrelated isolates (data not shown). To address this aim, amplifications of all MLVF loci were conducted separately and, after electrophoresis, the presence and intensity of the amplified bands were compared with the MLVF profiles obtained after multiplex PCR. It appeared that the combination of 1 previously published and 5 newly designed primers gave the best results (Table 1). Specifically, it was possible to reduce the background and increase the intensity of amplicons for all tested isolates by the use of the new primer combination. Moreover, in the case of one of the tested isolates, a spa amplicon was generated with the new primers, while the already published primers did not give an amplification product for this locus.
Reproducibility of the MLVF method.
In order to test the reproducibility of MLVF, the patterns were obtained from 12 isolates in two independent experiments with different DNA preparations and PCR amplifications and by running the samples on separate chips. In these experiments, MLVF showed excellent reproducibility (100%). Moreover, on each chip the same isolate (designated M2) was included to monitor the quality of DNA extraction, PCR amplification, and electrophoretic separation. Each time, the MLVF method produced the same banding pattern for isolate M2.
Criteria for defining MLVF clusters.
MLVF analysis identified 107 different banding patterns among 206 MRSA isolates (see Table S1 in the supplemental material). Thirty-two patterns were represented by two or more isolates (131 isolates in total). The remaining 75 patterns contained a single isolate. In order to determine rules for clustering the patterns into clonal groups, different cutoff values for the similarities between the MLVF banding profiles were tested. Using a similarity cutoff value of 68%, which was the value most frequently used in our laboratory for interpretation of the MLVF banding patterns obtained after conventional electrophoresis on agarose gels, the values for concordance (AR) between the MLVF clusters and the clonal complexes obtained by MLVA and spa typing were 0.763 and 0.764, respectively. With a cutoff value of 64%, the values for concordance (AR) between the MLVF clonal groups and those of MLVA and spa typing were higher than previously reported at 0.783 and 0.781, respectively. However, by applying more relaxed conditions to the MLVF interpretation, with the cutoff value equal to 60%, the values for concordance (AR) between the MLVF clusters and the MLVA and spa-CCs dropped and were 0.775 and 0.780, respectively. Thus, the MLVF clusters were most consistent with the clonal complexes generated by MLVA and spa typing when the cutoff value between the MLVF patterns was set at a level of 64%. With the 64% cutoff, 192 of the isolates were classified into 23 clusters designated C1 to C23, while 14 isolates had separate positions in the dendrogram (see Table S1 in the supplemental material). Among the 23 MLVF clusters, which were distinguished by applying the 64% similarity cutoff value, we found nine clusters (C3, C4, C5, C6, C8, C9, C16, C21, and C22) in which the isolates were unrelated by both MLVA and spa typing.
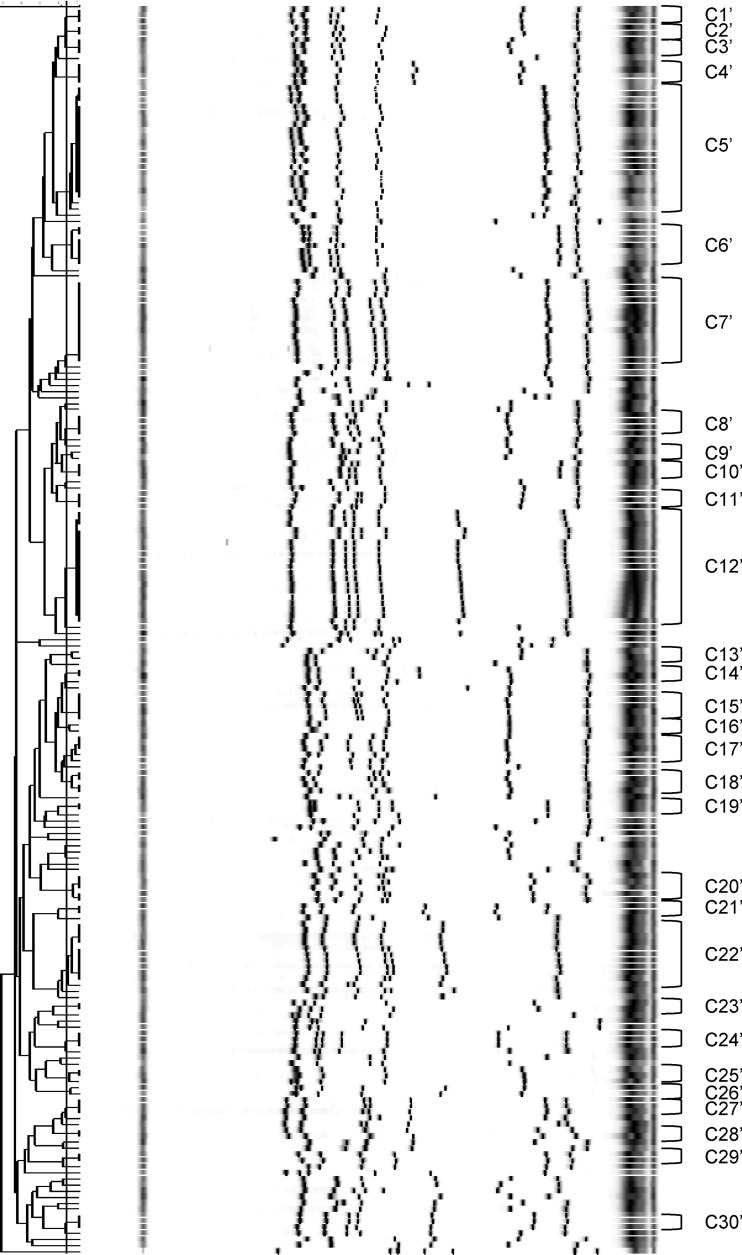
We subsequently searched for a level of similarity between MLVF patterns that would be in best concordance (AR) with the determined MLVA and spa types. Based on visual inspection of the MLVF dendrogram, we tested the cutoff values of 83%, 85%, 88%, and 90%; the concordance (AR) values for MLVF clusters and spa types were 0.787, 0.791, 0.797, and 0.786, respectively, whereas the concordance (AR) values for the MLVF clusters and MLVA types were 0.787, 0.793, 0.811, and 0.800, respectively. Therefore, the MLVF clusters were most consistent with the MLVA and spa types when the cutoff value between the MLVF banding profiles was set at the level of 88%. Based on the cutoff value of 88% (Fig. 3), 30 MLVF clusters comprising 145 isolates were determined and designated C1′ to C30′, whereas 61 isolates formed an outgroup (see Table S1 in the supplemental material). With the MLVF similarity cutoff value of 88%, any two isolates were always grouped together within a corresponding spa-CC and, with the exception of the isolates of MLVF clusters C9′ and C11′, always in a corresponding MLVA-CC. However, none of the “missing” isolates was clustered together with any other isolates by MLVA. Only MLVF was able to correctly cluster the outbreak isolates (MLVF cluster C12′ with spa type t041 and MLVA type 4). However, the spa and MLVA clusters identified an extra isolate (designated M75) which had been recovered from a patient 4 years after the outbreak. This additional isolate (M75) was a part of a MLVF cluster when the less stringent criteria for MLVF interpretation were applied (cluster C5).
Fig 3.
MLVF dendrogram of the study isolates generated by the UPGMA algorithm. Isolate clusters were delineated with an 88% similarity cutoff value. For clarity, only the MLVF clusters are indicated. MLVF clusters are shown in characters, e.g., C1′ denotes MLVF cluster 1.
Discriminatory power.
The MLVF approach was the most discriminatory method in this study, but in the case of MLVA, the difference was not statistically significant because of overlapping 95% confidence intervals (CI95) (0.98 [CI95, 0.972 to 0.987] and 0.969 [CI95, 0.96 to 0.979], respectively). The discriminatory power of spa typing was 0.959 (CI95, 0.949 to 0.969), and its resolution differed substantially from that of MLVF (nonoverlapping 95% confidence intervals). The isolates identical by MLVF (131 isolates) were in most cases (94.7%; 124/131) indistinguishable by spa typing (124 isolates). Compared to MLVA, spa typing had a lower but nevertheless comparable discriminatory power for subtyping MRSA isolates, since their 95% confidence intervals overlapped.
Concordance between methods.
The values for concordance between the typing methods compared are listed in Table 2. On the pattern/type level, the highest (and very good) concordance was found between the results produced by MLVA and spa typing (AR, 0.829). The same methods showed excellent clonal clustering concordance (AR, 0.938). The concordance between MLVF patterns and MLVA and spa types was much lower (AR, 0.668 and 0.649, respectively). This was not surprising, because the discriminatory ability of MLVF was much higher than that of MLVA and spa typing. Much better concordance levels were found when the MLVF clusters determined with the cutoff level of 64% and MLVA and spa clonal complexes were compared (AR, 0783 and 0.783, respectively). However, higher concordance levels were observed between the MLVF clusters defined by the use of the 88% cutoff value and MLVA and spa types (AR, 0.811 and 0.797, respectively).
Table 2.
Correlations between three typing methods according to adjusted Rand's and Wallace's coefficients
| Typing method | Adjusted Rand's coefficient |
Wallace's coefficient |
||||
|---|---|---|---|---|---|---|
| spa type/CC | MLVA type/CC | MLVF pattern/Cl-64%a/Cl-88%b | spa type/CC | MLVA type/CC | MLVF pattern/Cl-64%a/Cl-88%b | |
| spa type | 0.732/0.985 | 0.494/0.915/0.697 | ||||
| spa CC | 0.324/0.947 | 0.215/0.716/0.316 | ||||
| MLVA type | 0.829/0.465 | 0.971/0.998 | 0.562/0.944/0.806 | |||
| MLVA CC | 0.565/0.938 | 0.421/0.940 | 0.212/0.714/0.312 | |||
| MLVF pattern | 0.649/0.332 | 0.668/0.328 | 0.988/1.000 | 0.848/0.995 | ||
| MLVF Cl-64%a | 0.626/0.781 | 0.530/0.783 | 0.499/0.905 | 0.388/0.911 | ||
| MLVF Cl-88% | 0.797/0.456 | 0.811/0.451 | 0.949/1.000 | 0.827/0.995 | ||
MLVF clusters defined by the 64% cutoff value.
MLVF clusters defined by the 88% cutoff value.
MLVF performed on the level of patterns and clusters defined by a similarity cutoff value of 88% showed a complete probability (W, 1) of predicting the corresponding spa-CC (Table 2). Also, the MLVA type showed a high probability of predicting the spa-CC, with a Wallace's coefficient of 0.998. Other excellent Wallace's coefficient values were found for MLVF patterns and clusters with a similarity cutoff value of 88% to predict MLVA-CC (W, 0.995) as well as for MLVF patterns to predict spa types (W, 0.988) and for spa types to predict MLVA-CCs (W, 0.985). The lowest Wallace's coefficients were found for MLVA-CCs and spa-CCs to predict MLVF patterns (W, 0.212 and 0.215, respectively).
DISCUSSION
The MLVF method was originally designed in 2002 and published 1 year later (23). In the year 2002, the completed and unfinished genome sequences of only 6 S. aureus strains were publicly available through the Internet. During recent years, additional complete S. aureus genome sequences were released, which substantially enriched our knowledge about the genetic diversity of this species. Using this information, it was possible to design the new set of MLVF primers, which improved the efficiency of the method by reducing the background and enhancing the intensity of the bands in the patterns. Moreover, for some S. aureus strains it is now possible to obtain amplicons which had not been amplified using the previously described MLVF primers due to mismatches in the regions for which these primers were designed.
In 2005, Francois and colleagues (8) reported on the use of a multiple-locus, variable-number tandem-repeat-based method in conjunction with the Agilent BioAnalyzer for the typing of S. aureus isolates. They showed the usefulness of this approach in terms of performance and cost. Therefore, to improve the electrophoretic separation reproducibility and resolution of MLVF as well as couple the method with a rapid and automated analysis of data, the Agilent BioAnalyzer was applied in our present study. The BioAnalyzer sizes and quantitates 12 DNA samples on a disposable chip in approximately 40 min. The resulting data are produced in the form of an electropherogram, which graphically depicts spikes in fluorescence over time. For each peak in a pattern, the BioAnalyzer software calculates the corresponding size and concentration. An advantage of the BioAnalyzer software is that the exported data already contain normalized curves and strain information, which greatly speeds up the analysis of the results in comparison to the traditional agarose gel method. In practice, by applying microfluidic chips for MLVF, 12 samples can be analyzed in a single run within 1 h from start to finish, including the postanalysis data processing. By comparison, the previously established MLVF protocol (23), which utilizes conventional DNA electrophoresis, takes about 3 h. This includes electrophoresis of 16 samples and 4 external standards (in the first, 2 middle, and last lane positions of the gel to obtain reliable fragment position normalization) on an agarose gel (2 h 15 min), gel documentation (15 min), and dendrogram generation (30 min).
The accurate sizing of the DNA fragments by the BioAnalyzer allows the application of more stringent conditions (lower matching tolerance for pattern interpretation) in the clustering of banding patterns than the MLVF analysis by conventional electrophoresis. The accuracy is further enhanced by including upper and lower molecular weight markers in each sample, which allows detailed comparisons between samples analyzed with one chip and matching fragment patterns obtained with multiple chips. Therefore, the analysis using microfluidic chips not only enables the identification of identical S. aureus isolates by the same MLVF pattern but also allows a clear distinction of nonidentical isolates with DNA fragment positions differing slightly from each other. This is especially important in hospital settings with high rates of epidemic MRSA infections, where MLVF analysis with the BioAnalyzer is better able to show a person-to-person mode of strain transmission.
The total costs of all reagents and consumables for MLVF performed with the microfluidics-based BioAnalyzer platform (not including labor) are small and at least comparable with those of MLVF performed with conventional agarose gels. The DNA extraction procedure is the same for both approaches, and its costs amount to approximately €0.2 or $0.25 per sample. The main differences in the costs of the two approaches relate to the prices for PCR amplification and DNA electrophoresis. A clear advantage of analyses performed with the BioAnalyzer is the minimal sample consumption. Only 1 μl of PCR mixture is needed for a single run. In the case of conventional agarose gel analysis, a 5- to 10-μl volume of PCR mixture is needed to monitor the MLVF amplification products upon ethidium bromide or SYBR Safe staining with a UV transilluminator (15, 23). In total, the costs of PCR amplifications for MLVF analyses performed with the BioAnalyzer (5 μl final volume) amount to €0.4 or $0.5 per sample, while these costs amount to €1.6 to €4 or $2.1 to $5.2 (20-to-50-μl final volumes) for MLVF analyses employing conventional agarose gels. On the other hand, the BioAnalyzer chip electrophoresis is more expensive (€1.5 or $2 per sample) than the conventional electrophoresis (€0.3 or $0.4 per sample). Altogether, the costs of materials for MLVF performed with the Agilent BioAnalyzer amount to €2.1 or $2.75 per sample, while the costs for MLVF with conventional agarose gels amount to €2.1 to €4.5 or $2.75 to $5.85.
In the current study, we tested the clustering potential of the MLVF method. We particularly looked for those clustering criteria for the MLVF method that would be optimal in an outbreak setting. At the group level, the highest concordance was obtained when the cutoff value of the similarity between two MLVF patterns was set at 64%. However, with the 64% cutoff, we found nine MLVF clusters (C3, C4, C5, C6, C8, C9, C16, C21, and C22) in which the isolates were unrelated by both MLVA and spa typing. As effective control of MRSA outbreaks requires identification of true clusters of infected patients, clustering criteria must be stringent enough to differentiate outbreak isolates from nonoutbreak isolates. Therefore, we tested more stringent criteria in MLVF interpretation. When the cutoff value of 88% was applied, the MLVF clusters were the most consistent with the MLVA and spa types. The MLVF clusters obtained by applying a cutoff level of 88% never grouped isolates that were identified as being unrelated by MLVA and spa typing. In fact, the isolates of MLVF clusters defined by the 88% cutoff were always grouped in the corresponding spa-CCs and almost always in the corresponding MLVA-CCs. Therefore, for epidemiological typing, MLVF clusters defined with the cutoff value of 88% are the most suitable for detecting outbreaks as well as identifying sources and routes of transmission.
A cost analysis of all reagents and consumables indicates that the costs of MLVF performed with the Agilent BioAnalyzer amount to approximately €2.1 or $2.75 per sample, which is significantly lower than the costs of MLVA or spa typing (about €8 to €9 or $10 to $12). Also, the start-up costs for the instrumentation needed for MLVF are much lower than those for MLVA and spa typing. The costs for the microfluidics-based Agilent 2100 BioAnalyzer are a few times lower than those of DNA sequencers. Moreover, in laboratories that already possess equipment for the DiversiLab (bioMérieux, Marcy l'Etoile, France) repetitive element-PCR (rep-PCR) typing, the same device can be used for MLVF. Importantly, the MLVF procedure is faster and more straightforward to perform than the MLVA and spa typing procedures. All 3 of these typing methods can use the same DNA extraction protocols and involve PCR amplification. The MLVF and spa amplicons are obtained in a single tube, while the MLVA amplicons are obtained in 2 PCRs. The MLVF amplification products are directly separated by electrophoresis using the BioAnalyzer, while spa and MLVA amplicons involve PCR handling steps prior to electrophoretic separation on a DNA sequencer.
In conclusion, MLVF was shown to be cheaper, faster, more discriminatory, and easier to use than MLVA and spa typing. However, MLVF cannot replace MLVA and spa typing, because it does not produce portable data. In our hospital, MLVF is therefore used as the complementary method for spa typing. All MRSA isolates are characterized by spa typing as the first-line typing method. In those cases where different isolates with the same spa type are recovered from the patients, we use MLVF to conduct further subtyping of these isolates. Moreover, in outbreak situations where several MRSA-carrying patients are detected within a short period of time, MLVF and spa typing are conducted in parallel. In this way, MLVF is likely to become an important tool for enhanced MRSA control and prevention.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gerlinde N. Pluister from the Laboratory for Infectious Diseases and Perinatal Screening, National Institute for Public Health and the Environment, Bilthoven, the Netherlands, for technical support in the MLVA typing studies. Furthermore, we thank Viktoria Akkerboom from the Department of Medical Microbiology, University of Groningen, University Medical Center Groningen, the Netherlands, for excellent technical assistance.
This work was supported by the Interreg Iva-funded project EurSafety Heath-net (III-1-02=73), a Dutch-German cross-border network supported by the European Commission, the German federal states of Nordrhein-Westfalen and Niedersachsen, and the Dutch provinces of Overijssel, Gelderland, and Limburg.
Footnotes
Published ahead of print 9 May 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Aires-de-Sousa M, et al. 2006. High interlaboratory reproducibility of DNA sequence-based typing of bacteria in a multicenter study. J. Clin. Microbiol. 44:619–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carriço JA, et al. 2006. Illustration of a common framework for relating multiple typing methods by application to macrolide-resistant Streptococcus pyogenes. J. Clin. Microbiol. 44:2524–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cookson BD, et al. 2007. Evaluation of molecular typing methods in characterizing a European collection of epidemic methicillin-resistant Staphylococcus aureus strains: the HARMONY collection. J. Clin. Microbiol. 45:1830–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deurenberg RH, et al. 2009. Cross-border dissemination of methicillin-resistant Staphylococcus aureus, Euregio Meuse-Rhin region. Emerg. Infect. Dis. 15:727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farlow J, et al. 2002. Strain typing of Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii by using multiple-locus variable-number tandem repeat analysis. J. Clin. Microbiol. 40:4612–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farlow J, et al. 2001. Francisella tularensis strain typing using multiple-locus, variable-number tandem repeat analysis. J. Clin. Microbiol. 39:3186–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Francois P, et al. 2005. Use of an automated multiple-locus, variable-number tandem repeat-based method for rapid and high-throughput genotyping of Staphylococcus aureus isolates. J. Clin. Microbiol. 43:3346–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedrich AW, et al. 2008. EUREGIO MRSA-net Twente/Münsterland—a Dutch-German cross-border network for the prevention and control of infections caused by methicillin-resistant Staphylococcus aureus. Euro. Surveill. 13:pii=18965. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18965 [DOI] [PubMed] [Google Scholar]
- 10. Frothingham R, Meeker-O'Connell WA. 1998. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 144:1189–1196 [DOI] [PubMed] [Google Scholar]
- 11. Grundmann H, et al. 2010. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7:e1000215 doi:10.1371/journal.pmed.1000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grundmann H, Hori S, Tanner G. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hallin M, et al. 2007. Validation of pulsed-field gel electrophoresis and spa typing for long-term, nationwide epidemiological surveillance studies of Staphylococcus aureus infections. J. Clin. Microbiol. 45:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harmsen D, et al. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holmes A, et al. 2010. Comparison of two multilocus variable-number tandem-repeat methods and pulsed-field gel electrophoresis for differentiating highly clonal methicillin-resistant Staphylococcus aureus isolates. J. Clin. Microbiol. 48:3600–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karynski M, Sabat AJ, Empel J, Hryniewicz W. 2008. Molecular surveillance of methicillin-resistant Staphylococcus aureus by multiple-locus variable number tandem repeat fingerprinting (formerly multiple-locus variable number tandem repeat analysis) and spa typing in a hierarchic approach. Diagn. Microbiol. Infect. Dis. 62:255–262 [DOI] [PubMed] [Google Scholar]
- 18. Keim P, et al. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 182:2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klevytska AM, et al. 2001. Identification and characterization of variable-number tandem repeats in the Yersinia pestis genome. J. Clin. Microbiol. 39:3179–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Köck R, et al. 2009. Cross-border comparison of the admission prevalence and clonal structure of meticillin-resistant Staphylococcus aureus. J. Hosp. Infect. 71:320–326 [DOI] [PubMed] [Google Scholar]
- 21. Luczak-Kadlubowska A, et al. 2008. Usefulness of multiple-locus VNTR fingerprinting in detection of clonality of community- and hospital-acquired Staphylococcus aureus isolates. Antonie Van Leeuwenhoek 94:543–553 [DOI] [PubMed] [Google Scholar]
- 22. Malachowa N, et al. 2005. Comparison of multiple-locus variable-number tandem-repeat analysis with pulsed-field gel electrophoresis, spa typing, and multilocus sequence typing for clonal characterization of Staphylococcus aureus isolates. J. Clin. Microbiol. 43:3095–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabat A, et al. 2003. New method for typing Staphylococcus aureus strains: multiple-locus variable-number tandem repeat analysis of polymorphism and genetic relationships of clinical isolates. J. Clin. Microbiol. 41:1801–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sabat A, Malachowa N, Miedzobrodzki J, Hryniewicz W. 2006. Comparison of PCR-based methods for typing Staphylococcus aureus isolates. J. Clin. Microbiol. 44:3804–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schouls LM, et al. 2009. Multiple-locus variable number tandem repeat analysis of Staphylococcus aureus: comparison with pulsed-field gel electrophoresis and spa-typing. PLoS One 4:e5082 doi:10.1371/journal.pone.0005082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Belkum A, et al. 1997. Outbreak of amoxicillin-resistant Haemophilus influenzae type b: variable number of tandem repeats as novel molecular markers. J. Clin. Microbiol. 35:1517–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.