Abstract
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) was evaluated for testing susceptibility to caspofungin of wild-type and fks mutant isolates of Candida and Aspergillus. Complete essential agreement was observed with the CLSI reference method, with categorical agreement for 94.1% of the Candida isolates tested. Thus, MALDI-TOF MS is a reliable and accurate method to detect fungal isolates with reduced caspofungin susceptibility.
TEXT
Echinocandin drugs represent the newest class of antifungal agents. They act by inhibition of the 1,3-β-d-glucan synthase complex, which catalyzes the synthesis of 1,3-β-d-glucan in the fungal cell walls (5). All three available echinocandins—anidulafungin, caspofungin, and micafungin—are presumed to bind to the catalytic subunit of the enzyme complex, Fks, which is encoded by three related genes, FKS1, FKS2, and FKS3 (18). As echinocandins are not active at clinically relevant concentrations against Cryptococcus neoformans or non-Aspergillus molds, their clinical utility is largely restricted to the treatment of candidiasis and aspergillosis (9). Particularly, caspofungin was shown to be effective for treating esophageal candidiasis, candidemia, and invasive candidiasis (5). The drug is also effective as an empirical treatment of febrile neutropenia and as salvage therapy for invasive aspergillosis (3, 31), and it is the only echinocandin FDA approved for use in pediatric patients (29).
Resistance to caspofungin, as mediated by fks1 and/or fks2 enzyme modifications, has been described for Candida clinical isolates displaying high MICs (20), and, recently, breakthrough infections caused by Aspergillus fumigatus isolates with elevated minimum effective concentrations (MECs) have been reported (1, 15, 16). Clinical breakpoints for echinocandins and Candida have now been established by the Clinical and Laboratory Standards Institute (CLSI) Subcommittee for Antifungal Testing (23) and the European Committee on Antibiotic Susceptibility Testing (EUCAST) (“Anidulafungin: Rationale for the Clinical Breakpoints,” version 1.0, 2010; freely available from the EUCAST website at http://www.eucast.org), which serve as a more sensitive means of detecting isolates with acquired resistance mechanisms. EUCAST and CLSI have developed and standardized in vitro susceptibility testing methods (6, 7, 27, 28) to detect fungal strains with high MICs (or MECs) and fks mutations (10, 11, 24). However, the CLSI method is mainly restricted by the visual (and subjective) determination of yeast susceptibility endpoints (6), as well as are both the CLSI and EUCAST methods with regard to filamentous fungi (7, 28). Indeed, evaluation of the MEC when testing nonyeast fungi against echinocandins should provide more consistent and reproducible susceptibility data than the conventional MIC reading (7), although MEC determination remains technically troublesome.
The aim of the present study was to evaluate the matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) methodology—recently introduced in clinical microbiology laboratories—by using a composite correlation index (CCI) approach for testing caspofungin susceptibility of wild-type and fks mutant isolates of Candida and Aspergillus species.
A panel of 44 fungal isolates with and without known resistance-associated fks mutations were used throughout this work (Table 1). They included 34 Candida (14 Candida albicans, 12 Candida glabrata, 4 Candida parapsilosis, and 4 Candida krusei) isolates and 10 Aspergillus (6 Aspergillus fumigatus and 4 Aspergillus flavus) isolates. C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as control strains. Among the clinical isolates studied (n = 40), all isolates were referred to the authors' laboratories (UCSC or DSP), with the exception of isolates designated CP and CG, which were provided by A. Tavanti and C. Girmenia, respectively (Table 1). One FKS-containing A. fumigatus isolate was a laboratory mutant engineered in strain KU80ΔakuB (26). Isolate identification was performed by conventional microbiologic methods and, only for Aspergillus isolates, was confirmed by gene sequencing analyses (4). For all isolates, caspofungin susceptibility testing was performed by the broth microdilution method following the CLSI M27-A3 and M38-A2 guidelines (6, 7), and the MIC and MEC were determined using prominent (corresponding to 50%) growth inhibition or aberrant growth as the respective endpoint. For all isolates, FKS gene sequence analysis was performed as described elsewhere (12, 13, 26).
Table 1.
In vitro caspofungin susceptibilities of 44 isolates of Candida and Aspergillus species as determined by the CLSI reference test and MALDI-TOF MS methoda
| Species (nb) | Strain designationc | Phenotyped |
MIC (or MEC) (μg/ml)e | MPCCe | |
|---|---|---|---|---|---|
| Fks1 | Fks2 | ||||
| C. albicans (14) | UCSC13 | WT | 0.12 | 0.12 | |
| UCSC69 | WT | 0.06 | 0.12 | ||
| UCSC70 | WT | 0.12 | 0.12 | ||
| UCSC131 | WT | 0.12 | 0.12 | ||
| DSP1012 | D648Y | 2.67 | 1 | ||
| DSP1006 | F641L | 2 | 2 | ||
| DSP1007 | F641S | 4 | 4 | ||
| DSP1010 | S645F | 4 | 1 | ||
| DSP1011 | S645F + R1361R/H | 4 | 4 | ||
| DSP21 | S645P | 8 | 4 | ||
| DSP1009 | S645F | 4 | 8 | ||
| DSP1013 | P649H | 4 | 1 | ||
| DSP1040 | R1361H | 2 | 1 | ||
| DSP1014 | R1361R/H | 1 | 1 | ||
| C. glabrata (12) | UCSC91 | WT | WT | 0.03 | 0.03 |
| UCSC92B | WT | WT | 0.03 | 0.03 | |
| UCSC103 | WT | WT | 0.06 | 0.06 | |
| UCSC104 | WT | WT | 0.06 | 0.06 | |
| DSP38 | F625S | WT | 8 | 2 | |
| DSP155 | WT | F659V | 4 | 4 | |
| DSP41 | D632G | WT | 4 | 16 | |
| DSP33 | WT | D666E | 4 | 2 | |
| DSP32 | WT | D666G | 4 | 8 | |
| DSP34 | WT | P667T | 2 | 2 | |
| DSP39 | S629P | WT | 8 | 16 | |
| DSP30 | WT | S663P | 16 | 4 | |
| C. parapsilosis (4) | ATCC 22019 | WT | 0.25 | 0.25 | |
| CP14 | WT | 0.5 | 1 | ||
| CP18 | WT | 0.25 | 0.5 | ||
| CP147 | WT | 0.5 | 0.5 | ||
| C. krusei (4) | ATCC 6258 | WT | 0.125 | 0.5 | |
| UCSC28 | WT | 0.25 | 0.5 | ||
| DSP45 | F655F/C | 8 | 4 | ||
| DSP1023 | R361G (T657I + L660I) | 16 | 32 | ||
| A. fumigatus (6) | UCSC593 | WT | 0.06 | 0.125 | |
| CG221 | WT | 0.06 | 0.125 | ||
| CG277 | WT | 0.125 | 0.5 | ||
| CG295 | WT | 0.125 | 0.125 | ||
| DSPCEA17 | WT | 0.25 | 0.5 | ||
| DSPEMFR | S678P/S678P | 32 | 16 | ||
| A. flavus (4) | CBS 110.45 | WT | 0.03 | 0.125 | |
| CG192 | WT | 0.06 | 0.25 | ||
| CG196 | WT | 0.03 | 0.125 | ||
| CG217 | WT | 0.06 | 0.25 | ||
Isolates include clinical (n = 40), reference (n = 3), and laboratory mutant (n = 1) strains.
n, number of isolates tested.
Isolates are designated by source as follows: ATCC, American Type Culture Collection, Manassas, VA; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CG, culture strain collection of the Università La Sapienza, Rome, Italy; CP, culture strain collection of the Università of Pisa, Pisa, Italy; DSP, culture strain collection of the UMDNJ—New Jersey Medical School, Newark, NJ; and UCSC, culture strain collection of the Università Cattolica del Sacro Cuore (Rome, Italy).
WT, wild type at mutational hot spot regions of Fks1 and Fks2. Otherwise, the specific amino acid substitutions harbored by mutant strains are indicated.
Shown are geometric mean values (three repetitions from separate preparations) for MICs, MECs, and minimal profile change concentrations (MPCCs).
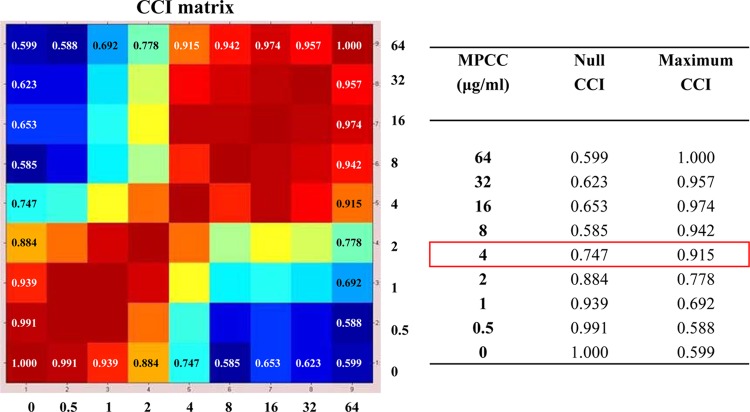
For MALDI-TOF MS-based caspofungin susceptibility assays, we adopted the protocol developed by Marinach et al. (17), with some modifications. Briefly, aliquots of conidial inoculum suspensions of approximately 1 × 106 cells per milliliter, as determined by a hemocytometric method (14), were added to RPMI, with serial dilutions (64 to 0.008 μg/ml) of caspofungin (pure substance provided by Merck), or to RPMI alone as a negative control, into 24-well plates and kept at 37°C under agitation for 15 h. The cells then were washed twice with sterile water and resuspended in 10% formic acid. One microliter of each fungal suspension was directly spotted, in duplicate, onto a polished steel target plate (Bruker Daltonics, Bremen, Germany), covered with 1 μl of absolute ethanol and 1 μl of a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile–2.5% trifluoroacetic acid (Bruker Daltonics), and allowed to air dry. Measurements were performed with a microflex LT mass spectrometer (Bruker Daltonics) within a mass range of 3,000 to 8,000 Da (2), and spectra were recorded in the positive linear mode as described previously (8). For each experimental condition (a total of 14 drug concentrations and 1 drug-free control), spectra were collected from 3 biological replicates (prepared from repeated cultivations on different days) and were automatically imported into the MALDI Biotyper 3.0 software (Bruker Daltonics) as raw data. We then used the CCI tool of MALDI Biotyper, a statistical method for analyzing the relationships between spectra according to Arnold and Reilly (2). The raw spectra were divided into 12 intervals of the same size (417 Da each), and the composition of cross-correlations and autocorrelations of all intervals (in terms of geometric mean) was used as a distance parameter between the spectra. CCI values around 1 represent a high conformance of spectra, while CCI values near 0 indicate a clear diversity of the spectra. The results were automatically viewed in a correlation matrix view and translated into a heat map, where closely related spectra are marked in hot colors and unrelated ones in cold colors, based on their respective high or low CCI values (Fig. 1). Thus, we matched for each concentration its spectrum similarity against each of the two extreme concentrations (null or maximum) of the drug according to Marinach et al. (17). This allowed determination of the minimal profile change concentration (MPCC) for caspofungin, a value defined as the lowest drug concentration at which a spectrum is more similar to the one observed at the maximum concentration than the spectrum observed at the null concentration (Fig. 1).
Fig 1.
Representative composite correlation index (CCI) matrix derived from selected mass spectra, which correspond to those for C. glabrata DSP155 cells (MIC of 4 μg/ml) exposed for 15 h at 37°C to serial caspofungin concentrations (including the null one), ranging from 0.5 to 64 μg/ml. By comparing spectra with one another at the indicated drug concentrations, numerical correlation indices were obtained, automatically visualized in a CCI matrix view, and translated into a heat map (on the left) in which closely related spectra are marked in hot colors and unrelated ones in cold colors. After matching for each concentration and its spectrum similarity against each of the two extreme concentrations (null or maximum) of the drug, the minimal profile change concentration (MPCC) was assessed as the CCI value at which a spectrum is more similar to the one observed at the maximum caspofungin concentration (maximum CCI) than the spectrum observed at the null caspofungin concentration (null CCI). The MPCC (4 μg/ml) and its relative null and maximum CCI values are shown on the right in the red box.
The new, CLSI species-specific clinical breakpoints for Candida caspofungin susceptibility (≤0.25 μg/ml for C. albicans, C. tropicalis, and C. krusei; ≤0.125 μg/ml for C. glabrata; and ≤2 μg/ml for C. parapsilosis) (23) were used to obtain the percentage of categorical agreement between the MALDI-TOF MS (MPCC) and reference (MIC) endpoints, while discrepancies among MPCCs and MICs (or MECs) of more than 2 dilutions (two wells) were used to calculate the percentage of essential agreement (25).
Table 1 shows the susceptibility of the studied isolates (wild-type and fks mutants) to the echinocandin caspofungin, as obtained with the CLSI and MALDI-TOF MS methods of testing. A high level of concordance between the values of MPCC and MIC (or MEC) was found, with full essential agreement (within 2 dilutions) for 100% of the isolates (both Candida and Aspergillus) tested. By applying the clinical breakpoints for caspofungin susceptibility/resistance recently proposed for the major species of Candida, such as C. albicans, C. glabrata, Candida tropicalis, C. krusei, and C. parapsilosis (23), all but two of our Candida isolates were correctly identified as susceptible (n = 12) or resistant (n = 20) by MALDI-TOF MS, leading to a categorical agreement of 94.1% with the reference CLSI method. Two C. krusei isolates were misclassified (minor errors) as intermediate by MALDI-TOF MS.
When the results were analyzed according to the type of fks mutation possessed, we found, interestingly, that two (DSP1012 and DSP1013) of five resistant C. albicans isolates (MICs of 2.67 and 4 μg/ml, respectively) that had an MPCC of 1 μg/ml harbored an Fks1 substitution, which belongs to those alterations (D648Y and P649H) shown to confer the lowest inhibition constant (Ki) values, compared to ones resulting in amino acid changes at Ser645 (S645P, S645F, and S645Y) (13). Similarly, the two (DSP33 and DSP38) of the three resistant C. glabrata isolates (MICs of 4 and 8 μg/ml, respectively) having the lowest MPCC value (2 μg/ml) did not harbor mutations at Ser629 (Fks1) or Ser663 (Fks2), which were indeed seen to display the highest 50% inhibitory concentration (IC50) values for all echinocandin drugs (12). This would add further support to the notion that an elevated MIC may be a result not only of characteristic mutations in FKS but also of other compensatory mechanisms (18). Therefore, it is possible that other cell-response/adaptive mechanisms (e.g., increase in cell wall chitin content) (30) have led to the high MIC values in at least some of our Candida isolates displaying low MPCCs, when they were tested using the CLSI reference method. Nonetheless, the MPCC values we obtained for the same isolates using the MALDI-TOF MS method might be a reflection of changes in the 1,3-β-d-glucan synthase affinity for caspofungin without strongly impacting relative susceptibility in the in vitro growth.
Thus, the MALDI-TOF MS-based susceptibility testing assay appears to correctly identify isolates of Candida with fks mutations and/or modification of the echinocandin sensitivity of the 1,3-β-d-glucan synthase enzyme complex, which would result in fungal cell glucan depletion. In this context, a prominent variation of protein composition induced by caspofungin to which fungal cells are subjected could be a predictor of resistance as valid as the prominent-inhibition endpoint currently used. In the absence of clinical breakpoints, recently Espinell-Ingroff et al. (11) proposed epidemiological cutoff values for six Aspergillus species, including A. fumigatus, and caspofungin to determine the relationship between resistant molecular mechanisms and non-wild-type MEC values. However, while it is uncommon to detect Aspergillus isolates with reduced caspofungin susceptibility or non-wild-type strains (11, 21), high caspofungin MECs (≥1 μg/ml) or FKS mutations for A. fumigatus were obtained from breakthrough infections in patients on caspofungin therapy (1, 16). In agreement with the MEC values observed here, MPCC values of 0.5 and 0.25 μg/ml captured, respectively, all of the clinical A. fumigatus and A. flavus isolates tested.
In summary, our data confirm previous findings showing that MALDI-TOF MS-based technology is a reliable and reproducible tool for antifungal susceptibility testing (17). Due to the paucity of isolates tested, especially resistant A. fumigatus, further studies will be required to establish whether MPCC will generally overestimate or underestimate caspofungin susceptibility of pathogenic fungi, in order to include it in a new generation of susceptibility tests (19). In practice, numerous strains per fungal species will need to be tested to show that MALDI-TOF MS produces the same wild-type MIC distributions as the CLSI method (21, 22) and, ultimately, to validate that the newly proposed method is equivalent to the reference one. To date, while the endpoint readings achievable with MALDI-TOF MS represent a slight time savings (15 h versus 24 h) over the CLSI method, MALDI-TOF MS has great advantage of eliminating subjective read-outs, which occur with the CLSI (and EUCAST) method when filamentous fungi are tested. A cost comparison of the MALDI-TOF MS with the reference method appears to show an economic disadvantage. However, if compared with commercial CLSI- or EUCAST-based methods that are currently used for antifungal susceptibility testing, MALDI-TOF MS is surely cost-effective for those mycology laboratories (nowadays ever numerous) that routinely use MALDI-TOF MS for species identification of clinical isolates. As shown with the use for identification purposes, it is plausible that interlaboratory comparisons using the same instrument, as well as comparisons of spectral data from different instruments (i.e., Saramis or Bruker Daltonics), will prove that MALDI-TOF MS is a widely exportable technology even when applied to antifungal susceptibility testing assays.
In conclusion, we propose MALDI-TOF MS as an alternative method that may aid in effective detection of fungal isolates with reduced caspofungin susceptibility, particularly for diagnostic microbiology laboratories equipped with MALDI-TOF MS instruments.
ACKNOWLEDGMENTS
We thank Markus Kostrzewa for critical reading of the manuscript and helpful discussions.
This work was supported by a grant from the Università Cattolica del Sacro Cuore (Fondi Linea D1, 2011).
Footnotes
Published ahead of print 25 April 2012
REFERENCES
- 1. Arendrup MC, et al. 2009. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 53:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnold RJ, Reilly JP. 1998. Fingerprint matching of E. coli strains with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of whole cells using a modified correlation approach. Rapid Commun. Mass Spectrom. 12:630–636 [DOI] [PubMed] [Google Scholar]
- 3. Bal AM. 2010. The echinocandins: three useful choices or three too many? Int. J. Antimicrob. Agents 35:13–18 [DOI] [PubMed] [Google Scholar]
- 4. Balajee SA, et al. 2009. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J. Clin. Microbiol. 47:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen SC, Sorrell TC. 2007. Antifungal agents. Med. J. Aust. 187:404–409 [DOI] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A2, 2nd ed Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. De Carolis E, et al. 29 August 2011. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. [Epub ahead of print.] doi:10.1111/j.1469-0691.2011.03599.x [DOI] [PubMed] [Google Scholar]
- 9. Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151 [DOI] [PubMed] [Google Scholar]
- 10. Desnos-Ollivier M, et al. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob. Agents Chemother. 52:3092–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espinel-Ingroff A, Fothergill Fuller J, Johnson E, Pelaez T, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for caspofungin and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob. Agents Chemother. 55:2855–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 53:3690–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guarro J, Pujol I, Aguilar C, Llop C, Fernández-Ballart J. 1998. Inoculum preparation for in-vitro susceptibility testing of filamentous fungi. J. Antimicrob. Chemother. 42:385–387 [DOI] [PubMed] [Google Scholar]
- 15. Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938–944 [DOI] [PubMed] [Google Scholar]
- 16. Madureira A, et al. 2007. Breakthrough invasive aspergillosis in allogeneic haematopoietic stem cell transplant recipients treated with caspofungin. Int. J. Antimicrob. Agents 30:551–554 [DOI] [PubMed] [Google Scholar]
- 17. Marinach C, et al. 2009. MALDI-TOF MS-based drug susceptibility testing of pathogens: the example of Candida albicans and fluconazole. Proteomics 9:4627–4631 [DOI] [PubMed] [Google Scholar]
- 18. Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perlin DS. 2009. Antifungal drug resistance: do molecular methods provide a way forward? Curr. Opin. Infect. Dis. 22:568–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perlin DS. 2011. Current perspectives on echinocandin class drugs. Future Microbiol. 6:441–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfaller MA, et al. 2010. Wild-type minimum effective concentration distributions and epidemiologic cutoff values for caspofungin and Aspergillus spp. as determined by Clinical and Laboratory Standards Institute broth microdilution methods. Diagn. Microbiol. Infect. Dis. 67:56–60 [DOI] [PubMed] [Google Scholar]
- 22. Pfaller MA, et al. 2010. Wild-type MIC distributions and epidemiological cutoff values for the echinocandins and Candida spp. J. Clin. Microbiol. 48:52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfaller MA, et al. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14:164–176 [DOI] [PubMed] [Google Scholar]
- 24. Pfaller MA, et al. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Posteraro B, et al. 2009. Reliability of the Vitek 2 yeast susceptibility test for detection of in vitro resistance to fluconazole and voriconazole in clinical isolates of Candida albicans and Candida glabrata. J. Clin. Microbiol. 47:1927–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rocha EM, Garcia-Effron G, Park S, Perlin DS. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:4174–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez-Tudela JL, et al. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398–405 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez-Tudela JL, et al. 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982–984 [DOI] [PubMed] [Google Scholar]
- 29. VandenBussche HL, Van Loo DA. 2010. A clinical review of echinocandins in pediatric patients. Ann. Pharmacother. 44:166–177 [DOI] [PubMed] [Google Scholar]
- 30. Walker LA, Gow NA, Munro CA. 2010. Fungal echinocandin resistance. Fungal Genet. Biol. 47:117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walsh TJ, et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]