Abstract
Plasmodium knowlesi infection with low parasitemia presents a diagnostic challenge, as rapid diagnostic tests are often negative and identification to the species level by microscopy is difficult. P. knowlesi malaria in a traveler is described, and real-time PCR is demonstrated to support fast and reliable diagnosis and identification to the species level.
CASE REPORT
In July 2011, a 32-year-old woman with a blank medical history presented to her general practitioner with complaints of spiking fever with chills, nausea with vomiting, severe headache, and a backache. One day before, she had returned from a 3-week vacation in Malaysia. During the first week of the vacation, she had done a 2-day jungle trek in Borneo, where she had been repeatedly bitten by mosquitoes. Advised by a Dutch travel clinic, she had not used malaria prophylaxis. Three days before her return to The Netherlands, her complaints started. The patient had visited a local doctor and been prescribed ciprofloxacin at 500 mg three times a day and acetaminophen with minimal relief of symptoms.
On request of the general practitioner, a malaria diagnosis was performed. The thick blood film was reported dubiously positive, with both a negative thin blood film and a negative antigen test (Binax NOW Malaria, Inverness Medical). Being severely ill, possibly due to malaria, the patient was sent to the first aid department of our hospital for further examination.
There, she presented as a sick, hemodynamically stable patient with complaints of fever spiking to 40.2°C. Her physical examination was normal. Laboratory results revealed a normal hemoglobin concentration of 8.4 mmol/liter (reference range, 7.5 to 10.0 mmol/liter), thrombocytopenia at 72 platelets/nl (150 to 400/nl), leukopenia at 3.5 leukocytes/nl (4.0 to 10.0/nl), and a C-reactive protein of level of 190 mg/liter (<6.0 mg/liter). The total bilirubin level was 12 μmol/liter (<17 μmol/liter), the lactate dehydrogenase level was 330 U/liter (<250 U/liter), the aspartate aminotransferase level was 76 U/liter (<30 U/liter), the alanine aminotransferase level was 80 U/liter (<35 U/liter), the alkaline phosphatase level was 65 IU/liter (40 to 120 IU/liter), the γ-glutamyltransferase level was 66 U/liter (<40 U/liter), and the prothrombin time was 15.4 s (12 to 14.5 s). Urinalysis was normal.
A second malaria test was performed. An antigen test (Binax NOW Malaria) was negative; in both thick and thin blood films, several malaria parasites were observed with a parasite index of 0.0005% (Fig. 1A to C). Although not completely typical, the morphology of the parasites resembled that of Plasmodium malariae. Combining the patient's vacation destination, her severe symptoms, and the morphological resemblance of her parasites to P. malariae, malaria infection with Plasmodium knowlesi was suspected. A blood specimen was sent to a specialized laboratory for molecular diagnosis and correct identification to the species level.
Fig 1.
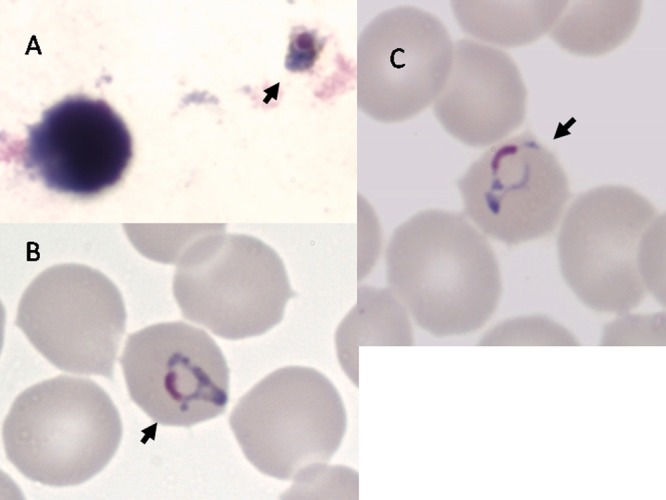
Giemsa staining of P. knowlesi parasites in thick (A) and thin (B and C) films prepared on the day of hospital admission. Arrows point to parasites. Films and stains were prepared by standard techniques. Magnification, ×1,000.
The patient was admitted to the hospital. Because the species of the infecting malaria parasite was still unknown and (co)infection with Plasmodium falciparum could not be ruled out completely, treatment with a fixed does of atovaquone (1000 mg/day) and proguanil (400 mg/day) once a day for 3 days was started. One day after admission, laboratory results showed a hemoglobin level decrease to 7.7 mmol/liter with slow improvement of liver test results. One day after the start of treatment, a thick blood film was still positive with a parasite index of 0.0002%; on the 3 following days, thick blood films were negative. The patient's fever and headache decreased after 1 day of treatment; after 2 days, her symptoms cleared up. Four days after admission, the patient was discharged in good condition. Follow-up showed no complications except temporary fatigue for 3 to 4 weeks. Blood and urine cultures remained negative.
Two days after admission, PCR results were available. A generic real-time PCR for the Plasmodium genus targeting the small-subunit rRNA gene (9) was positive. Specific real-time PCRs for the small-subunit rRNA genes of P. falciparum, P. vivax, P. ovale, and P. malariae (9) were negative, but a novel real-time PCR targeting the P. knowlesi S-type small-subunit rRNA gene was positive. This PCR mixture contained primers NVPK-F (GCATCATAATCCAGTTTTATG) and NVPK-R (TACCTTGTACCTAATAATACTTGG) at 0.9 μM each, 0.2 μM locked nucleic acid probe NVPK-P (5′-Cyan500-CAGGGAATAGAG+G+GTTG-BlackB Quencher-3′ [plus signs represent locked bases]), and uracil DNA glycosylase (Fermentas) in 1× Roche probe master buffer. Reactions were performed in a Roche LightCycler 480 system. The analytical sensitivity of this PCR is 5 target copies per reaction, which equals <1 parasite. Its specificity was 100% with 88 samples from patients suspected of having malaria. Sequence analysis of both the generic PCR product and the P. knowlesi PCR product confirmed that P. knowlesi sequences were amplified by both PCRs. PCR and thick blood films were negative 1 month after discharge.
P. knowlesi is the emerging fifth malaria parasite infecting humans (6, 11). The first reported case of natural infection of a human was that of an American traveler returning from peninsular Malaysia in 1965 (6, 11). Only recently has a higher incidence of P. knowlesi infections of humans been reported in Southeast Asia (6). The great majority of cases are from Malaysian Borneo, where 120 cases of naturally acquired P. knowlesi infection were described in a period of 8 years (10). In western countries, fewer than 10 travelers returning with P. knowlesi infection have been described thus far (6).
In Malaysia, patients with P. knowlesi infection typically presented with a nonspecific febrile illness with daily fever and chills (6). Other frequent symptoms include headache, rigors, malaise, myalgia, abdominal pain, breathlessness, and productive cough (6). Tachypnea, pyrexia, and tachycardia were common clinical signs. All of the patients had thrombocytopenia at hospital admission or on the following day, yet none of the patients had clinical coagulopathy (6). At hospital admission, only a few of the patients had anemia, whereas mild hepatic dysfunction was relatively common (6). Applying the World Health Organization criteria for P. falciparum infection, P. knowlesi infection was evaluated as severe in 7% of the cases (6). As described above, our patient experienced most of these symptoms and also had thrombocytopenia with no symptoms of clinical coagulopathy. At presentation, she already had some mild hepatic dysfunction, and during hospital admission, she developed mild anemia.
Correct identification to the species level is important, as the symptoms of P. knowlesi can be more severe than those of P. malariae infection (6). P. knowlesi has a short life cycle of 24 h, and erythrocyte invasion is not restricted to young or old cells, which allows a high level of parasitemia, and the development of the parasite in erythrocytes is asynchronous (6). Early trophozoites of P. knowlesi are morphologically similar to those of P. falciparum, and all other stages resemble those of P. malariae (3). Therefore, P. knowlesi infection is easily misdiagnosed as P. malariae infection by conventional microscopy (6, 10). Rapid diagnostic tests have low sensitivity for P. knowlesi (6).
Currently, PCR is the method of choice for differentiating between P. knowlesi and P. malariae infections or coinfection with either species and P. falciparum. Real-time PCR is faster than conventional PCR and sequence analysis (7). Current combinations of species-specific real-time PCRs for the detection of the four classical Plasmodium species in use in reference centers (9) should therefore be extended to include P. knowlesi. Apart from the real-time PCR described here, similar PCRs have been described that await evaluation in a clinical setting (1, 4, 8).
The majority of Malaysian patients with P. knowlesi infection were treated effectively with chloroquine (6). In western travelers also, the combination of quinine and doxycycline, a fixed dose of atovaquone and proguanil, and monotherapy with mefloquine have been used (6).
Our patient was successfully treated with a combination of atovaquone and proguanil, Malarone, that is also effective for the treatment of uncomplicated P. falciparum and P. malariae infections (2). Both fever and other symptoms disappeared within a few days after treatment started, with no residual symptoms. The effectiveness of Malarone in P. knowlesi infection has been reported in only one patient before (5). Treatment with Malarone can be considered when P. falciparum initially cannot be ruled out in cases of suspected P. knowlesi infection. When the differential diagnosis is infection with P. knowlesi or P. malariae, chloroquine is the preferred treatment (6). Our patient did not use malaria prophylaxis, as was true of all other western travelers reported with P. knowlesi infections (6).
This case report illustrates that clinicians should be aware of infection with P. knowlesi when microscopic examination suggests P. malariae but the patient has severe disease, considerable parasitemia, or a history of visiting woods or their vicinity in Southeast Asia.
Footnotes
Published ahead of print 9 May 2012
REFERENCES
- 1. Babady NE, Sloan LM, Rosenblatt JE, Pritt BS. 2009. Detection of Plasmodium knowlesi by real-time polymerase chain reaction. Am. J. Trop. Med. Hyg. 81:516–518 [PubMed] [Google Scholar]
- 2. CDC 2011. Treatment of malaria (guidelines for clinicians). Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/malaria/resources/pdf/clinicalguidance.pdf [Google Scholar]
- 3. Daneshvar C, et al. 2010. Clinical and parasitological response to oral chloroquine and primaquine in uncomplicated human Plasmodium knowlesi infections. Malar. J. 9:238 doi:10.1186/1475-2875-9-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Divis PC, Shokoples SE, Singh B, Yanow SK. 2010. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar. J. 9:344 doi:10.1186/1475-2875-9-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Figtree M, et al. 2010. Plasmodium knowlesi in human, Indonesian Borneo. Emerg. Infect. Dis. 16:672–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kantele A, Jokiranta TS. 2011. Review of cases with the emerging fifth human malaria parasite, Plasmodium knowlesi. Clin. Infect. Dis. 52:1356–1362 [DOI] [PubMed] [Google Scholar]
- 7. Mackay IM. 2004. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infect. 10:190–212 [DOI] [PubMed] [Google Scholar]
- 8. Oddoux O, et al. 2011. Identification of the five human Plasmodium species including P. knowlesi by real-time polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 30:597–601 [DOI] [PubMed] [Google Scholar]
- 9. Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. 2009. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J. Clin. Microbiol. 47:975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh B, et al. 2004. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363:1017–1024 [DOI] [PubMed] [Google Scholar]
- 11. Vythilingam I, et al. 2008. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit. Vectors 1:26 doi:10.1186/1756-3305-1-26 [DOI] [PMC free article] [PubMed] [Google Scholar]


