Abstract
Phosphatidylinositol 3-kinase (PI3-K) amplification and phosphatase and tensin homolog (PTEN) deletion-caused Akt activation contribute to the development of prostate cancer. Mammalian target of rapamycin complex 2 (mTORC2) is a kinase complex comprised of mTOR, Rictor, mSin1, mLST8/GβL and PRR5 and functions in the phosphorylation of Akt at Ser473. Herein, we report that mTORC2 plays an important role in PC3 androgen refractory prostate cell proliferation and anchorage-independent growth. Aloe-emodin, a natural compound found in aloe, inhibited both proliferation and anchorage-independent growth of PC3 cells. Protein content analysis suggested that activation of the downstream substrates of mTORC2, Akt and PKCα, was inhibited by aloe-emodin treatment. Pull-down assay and in vitro kinase assay results indicated that aloe-emodin could bind with mTORC2 in cells and inhibit its kinase activity. Aloe-emodin also exhibited tumor suppression effects in vivo in an athymic nude mouse model. Collectively, our data suggest that mTORC2 plays an important role in prostate cancer development and aloe-emodin suppresses prostate cancer progression by targeting mTORC2.
Introduction
Prostate cancer is the second most common cause of cancer-related death in men in the USA. Accumulating evidence indicates that phosphatidylinositol 3-kinase (PI3-K) and phosphatase and tensin homolog (PTEN) play an important role in prostate cancer development (1,2). PI3-K protein and messenger RNA levels are increased in prostate cancer and sometimes mutations are found (3,4). PI3-K also plays an important role in the transition of prostate cancer cells from androgen-dependence to androgen-independence (5). PTEN hemizygous or homozygous deletions are frequently detected in many prostate cancer cell lines and primary prostate cancers (6,7). Loss of the PTEN protein is a pathological marker of poor prognosis in prostate cancer (6,8). Both PI3-K amplification and PTEN deletion consistently activate Akt, which plays a central role in a number of cell signaling pathways that are important in cancer cell survival (9).
The activation of Akt requires phosphorylation at Thr308 by 3-phosphoinositide-dependent kinase 1 (PDK1) and at Ser473 by mTORC2. mTORC2 is a complex comprised of mammalian target of rapamycin (mTOR), rapamycin-insensitive component of mTOR (Rictor), mSin1, mLST8/GβL and Protor/PRR5. A recent report indicated that ribosomes are required for mTORC2 signaling (10). Besides mTORC2, mTOR is also a member of the mTOR complex 1 (mTORC1), which regulates cell growth by controlling the activity of the S6 kinases and the 4E-BP proteins. mTORC2 is implicated in actin cytoskeleton regulation, as well as phosphorylation of Akt at Ser473 and PKCα at Thr638 (11,12).
Recent studies indicated that inhibition of mTORC1 can induce Akt (Ser473) phosphorylation in some cancer cell lines and patient tumors (13–16), an event that may attenuate tumor responses to therapy (17–19). Further investigations led to the discovery of a negative feedback loop between mTOR/S6K1 and PI3-K, whereby mTOR/S6K1 activation attenuates PI3-K signaling by suppressing the function of insulin receptor substrate-1 (IRS1), a mediator of insulin receptor-dependent activation of PI3-K (14,20). The inhibition of mTORC1 is proposed to relieve the inhibition of the PI3-K pathway through inactivation of S6K1, thereby activating Akt (18,21,22). mTORC2, which is directly responsible for Akt phosphorylation, may be an ideal target for prostate cancer prevention or therapy against cancers driven by PI3-K activation or PTEN loss. Furthermore, previous studies have shown that mTORC2 is not required for survival of mouse embryonic fibroblasts or development of Drosophila embryos, whereas transformed human prostate epithelial cells lacking PTEN require mTORC2 to form tumors when injected into nude mice (12,23).
Aloe-emodin is an ingredient from aloe and Rheum palmatum. Previous studies demonstrate that aloe-emodin exerts antiproliferation effects and induces cellular apoptosis (24,25). Here, we found that aloe-emodin inhibited proliferation and anchorage-independent growth of the androgen refractory prostate cancer cell line PC3. In vitro kinase assay results showed that aloe-emodin can bind with mTORC2 and inhibit its kinase activity. Knocking down Rictor expression also strongly attenuated PC3 proliferation and anchorage-independent growth. Aloe-emodin inhibited PC3 growth in vivo by decreasing Akt (Ser473) phosphorylation. Therefore, these data demonstrated that aloe-emodin suppressed androgen refractory prostate cancer cell growth by targeting mTORC2.
Materials and methods
Materials
Aloe-emodin (>95% purity) and other chemical reagents, including Tris, NaCl and sodium dodecyl sulfate (SDS), for molecular biology and buffer preparation, were purchased from Sigma–Aldrich (St Louis, MO). CNBr-Sepharose 4B and glutathione-Sepharose 4B beads were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Antibodies for Western blot analysis were from Cell Signaling Technology (Beverly, MA), Santa Cruz Biotechnology (Santa Cruz, CA) or Upstate Biotechnology (Charlottesville, VA).
Cell culture
PC3 prostate cancer cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). PC3 cells were propagated in F-12K medium (Cellgro, Manassas, VA) containing 10% fetal bovine serum (Gibco, Grand Island, NY) and 1 IU penicillin/1 μl/ml streptomycin (Cellgro) at 37°C in a humidified incubator with 5% CO2. PC3 cells were cytogenetically tested and authenticated before the cells were frozen. Each vial of frozen cells was thawed and maintained in culture for a maximum of 8 weeks. Enough frozen vials were available for each cell line to ensure that all cell-based experiments were conducted on cells that had been tested and in culture for ≤8 weeks.
MTS assay
PC3 cells (3 × 103) were seeded into 96-well plates in 100 μl of 10% fetal bovine serum F-12K medium and incubated in a 37°C in a 5% CO2 incubator. After culturing for 12 h, different concentrations of aloe-emodin were added to each well. After incubation for another 24, 48, 72 or 96 h, 20 μl of the CellTiter96 Aqueous One Solution (Promega, Madison, WI) was added to each well and cells were then incubated for another 2 h at 37°C. Absorbance was measured at 490 and 690 nm.
Western blotting
For Western blot analysis, cells (2 × 106) were cultured in a 10-cm dish for 24 h. The cells were then treated with various concentrations of aloe-emodin (0, 2.5, 5, 10 or 15 μM) for 24 h. The protein concentration was determined and lysate protein (30 μg) was subjected to 10% SDS–polyacrylamide gel electrophoresis. After transferring proteins, membranes were incubated with a specific primary antibody at 4°C overnight. Protein bands were visualized by a Chemiluminescence Detection Kit (Amersham Pharmacia Biotech) after hybridization with a horseradish peroxidase-conjugated secondary antibody.
mTOR in vitro kinase assay
The glutathione-S-transferase-tagged fusion Akt1 proteins were purified using glutathione-Sepharose 4B beads and eluted with 10 mM reduced glutathione in 50 mM Tris–HCl (pH 8.0) buffer. Active mTOR (1362-end, 250 ng; Millipore, Billerica, MA) was incubated with dimethyl sulfoxide or aloe-emodin and then reacted with purified Akt1 fusion proteins (1 μg). Reactions were conducted in kinase buffer containing 50 μM unlabeled ATP with or without 10 μCi of [γ-32P]ATP at 30°C for 30 min. Reactions were terminated and proteins resolved by 8% SDS–polyacrylamide gel electrophoresis and visualized by autoradiography.
mTORC2 in vitro kinase assay
mTORC2 was pulled down with a Rictor antibody as described by Sarbassov et al. (11). Purified Akt1 fusion proteins (1 μg) were used for an in vitro kinase assay. Reactions were conducted in kinase buffer containing 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 100 mM potassium acetate, 1 mM MgCl2 and 50 μM ATP at 30°C for 30 min. Reactions were terminated and proteins resolved by 8% or 6% SDS–polyacrylamide gel electrophoresis and visualized by Western blotting.
PI3-K in vitro kinase assay
The PI3-K in vitro kinase assay was performed as described (26). Briefly, 100 ng active PI3-K (Millipore) was incubated with dimethyl sulfoxide or aloe-emodin for 15 min and then reacted with phosphatidylinositol sodium salt (MP Biomedical, Solon, OH). Reactions were performed in kinase buffer containing 50 μM unlabeled ATP with or without 10 μCi of [γ-32P]ATP at 30°C for 20 min. Reactions were terminated and resolved by thin layer chromatography (Merck, Whitehouse Station, NJ) and visualized by autoradiography.
Anchorage-independent cell growth
Cells (8 × 103/ml) were exposed to aloe-emodin (0–15 μM) in 1 ml of 0.33% basal medium Eagle's agar containing 10% fetal bovine serum. The cultures were maintained at 37°C in a 5% CO2 incubator for 14 days, and the cell colonies were counted under a microscope with the aid of the Image-Pro Plus software (v. 6) program (Media Cybernetics, Silver Spring, MD) as described by Colburn et al. (27).
Pull-down assays
For the ex vivo pull-down assay, a cellular supernatant fraction of PC3 cells (500 μg) was incubated with aloe-emodin-Sepharose 4B (or Sepharose 4B alone as a control) beads (100 μl, 50% slurry) in reaction buffer (50 mM Tris pH 7.5, 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1 mM dithiothreitol, 0.01% Nonidet P-40, 2 μg/ml bovine serum albumin, 0.02 mM phenylmethylsulfonyl fluoride and 1 μg protease inhibitor mixture). After incubation with gentle rocking overnight at 4°C, the beads were washed five times with buffer (50 mM Tris pH 7.5, 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1 mM dithiothreitol, 0.01% Nonidet P-40 and 0.02 mM phenylmethylsulfonyl fluoride) and proteins bound to the beads were analyzed by Western blotting.
In vivo tumor growth assay
Athymic nude mice (BALB/c nude mouse, 6 weeks old) were from Orient Bio (Jungwon-gu, Gyeonggi-Do, Republic of Korea). Animals were maintained under ‘specific pathogen free’ conditions and all animal studies were conducted according to guidelines approved by the KRIBB-IACUC (Korea Research Institute of Bioscience & Biotechnology—Institutional animal care and use committee). Animals were acclimated for 2 weeks before the study and had free access to food and water. The animals were housed in climate-controlled quarters with a 12-h light/12-h dark cycle. Animals were randomly assigned to the following groups: vehicle group (n = 12); 10 mg/kg aloe-emodin group (n = 12); 50 mg/kg aloe-emodin group (n = 12) and 50 mg/kg aloe-emodin control group (n = 12). Each mouse was administered aloe-emodin (10 or 50 mg/kg body weight in 100 μl of 20% PEG400 in autoclaved phosphate-buffered saline as vehicle) or only vehicle five times per week by intraperitoneal injection. After 3 days of treatment, PC3 cells (1 × 106) were injected subcutaneously into the right flank of mice in the respective groups. Following injection, mice continued to be administered aloe-emodin or vehicle. Mice in the 50 mg/kg aloe-emodin control group were not injected with cells but maintained for comparison of body weight and tumor development. Mice were weighed and tumors measured by caliper three times per week. Tumor volume was calculated from measurements of two diameters of the individual tumor according to the following formula: tumor volume (mm3) = (length × width × width/2). Mice were monitored until day 28 and at that time, mice were euthanized and tumors extracted.
Statistical analysis
All quantitative data are expressed as means ± SE or SD as indicated. The one-way analysis of variance was used for statistical analysis. A probability of P <0.05 was used as the criterion for statistical significance.
Results
Akt activity is increased in prostate cancer cell lines and the mTOR complex2 regulates Akt phosphorylation and is required for PC3 prostate cancer cell growth
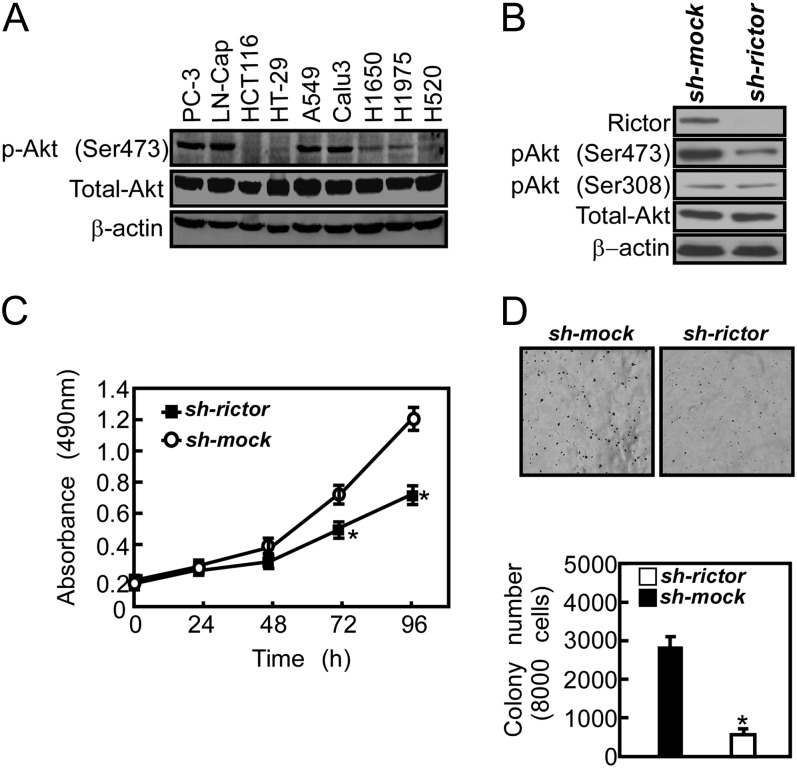
To determine whether Akt activity is directly associated with the tumorigenic properties of cancer cells, we measured the phosphorylation level of Akt at Ser473 and Thr308 in several human cancer cell lines. Akt phosphorylation at Ser473 was increased in prostate cancer cell lines and in some lung cancer cell lines (Figure 1A). These data suggested that Akt phosphorylation at Ser473 might be associated with tumorigenic potential of prostate cancer. Based on the finding, we investigated whether knocking down the upstream kinase of Akt would affect proliferation of prostate cancer cells. We established a PC3 cell line stably expressing short hairpin RNA targeting Rictor, a component of mTORC2. The specificity of sh-rictor was confirmed by Western blotting (Figure 1B). Knocking down Rictor expression suppressed the phosphorylation of Akt at Ser473, but not at Thr308. Cell proliferation results showed that proliferation was delayed after knocking down Rictor (Figure 1C). We thus examined whether knocking down Rictor would affect cell growth under anchorage-independent conditions. Our results indicated that knocking down Rictor in PC3 cells resulted in fewer colonies being formed in soft agar compared with control cells (Figure 1D).
Fig. 1.
Akt is activated in prostate cancer cell lines and knocking down Rictor expression suppresses proliferation and anchorage-independent growth of PC3 prostate cancer cells. (A) Western blot analysis of Akt phosphorylation in various cancer cell lines. PC3 and LNCaP: prostate carcinoma; HCT116 and HT29: colorectal carcinoma; A549, Calu3, H1650 and H1795: lung adenocarcinoma; H520: lung squamous cell carcinoma. (B) PC3 cells were transfected with an sh-mock or sh-rictor plasmid and stable colonies were selected with puromycin. Knockdown of Rictor expression and Akt phosphorylation were analyzed by Western blotting. (C) Proliferation was determined at 24-h intervals up to 96 h in sh-mock or sh-rictor stably transfected cells. Data are shown as means ± SD from three independent experiments. The asterisk indicates a significant (P < 0.05) decrease in proliferation compared with the control group. (D) Colony formation in soft agar using sh-mock or sh-rictor stably transfected cells. Cells were grown in soft agar for 14 days and then colonies were counted using a microscope and the Image-Pro PLUS software program (v.4). Data are shown as means ± SD from three independent experiments and the asterisk indicates a significant (P < 0.05) decrease in colony formation compared with sh-mock cells.
Aloe-emodin suppresses proliferation and anchorage-independent growth of PC3 cells
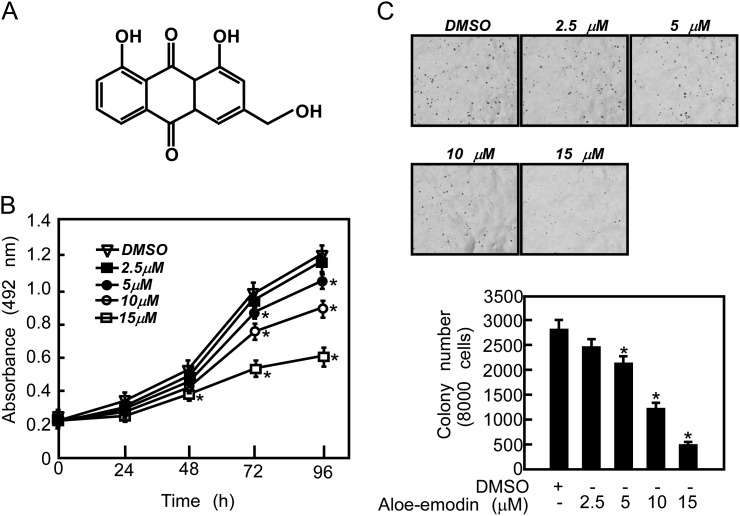
Aloe-emodin is an anthraquinone present in the aloe plant (Figure 2A). Aloe-emodin exhibits antitumor effects against gastric cancer and hepatoma cell lines (28,29). Our data indicated that PC3 cell proliferation was inhibited by aloe-emodin in a dose-dependent manner with a maximal concentration at 15 μM (Figure 2B). Furthermore, we examined the effect of aloe-emodin on anchorage-independent growth of PC3 cells. Aloe-emodin-treated cells showed an impaired anchorage-independent growth capability, leading to a dose-dependent reduction of colony formation (Figure 2C). However, the effect was not due to toxicity (Supplementary Figure 1, available at Carcinogenesis Online).
Fig. 2.
Aloe-emodin suppresses proliferation and anchorage-independent growth of PC3 cells. (A) Chemical structure of aloe-emodin. (B) Aloe-emodin inhibits cell proliferation. PC3 cells (3 × 103 cells per well) were treated with the indicated dose of aloe-emodin and proliferation was measured at the indicated time point by MTS as described in Materials and methods. Data are shown as means ± SD and the asterisk indicates a significant (P < 0.05) decrease in proliferation compared with untreated control cells. (C) Aloe-emodin suppresses anchorage-independent growth of PC3 cells. Representative photographs are shown (top panels). Colonies were counted and data are shown as means ± SD from three independent experiments. The asterisk indicates a significant (P < 0.05) decrease in colony formation in cells treated with aloe-emodin compared with the dimethyl sulfoxide-treated group.
Aloe-emodin attentuates mTORC2-mediated downstream signaling
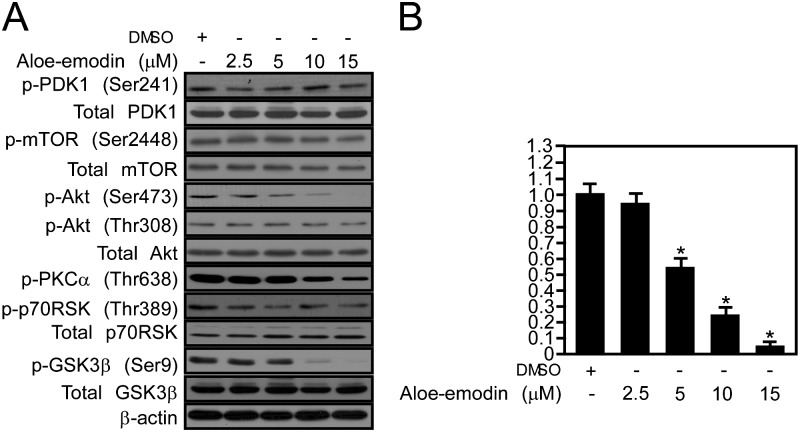
To identify the mechanism and molecular target of aloe-emodin, we treated PC3 cells with various amounts of aloe-emodin for 24 h. The data indicated that Akt phosphorylation at Ser473 was inhibited by aloe-emodin in a dose-dependent manner. The phosphorylation of GSK3β at Ser9, directly downstream of Akt, was also inhibited, but the phosphorylation level of p70S6k at Thr389 was not affected (Figure 3). In addition, aloe-emodin had no effect on the mitogen-activated protein kinase pathway, including phosphorylation of extracellular signal-regulated kinases and total protein level of p38, c-jun N-terminal kinases or extracellular signal-regulated kinases (Supplementary Fig ure 2, available at Carcinogenesis Online).
Fig. 3.
Aloe-emodin suppresses mTORC2-mediated downstream signaling. (A) PC3 cells were treated for 24 h with the indicated dose of aloe-emodin. The levels of phosphorylated and total proteins were visualized by Western blotting with specific primary and horseradish peroxidase-conjugated secondary antibodies. Each experiment was repeated three times. (B) The density of phosphorylated Akt at Ser473 and actin was measured using the ImageJ software program (v. 1.37V; NIH). The value of dimethyl sulfoxide-treated pAkt (Ser473) is marked as 1. The other values were obtained by comparison with the ‘dimethyl sulfoxide-treated control’.
Aloe-emodin binds with the mTOR complex2
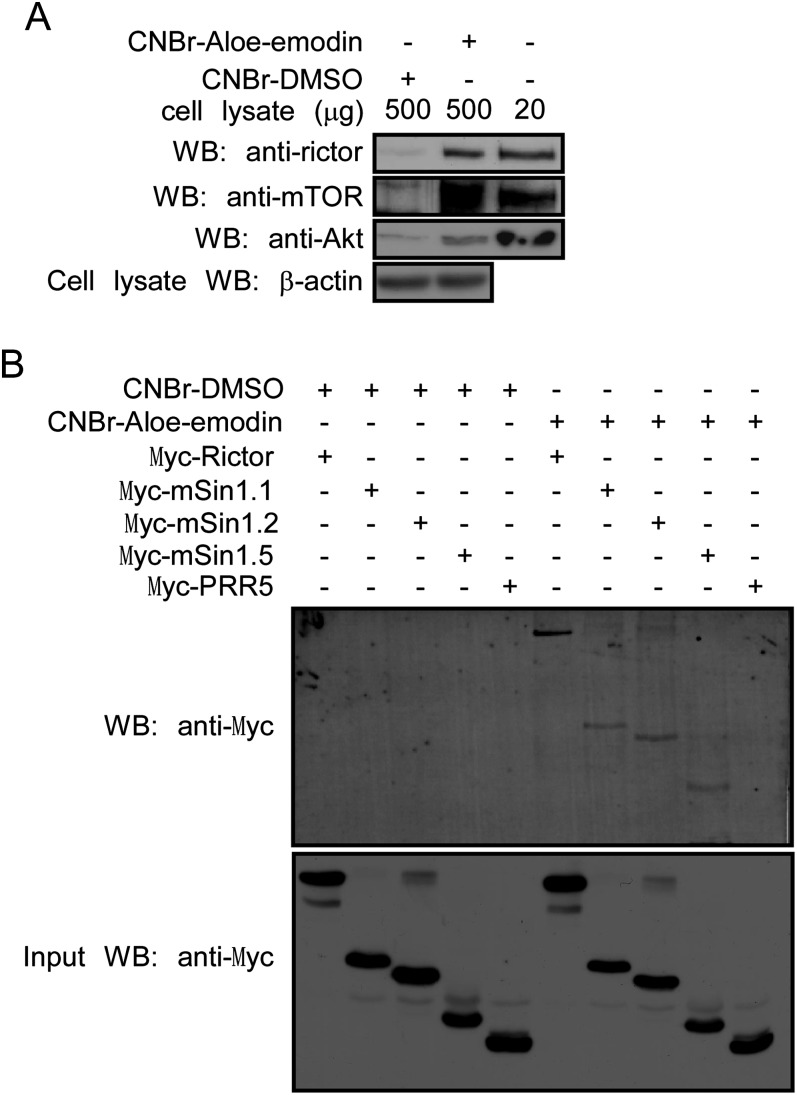
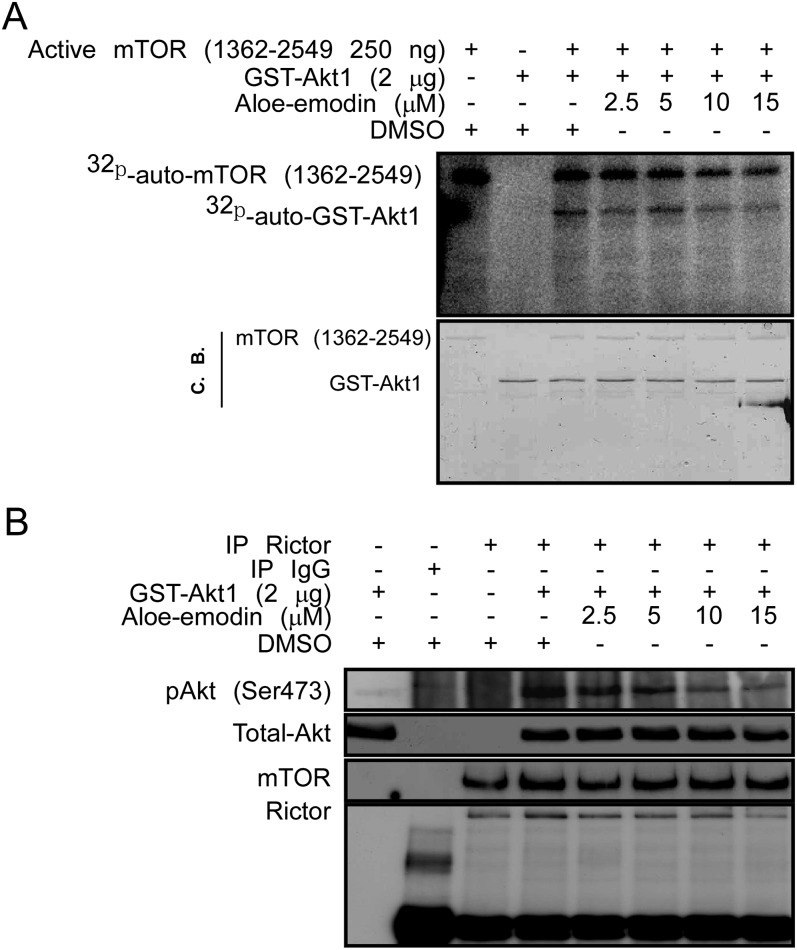
mTORC2 plays a pivotal role in PTEN loss and prostate cancer development and it phosphorylates Akt at Ser473 and PKCα at Thr638 (12,30). Based on our Western blotting data, we speculated that mTORC2 might be a molecular target of aloe-emodin. To test this idea, we performed an ex vivo pull-down assay using aloe-emodin-conjugated Sepharose 4B beads. Results revealed that aloe-emodin-conjugated beads, but not Sepharose beads alone, can pull down endogenous Rictor and mTOR together. Furthermore, the Akt protein can also be pulled down with weak binding compared with input cell lysate (Figure 4A).
Fig. 4.
Aloe-emodin binds with mTORC2. (A) Aloe-emodin binds with endogenous mTORC2 in cells. PC3 cell lysates (500 μg) were pulled down with CNBr or CNBr-aloe-emodin-conjugated beads. The pulled down proteins were visualized using antibodies to detect Rictor, mTOR and Akt. (B) Aloe-emodin binds with ectopically expressed Rictor or mSin1 isoforms in cells. Plasmids containing myc-Rictor, myc-mSin1.1, myc-mSin1.2, myc-mSin1.5 or myc-PRR5 were ectopically overexpressed in 293 cells. The overexpression protein level was visualized by Western blotting and showed that the same amount of overexpressed protein was pulled down with CNBr or CNBr-aloe-emodin beads. The pulled down proteins were visualized by a myc-specific primary and horseradish peroxidase-conjugated secondary antibodies.
To further identify the direct binding target of aloe-emodin with mTORC2, we constructed myc-tagged mammalian expression plasmids containing Rictor, mSin1.1, mSin1.2, mSin1.5 and PRR5. These plasmids were, respectively, transfected into HEK 293 cells and the overexpressed proteins were analyzed by Western blot. The same amounts of overexpressed proteins were used for an ex vivo binding assay with aloe-emodin-conjugated Sepharose 4B beads. Results indicated that aloe-emodin strongly pulled down the overexpressed Rictor and also weakly binds with mSin1 isoforms, but not with PRR5 (Figure 4B).
Aloe-emodin inhibits mTORC2 kinase activity in vitro
Based on our results showing that aloe-emodin can directly bind with mTORC2, we determined whether aloe-emodin can inhibit mTORC2 kinase activity in vitro. We first examined whether aloe-emodin can inhibit mTOR kinase by using glutathione-S-transferase-Akt1 as substrate and found that mTOR kinase activity could not be inhibited by aloe-emodin in vitro (Figure 5A). Next, we evaluated the effect of aloe-emodin on mTORC2 kinase activity by using glutathione-S-transferase-Akt1 as a substrate. Our results indicated that the phosphorylation of Akt1 at Ser473 was suppressed by aloe-emodin in a dose-dependent manner (Figure 5B).
Fig. 5.
Aloe-emodin inhibits mTORC2 kinase activity in vitro. (A) Aloe-emodin has no effect on mTOR protein abundance. The effect of aloe-emodin on mTOR kinase activity was measured by an in vitro kinase assay using [γ-32P] ATP and a glutathione-S-transferase-Akt1 protein as substrate. 32P-labeled glutathione-S-transferase-Akt1 was visualized by autoradiography as described in Materials and methods. (B) Aloe-emodin inhibits mTORC2 kinase activity. The effect of aloe-emodin on mTORC2 kinase activity was measured by an in vitro kinase assay using a glutathione-S-transferase-Akt1 protein. The phosphorylation level of Akt (Ser473) was visualized by western blotting.
PI3-K is another kinase strongly associated with Akt activation. To determine whether aloe-emodin affects PI3-K activity, we performed an in vitro kinase assay with PIP2 as substrate. The data suggested that the phosphorylation signal did not change with aloe-emodin treatment (Supplementary Figure 3, available at Carcinogenesis Online).
Aloe-emodin suppresses tumor growth by inhibiting mTOR complex2 activity
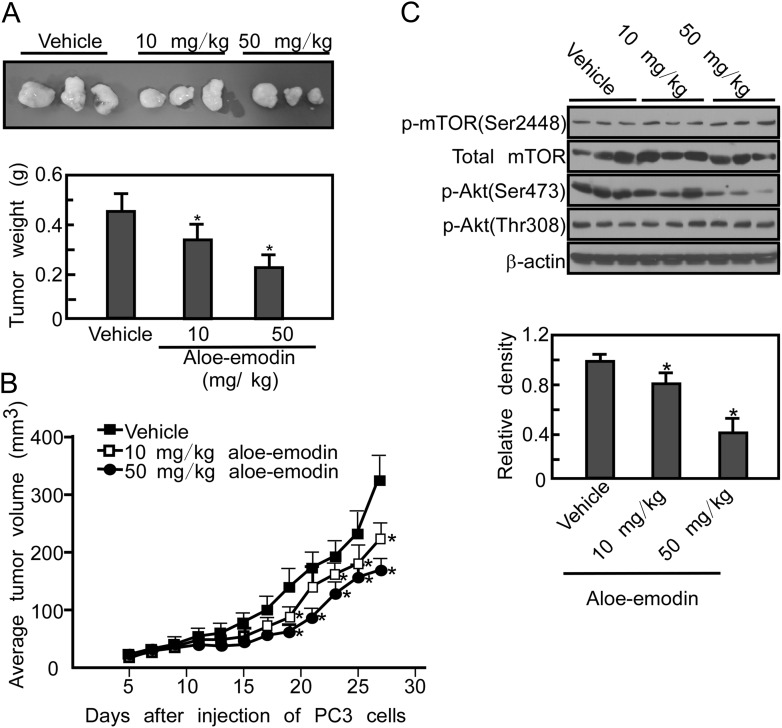
Based on our in vitro and ex vivo results, we next determined whether aloe-emodin could suppress tumor growth in vivo. The results show that the mean tumor weight was decreased in the aloe-emodin-treated group (Figure 6A, P < 0.05) and the mean tumor volume in the vehicle-treated group increased faster than that in the aloe-emodin-treated group (Figure 6B, P < 0.05). The body weights of vehicle-treated and aloe-emodin-treated mice (50 mg/kg) were not different (32.4 ± 0.70 g versus 31.8 ± 0.61 g, P ≥ 0.05). Tumor extracts from vehicle-treated and aloe-emodin-treated mice (i.e. euthanized on the same day of the experiment) were prepared and phosphorylation of Akt was analyzed. Western blotting analysis revealed that the aloe-emodin-treated tumor extracts exhibited substantially decreased Akt phosphorylation at Ser473 compared with vehicle-treated tumors (Figure 6C).
Fig. 6.
Aloe-emodin suppresses tumor growth by inhibiting mTORC2 activity. (A) The total average tumor weight in the aloe-emodin-treated group is significantly less (*P < 0.05) than that of the vehicle-treated group. Tumors were extracted and weighed after mice were killed. Data are shown as means ± SD and significant differences were determined by one-way analysis of variance. (B) Total average tumor volume in the aloe-emodin-treated group was significantly (*P < 0.05) less than that of the vehicle-treated group. Tumor volume was measured and recorded three times a week throughout the study. Data are shown as means ± SD. (C) Expression of total and phosphorylated mTOR and phosphorylated Akt was assessed by Western blot analysis in vehicle- and aloe-emodin-treated tumor tissues. The relative density was compared with actin as indicated. Data are shown as means ± SE.
Discussion
An accumulation of evidence reveals that PI3-K activation and PTEN loss play an important role in prostate tumorigenesis. Both these alterations strongly activate Akt, which plays a vital role in cell survival and cell growth. mTORC2 is known to be responsible for phosphorylation of Akt at Ser473 resulting in its full activation. Furthermore, mTORC2 is not required for survival of mouse embryonic fibroblasts and development of Drosophila embryos but is very important for transformed human prostate epithelial cells lacking PTEN to form tumors (12,23). Consistent with these findings, our observations showed that Akt is strongly activated in androgen-dependent cells (LNCaP) and androgen-refractory cells (PC3) (Figure 1A).
Rictor is a unique binding partner of mTORC2 and is known to play an important role in cell proliferation and tumor metastasis (31,32). Knocking down Rictor in PC3 cells inhibited proliferation and impaired anchorage-independent colony formation (Figure 1C). Phosphorylation of Akt at Ser473 was decreased, but not totally blocked, which is consistent with a recent observation that IkappaB kinase epsilon and TANK-binding kinase 1 (IKKepsilon/TBK1) also play a role in Akt phosphorylation (33). Taken together, these studies indicate that mTORC2 might be a promising target for both chemoprevention and chemotherapy of prostate cancer.
Aloe-emodin is a natural compound from aloe, which exhibits antitumor effects (34). Indeed, we found that aloe-emodin can inhibit PC3 cell proliferation and anchorage-independent growth (Figure 2A and B, Supplementary Figure 1, available at Carcinogenesis Online). Phosphorylation of Akt at Ser473 and PKCα at Thr638 was strongly inhibited by aloe-emodin treatment. The phosphorylation of p70S6K and mitogen-activated protein kinases was not affected (Figure 3 and Supplementary Figure 3, available at Carcinogenesis Online). These results suggest that aloe-emodin probably targets mTORC2. Results of an ex vivo pull-down assay with aloe-emodin-conjugated beads confirm our hypothesis and indicate that aloe-emodin can bind with mTORC2 (Figure 4A and B). Notably, aloe-emodin can inhibit mTORC2 kinase activity in vitro, but not PI3-K or mTOR kinase activities (Figure 5A and B, Supplementary Figure 2, available at Carcinogenesis Online).
Our results herein are noteworthy in that prostate cancer cell growth is suppressed by aloe-emodin in vivo. Moreover, the low in vivo toxicity and tumor inhibitory activity of aloe-emodin observed in nude mice suggest that aloe-emodin is an effective chemopreventive agent against prostate cancer (Figure 6A and B). In conclusion, we showed here that mTORC2 is closely associated with prostate cancer cell growth. We also provided clear evidence showing that aloe-emodin effectively suppresses anchorage-independent cell growth and in vivo tumor growth in PC3 cancer cell-bearing nude mice by inhibiting Akt activity. Collectively, these findings support the anticancer efficacy of aloe-emodin through its targeting of mTORC2 for the inhibition of prostate cancer progression.
Supplementary material
Supplementary Figures 1–3 can be found at http://carcin.oxfordjournals.org/
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2010-0029233). or Leap Research Program (No. 2010-0029233). -WCI: World Class Institute Program founded by the Korea Research Foundation, Ministry of Education, Science and Technology. or WCI 2009-002.
Funding
Hormel Foundation and National Institutes of Health (CA120388, CA077646, CA027502, R37CA081064 and ES016548).
Supplementary Material
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- mTOR
mammalian target of rapamycin
- PI3-K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homolog
- SDS
sodium dodecyl sulfate
References
- 1.Deocampo ND, et al. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 2003;28:145–153. [PubMed] [Google Scholar]
- 2.Gao N, et al. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem. Biophys. Res. Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 3.Agell L, et al. PI3K signaling pathway is activated by PIK3CA mRNA overexpression and copy gain in prostate tumors, but PIK3CA, BRAF, KRAS and AKT1 mutations are infrequent events. Mod. Pathol. 2011;24:443–452. doi: 10.1038/modpathol.2010.208. [DOI] [PubMed] [Google Scholar]
- 4.Sun X, et al. Genetic alterations in the PI3K pathway in prostate cancer. Anticancer Res. 2009;29:1739–1743. [PubMed] [Google Scholar]
- 5.Murillo H, et al. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 6.Sircar K, et al. PTEN genomic deletion is associated with p-Akt and AR signalling in poorer outcome, hormone refractory prostate cancer. J. Pathol. 2009;218:505–513. doi: 10.1002/path.2559. [DOI] [PubMed] [Google Scholar]
- 7.McCall P, et al. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br. J. Cancer. 2008;99:1296–1301. doi: 10.1038/sj.bjc.6604680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 9.de Souza PL, et al. Role of the Akt pathway in prostate cancer. Curr. Cancer Drug Targets. 2009;9:163–175. doi: 10.2174/156800909787581006. [DOI] [PubMed] [Google Scholar]
- 10.Zinzalla V, et al. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Sarbassov DD, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 12.Guertin DA, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell A, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J. Clin. Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun SY, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 16.Tabernero J, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J. Clin. Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 17.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Shaw RJ, et al. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 19.Rosen N, et al. AKT and cancer—is it all mTOR? Cancer Cell. 2006;10:254–256. doi: 10.1016/j.ccr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, et al. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 21.Harrington LS, et al. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem. Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Wan X, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 23.Hietakangas V, et al. Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 2007;21:632–637. doi: 10.1101/gad.416307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J, et al. Growth inhibitory effects of gastric cancer cells with an increase in S phase and alkaline phosphatase activity repression by aloe-emodin. Cancer Biol. Ther. 2007;6:85–88. doi: 10.4161/cbt.6.1.3553. [DOI] [PubMed] [Google Scholar]
- 25.Acevedo-Duncan M, et al. Aloe-emodin modulates PKC isozymes, inhibits proliferation, and induces apoptosis in U-373MG glioma cells. Int. Immunopharmacol. 2004;4:1775–1784. doi: 10.1016/j.intimp.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Lee DE, et al. 7,3',4'-Trihydroxyisoflavone inhibits epidermal growth factor-induced proliferation and transformation of JB6 P+ mouse epidermal cells by suppressing cyclin-dependent kinases and phosphatidylinositol 3-kinase. J. Biol. Chem. 2010;285:21458–21466. doi: 10.1074/jbc.M109.094797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colburn NH, et al. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc. Natl Acad. Sci. USA. 1981;78:6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo J, et al. Suppression of C-myc expression associates with anti-proliferation of aloe-emodin on gastric cancer cells. Cancer Invest. 2008;26:369–374. doi: 10.1080/07357900701788130. [DOI] [PubMed] [Google Scholar]
- 29.Kuo PL, et al. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci. 2002;71:1879–1892. doi: 10.1016/s0024-3205(02)01900-8. [DOI] [PubMed] [Google Scholar]
- 30.Guertin DA, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roulin D, et al. Targeting mTORC2 inhibits colon cancer cell proliferation in vitro and tumor formation in vivo . Mol. Cancer. 2010;9:57. doi: 10.1186/1476-4598-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F, et al. mTOR complex component Rictor interacts with PKCzeta and regulates cancer cell metastasis. Cancer Res. 2010;70:9360–9370. doi: 10.1158/0008-5472.CAN-10-0207. [DOI] [PubMed] [Google Scholar]
- 33.Xie X, et al. IkappaB kinase epsilon and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc. Natl Acad. Sci. USA. 2011;108:6474–6479. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pecere T, et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000;60:2800–2804. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.