Abstract
Nasopharyngeal carcinoma (NPC) has the highest metastatic potential among head and neck cancers. Distant metastasis is the major cause of treatment failure. The role of interleukin-8 (IL-8) in NPC progression remains unknown. Our multivariate survival analyses of 255 patients with NPC revealed that higher IL-8 expression in primary NPC tissue was an independent prognostic factor for overall survival, disease-free survival, and distant metastasis-free survival of the patients. In vitro study revealed that IL-8 was highly expressed in the established high-metastasis NPC clone S18 relative to the low-metastasis cells. Suppression of IL-8 by short-hairpin RNA reduced the expression of IL-8 in S18 cells and subsequently inhibited migration, invasion, and hepatic metastasis of the cells without influencing cellular growth. Overexpression of IL-8 in S26 cells resulted in increased migration, invasion, and metastasis capabilities of the cells without affecting cellular growth. Exogenous IL-8 enhanced the migration and invasion of low-metastasis CNE-2 cells in a dose-dependent manner. An epithelial–mesenchymal transition (EMT) could be induced by IL-8 in various NPC cell lines. The high level of phosphorylated AKT in S18 cells could be suppressed by knocking down IL-8 expression. Further, IL-8-promoted migration and invasion could be abolished by either the application of the phosphoinositide-3-kinase inhibitor LY294002 or the knock down of AKT expression by using small-interfering RNA. In summary, IL-8 serves as an independent prognostic indicator of overall survival, disease-free survival, and metastasis-free survival for patients with NPC. IL-8 promotes NPC metastasis via autocrine and paracrine means, involving activation of AKT signaling and inducing EMT in NPC cells.
Introduction
Nasopharyngeal carcinoma (NPC) has a high incidence rate in southern China and southeast Asia, especially in the descendants of the Bai Yue people (1, 2). Among head and neck cancers, NPC has the highest metastasis rate (3–5): at the time of diagnosis: 74.5% of patients present with regional lymph node metastasis and 19.9% present with distant metastasis (6, 7). Distant metastasis is therefore the major cause of treatment failure, although NPC is sensitive to radiotherapy. The molecular mechanisms regulating NPC metastasis are not fully understood. A well-established metastatic cellular model has been used to explore the cellular and molecular mechanisms underlying NPC metastasis (8–10). In this model, a high-metastasis cellular clone, S18, isolated from the NPC cell line CNE-2, was used for comparison with the low-metastasis clone S26, as well as with their low-metastasis parental cell line, CNE-2.
Interleukin 8 (IL-8; alternatively known as CXCL8) is a proinflammatory cysteine-X-cysteine (CXC) chemokine. The biological effects of IL-8 are mediated through binding to two cell-surface G-protein-coupled receptors called IL-8 receptor A (CXCR1) and IL-8 receptor B (CXCR2) (11). IL-8 was originally discovered as a leukocyte chemoattractant (12, 13). Studies have shown that IL-8 induces angiogenesis (14–16), and it promotes tumor growth and metastasis in melanoma (17–20), bladder cancer (21, 22), and ovarian cancer (23). Increased serum IL-8 level can even precede the diagnosis of lung cancer by several years (24).
Epstein–Barr virus infection has been closely linked to NPC (25–27). It has been observed that IL-8 expression in NPC cells can also be induced by Epstein–Barr virus proteins (28–30). However, it is undetermined whether high IL-8 expression level in NPC is an independent prognostic factor (31, 32). It is also not clear whether IL-8 can promote the progression of NPC. The goal of this study was to investigate the prognostic value of IL-8 in NPC, as well as the role of IL-8 in promoting NPC metastasis, hoping to reveal an effective target for prevention of NPC progression.
The Akt family of serine–threonine kinases consists of three members: Akt 1/PKBα, Akt 2/PKBβ, and Akt 3/PKBγ. Two specific amino acid residues, threonine 308 and serine 473, located in the kinase domain and C-terminal hydrophobic domain, respectively, can be phosphorylated upon full activation of AKT (33). It has been reported that irradiation of NPC cells can activate AKT (34). Activation of AKT by IL-8 signaling has been shown in prostate cancer cell lines (35, 36). It is unknown whether IL-8 can also induce AKT activation and further promote metastasis in NPC.
Materials and methods
Human tumor tissues and tissue microarray
Formalin-fixed and paraffin-embedded NPC tissues obtained before treatment were retrieved from the Department of Pathology, Sun Yat-sen University Cancer Center (SYSUCC), with prior written consent from the patients and the approval of the Institutional Clinical Ethics Review Board at SYSUCC. The tissue microarrays contained qualified primary NPC samples from 255 patients diagnosed at SYSUCC between 2 January 1998 and 5 December 2002. The patients’ median age was 46 years (range 17–77), and 75.3% were males. All patients underwent radiotherapy, with doses of 70–74 Gy to the primary NPC, 60–64 Gy to the involved neck areas, and 50 Gy to the uninvolved neck. Platinum-based chemotherapy was given to the patients with late-stage disease (stages III and IV) as induction chemotherapy (2–3 cycles) or concurrent chemoradiotherapy. Tissue microarray analyses were conducted according to the published methods (1). Tissue spots with less than 5% tumor tissue in the whole spot area were excluded.
Antibodies
Rabbit anti-IL-8 antibody was purchased from Abnova. Mouse anti-phospho-AKT (Ser473), rabbit anti-AKT (pan), and rabbit anti-vimentin antibody were from Cell Signaling Technology. Mouse anti-β-actin antibody was from Sigma (MO, USA); mouse anti-E-cadherin and rabbit anti-fibronectin antibodies from Abcam; mouse anti-N-cadherin antibody from BD Biosciences; and anti-mouse and anti-rabbit peroxidase-conjugated secondary antibodies from Promega.
Immunohistochemistry and histological evaluation
Immunohistochemical staining of IL-8 was performed as described previously (1). Briefly, paraffin-embedded tissues were sectioned at 5 µm and antigen-retrieved in citrate buffer (pH 6.0) at 100°C for 20min, followed by sequential incubations with polyclonal rabbit anti-IL-8 antibody (Abnova, Taiwan) at 1:200 dilution, secondary antibody, and finally chromogenic substrate (DAKO, Denmark). The immunoreactions were evaluated independently by two pathologists. Cytoplasmic and membranous staining intensity were categorized: absent staining as 0, weak as 1, moderate as 2, and strong as 3. The percentage of stained cells was categorized as no staining = 0, 1–10% of stained cells = 1, 11–50% = 2, 51–80% = 3, and 81–100% = 4. The staining score for each tissue was calculated by multiplying the staining value by the percentage category value, and the average of the scores from the two referees was used as the final score. For histological evaluation, mouse liver metastatic nodules were resected and fixed in 4% paraformaldehyde, followed by routine histology evaluation.
Cell culture and cellular growth curve
The human NPC cell lines CNE-2 and the CNE-2 subclones S18 and S26 as well as the low-metastasis line HONE-1, were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum at 37°C and 5% CO2. To plot the cellular growth curve, 5×104 cells suspended in 2ml of medium were seeded into a 6-well plate and cultured under normal conditions. At various time points after seeding, the cells in each well were trypsinized and counted.
Animals and liver metastasis assay
All animal experiments were approved by the Sun Yat-sen University Cancer Center Institutional Animal Care and Usage Committee. Mice used in this study were housed under standard conditions and cared for according to the institutional guidelines.
Liver metastatic capacity was determined by injecting cells into the spleen of 5- to 6-week-old male nude mice obtained from the Shanghai Institutes for Biological Sciences (Shanghai, China). Briefly, mice were anesthetized by continuous inhalation of isoflurane, and 2×105 NPC cells in 30 μl of 33% Matrigel (Becton Dickinson, NJ, USA) were injected into the spleens of laparotomized mice using insulin syringes (Becton Dickinson). At 28 days after tumor cell inoculation, the experiment was terminated. The liver and spleen of each mouse were weighed and the metastatic nodules in each liver were counted.
Lentivirus transduction studies
Lentiviral production and transductions were performed as described previously (1). Briefly, for IL-8 knockdown studies, cell lines stably expressing IL-8 short-hairpin RNA (shRNA) or a scrambled non-target shRNA were established. The targets for human IL-8 shRNA were, for IL-8 KD 1, 5′-CAAGGAGTGCTAAAGAACTTA-3′, and for IL-8 KD 2, 5′-GCAGAGGGTTGTGGAGAAGTT-3′. The shRNA targeting the 5′-GTCTCCACGCGCAGTACATTT-3′ sequence served as the negative control (scrambled non-target shRNA). Quantitative PCR (qPCR) and enzyme-linked immunosorbent assay (ELISA) were performed to determine the knockdown efficiency of IL-8. For IL-8 overexpression studies, the coding sequence of IL-8 was subcloned into the pENTR⁄D-TOPO plasmid followed by recombination with the pLenti6/V5-DEST vector according to the manufacturer’s instructions (Invitrogen, CA, USA). The primers used for PCR amplification of the IL-8 coding sequence were forward, 5′-CACCATGACTTCCAAGCTGGC-3′, and reverse, 5′-TTATGAATTCTCAGCCCTCTTC-3′. Lentiviral particles were packaged and used for cell transductions as described above, and ELISA was carried out to determine the efficiency of overexpression.
Immunoblotting
As described previously (9), cells were lysed in RIPA buffer. An equal amount of protein in each sample was mixed with Laemmli buffer and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis separation, followed by blotting onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA) after measurement of protein concentration. Membranes were incubated with primary antibodies at 4°C. Protein bands were detected using a chemiluminescence kit (Pierce, IL, USA).
ELISA
We plated 2×106 cells in 100-mm culture plates and incubated for 48h in a regular medium. The medium was then replaced with a serum-free medium (10ml) and the cells were incubated for an additional 12h. Conditioned medium was collected and subjected to centrifugation, followed by filtration at 0.45 µm to remove the debris. Secreted human IL-8 concentration in the conditioned medium was measured using a sandwich ELISA kit (R&D, MN, USA). Experiments were performed in triplicate.
Real-time qPCR and semi-quantitative reverse transcription-PCR
As described previously (9), total RNA was extracted from cells followed by reverse transcriptions. Fast SYBR Green Master Mix was used to determine the threshold cycle (Ct) value of each sample in the CFX96 real-time PCR detection system (Bio-Rad, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the normalization gene in these studies. The relative expression levels of the target genes were given by 2ΔCt (Ct of GAPDH minus the Ct of the target gene). The sequences of the PCR primers used for amplifications of GAPDH, IL8, CXCR1, and CXCR2 were as follows:
GAPDH forward: 5′-AAGGTCATCCCTGAGCTGAA-3′;
GAPDH reverse: 5′-TGACAAAGTGGTCGTTGAGG-3′;
IL8 forward: 5′-CTCCAAACCTTTCCACCCC-3′;
IL8 reverse: 5′-GATTCTTGGATACCACAGAGAATG-3′;
CXCR1 forward: 5′-CGTCTGTCAATGTCTCTTCCAACC-3′;
CXCR1 reverse: 5′-GATAGTGCCTGTCCAGAGCCAG-3′;
CXCR2 forward: 5′-CTTCACAGTCACATTCCAAGCCTC-3′;
CXCR2 reverse: 5′-GCACCAGGGCAAGCTTTCTAAAC-3′.
PCR amplifications of GAPDH, CXCR1, and CXCR2 were performed under the following conditions: denature at 95°C for 30 s, anneal at 58°C for 30 s, and extend at 72°C for 30 s; reactions were carried out for 30 cycles. The experiments were performed in triplicate.
Migration and invasion assays
Migration and invasion assays were performed as described previously (9, 10). Briefly, cells were plated into cell culture inserts with 8-µm microporous filters (Becton Dickinson) coated with (invasion) or without (migration) Matrigel and incubated for 24h. Cells in the upper filters (inside the inserts) were removed, and the migrated or invaded cells in the lower filters (outside the inserts) were fixed in methanol, stained with crystal violet, and counted under a microscope. The number of migrated or invaded cells in five random optical fields (100× magnification) of each filter from triplicate inserts was averaged. For S18 cells, 2×104 cells were plated into each insert. For S26, HONE-1, and CNE-2 cells, 5×104 cells were plated. For recombinant IL-8 (R&D) stimulation assays, recombinant IL-8 was added to the lower chambers as an attractant. To test the effect of AKT dephosphorylation on the migratory and invasive abilities of cells, cancer cells were pretreated with 20 µM LY294002 (Sigma) for 30min before performing the migration and invasion assays. Experiments were performed in triplicate.
siRNA transfection
Small-interfering RNA (siRNA) targeting AKT and a scrambled siRNA were purchased from Cell Signaling Technology (Cat No.: #6510). The target of AKT siRNA is 5′-GCACCTTCATTGGCTACAA-3′, and the siRNA targeting the sequence 5′-CGTACGCGGAATACTTCGA-3′ served as the negative control (scrambled non-target siRNA). For siRNA transfections, 2×105 cells suspended in 2ml of medium were seeded into a 6-well plate. After incubation for 20h, cells were transfected with 50nM siRNAs by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Gene silencing efficiency was measured using immunoblotting at the indicated time points after transfection. For S26 and CNE-2 cells, migration and invasion assays were performed 18h after siRNA transfection.
Statistics
Student’s t-test was used to compare two independent groups of data. The median immunohistochemical staining score was used as the cutoff value to divide the patients into low and high IL-8 expression groups. Chi-square tests were applied to analyze the relationship between IL-8 expression and clinicopathological status. Kaplan–Meier survivals were plotted and log-rank tests were performed. The significance of several variables for survival was analyzed using the Cox regression model in a multivariate analysis. A P value < 0.05 was considered statistically significant in all cases.
Results
IL-8 is an independent prognostic factor for patients with NPC
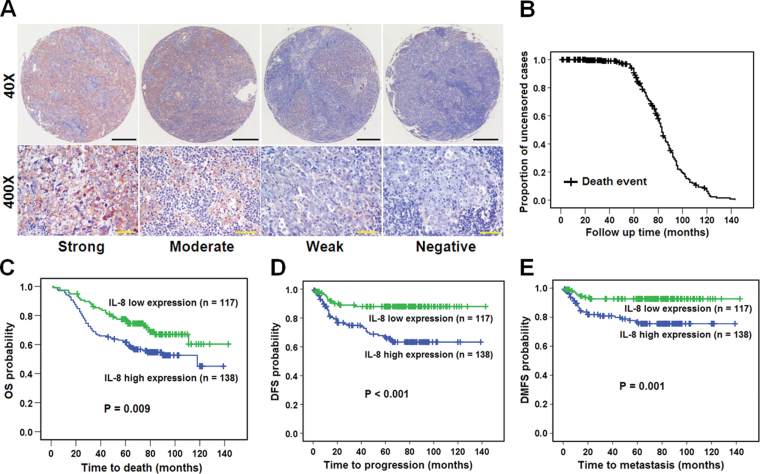
The clinicopathological characteristics of the 255 patients with NPC are shown in Supplementary Table 1 is available at Carcinogenesis Online. Survival analyses showed that a higher level of IL-8 expression significantly correlated with shorter overall survival, disease-free survival, and distant-metastasis-free survival in patients with NPC (Supplementary Table 2 and Figure 1 is available at Carcinogenesis Online). Multivariate analyses further showed that a high IL-8 level in the primary NPC was an independent prognostic factor for overall survival, disease-free survival, and distant metastasis-free survival of patients with NPC (Table I). All of these findings suggest that IL-8 may have an important role in promoting more aggressive behavior of NPC cells.
IL-8 is highly expressed in high-metastasis NPC cells
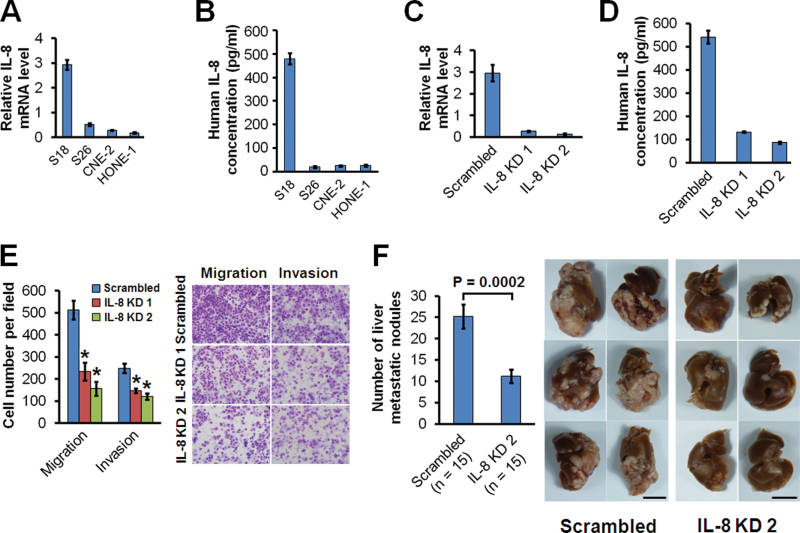
In order to investigate the role of IL-8 in NPC progression, we used the S18 clone, which has a high metastasis capability as evaluated by the lymph node metastasis model (9, 10). The mRNA level for IL-8 was found to be increased in S18 cells in comparison to that in low-metastasis cell lines (Figure 2A), although all of the lines expressed the IL-8 receptors CXCR1 and CXCR2 (Supplementary Figure 1 is available at Carcinogenesis Online). The secreted IL-8 protein level was also upregulated in S18 cells in comparison to that of low-metastasis cell lines (Figure 2B). To better explore the role of IL-8 in regulating NPC metastasis, we used a hepatic metastasis model in nude mice created by intrasplenic injection of cancer cells to generate a primary tumor and subsequent hepatic metastases. S18 cells had an increased liver metastasis capability relative to low-metastasis S26 cells (Supplementary Figure 2A and B is available at Carcinogenesis Online), which was parallel to the increase in IL-8 expression.
Fig. 2.
IL-8 expression level correlates with the in vivo metastasis ability of NPC cells. (A) Relative mRNA levels of IL-8 in NPC cells using quantitative PCR. (B) Concentration of secreted IL-8 in conditioned medium as measured by ELISA. (C) mRNA levels of IL-8 in S18 cells expressing IL-8 shRNAs (IL-8 KD1 and KD2) or scrambled shRNA as measured using quantitative PCR. (D) Concentration of secreted IL-8 in conditioned medium of S18 cells expressing IL-8 shRNAs or scrambled shRNA as measured by ELISA. (E) Migratory and invasive abilities of S18 cells expressing IL-8 shRNAs or scrambled shRNA evaluated by Transwell assay. *P < 0.001 relative to scrambled controls (Student’s t-test). Photomicrographs are at 100× (right panel). Bars correspond to mean ± standard deviation (SD) of three independent experiments. (F) In vivo liver metastasis ability of S18 cells expressing IL-8 shRNA or scrambled shRNA evaluated in nude mice. Bars correspond to mean ± standard error (SE), with P value calculated using Student’s t-test (left panel). The six livers having the largest number of metastatic nodules from each group are shown in the right panel. Scale bars, 1cm.
Fig. 1.
High IL-8 expression correlates with shorter overall survival, disease-free survival, and distant-metastasis-free survival in patients with NPC. (A) Tissue microarrays representative of those from 255 patients with NPC diagnosed at the M0 stage immunohistochemically stained using antibody against IL-8, at low (40×) and high (400×) magnification in a light microscope. Black scale bars0 500 µm; yellow scale bars, 100 µm. (B) The median follow-up time of these 255 patients was 69 months. (C) Overall survival (OS) curve. (D) Disease-free survival (DFS) curve. (E) Distant-metastasis-free survival (DMFS) curve. P values were calculated by the log-rank test.
Suppression of IL-8 inhibits migration, invasion, and metastasis of NPC cells
To clarify whether IL-8 has a causal relationship with the metastatic capability of NPC cells, we stably suppressed IL-8 expression in S18 cells using shRNAs. Suppression of IL-8 mRNA (Figure 2C) dramatically reduced the IL-8 protein secreted into the culture medium (Figure 2D) and subsequently inhibited the migration and invasion of S18 cells in vitro (Figure 2E). In vitro cellular growth of S18 was not influenced by knocking down IL-8 (Supplementary Figure 3A is available at Carcinogenesis Online). To further confirm that IL-8 was critical for in vivo regulation of NPC metastasis, we used the animal liver metastasis model. Figure 2F shows that liver metastasis was dramatically inhibited by suppressing IL-8 expression.
Overexpression of IL-8 promotes migration, invasion, and metastasis of NPC cells
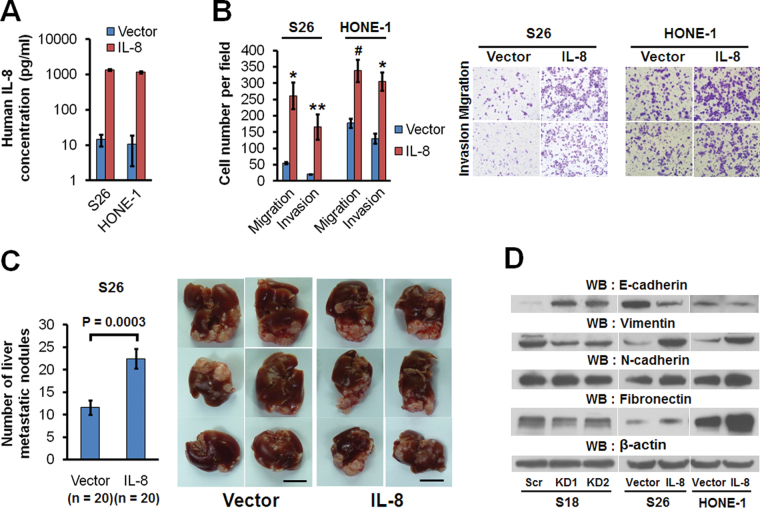
Stable overexpression of IL-8 in low-metastasis S26 and HONE-1 cells resulted in a high level of IL-8 secreted into the culture medium (Figure 3A), as well as enhanced migration and invasion (Figure 3B), but cellular growth was not influenced (Supplementary Figure 3B and C is available at Carcinogenesis Online). More importantly, animal experiments showed that overexpression of IL-8 dramatically enhanced NPC metastasis in vivo (Figure 3C). These results showed that IL-8 can promote NPC migration, invasion, and metastasis via an autocrine secretion mechanism.
Fig. 3.
Overexpression of IL-8 promotes migration, invasion, metastasis, and EMT in NPC cells. (A) The concentration of secreted IL-8 in conditioned medium of S26 and HONE-1 cells expressing IL-8 or empty vector. (B) Migratory and invasive abilities of S26 and HONE-1 cells expressing IL-8 or empty vector as evaluated by Transwell assay. *P < 0.001, **P = 0.0027, # P = 0.0016 relative to that of vector as controls (Student’s t-test). Photomicrographs are at 100× (right panel). Bars correspond to mean ± SD of three independent experiments. (C) In vivo liver metastasis ability of S26 cells expressing IL-8 or empty vector as evaluated in nude mice. Bars correspond to mean ± SE, with P value calculated using Student’s t-test (left panel). The six livers having the largest number of metastatic nodules from each group are shown (right panel). Scale bars, 1cm. (D) Immunoblots were performed with the indicated antibodies in whole-cell lysates.
IL-8 induces epithelial–mesenchymal transition in NPC cells
The epithelial–mesenchymal transition (EMT) is a crucial step for cancer cells in gaining metastasis capability (37). As shown in Figure 3D, knocking down IL-8 in high-metastasis S18 cells resulted in upregulation of the epithelial marker E-cadherin and downregulation of the mesenchymal markers vimentin and fibronectin. On another hand, overexpression of IL-8 in the low-metastasis S26 cells and HONE-1 cells downregulated the epithelial marker E-cadherin and upregulated the mesenchymal markers vimentin, N-cadherin, and fibronectin.
AKT signaling is essential for IL-8 promotion of cellular motility in NPC cells
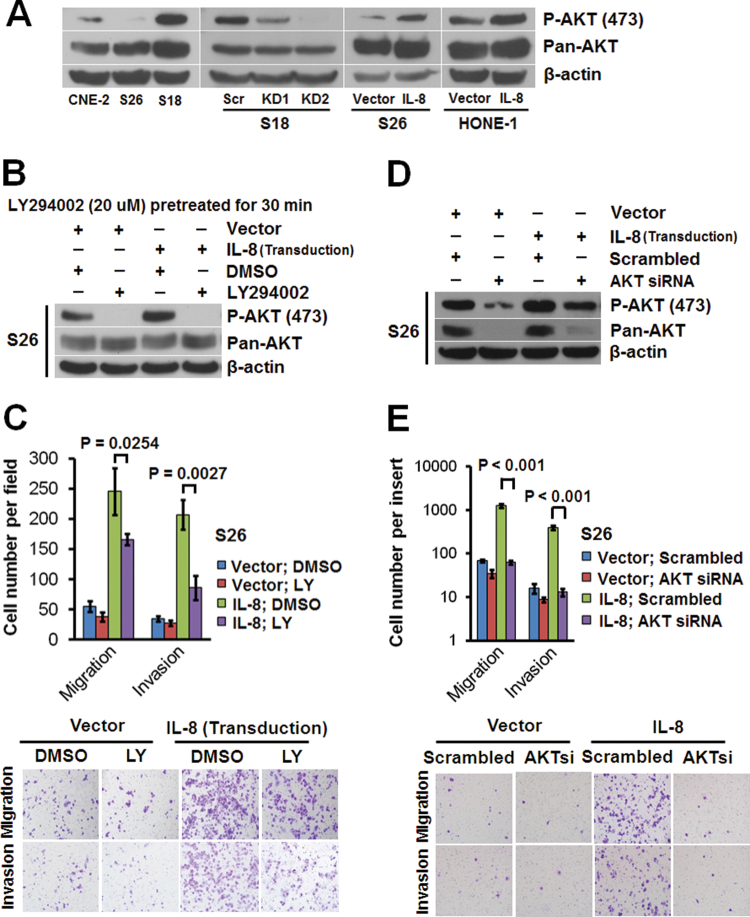
High levels of total AKT and phosphorylated AKT were found in S18 cells. Knocking down IL-8 in S18 cells could dramatically reduce phospho-AKT level and also slightly affect total AKT level (Figure 4A). Overexpression of IL-8 in S26 and HONE-1 cells upregulated phospho-AKT (Figure 4A); this effect could be abolished by the phosphoinositide-3-kinase inhibitor LY294002 (Figure 4B). The total AKT levels were also slightly increased by IL-8 overexpression in the low-metastasis S26 and HONE-1 cells (Figure 4A). The enhanced migration and invasion in S26 cells caused by overexpressing IL-8 was suppressed by LY294002 (Figure 4C), but it could also be abolished by knocking down AKT using siRNA (Figure 4D,4E). All of these findings confirmed that the IL-8 promotion of cellular motility in NPC cells depends on AKT signaling.
Fig. 4.
IL-8 promotes NPC cell migration and invasion through AKT activation. (A) Immunoblots of whole-cell lysates using anti-phospho-AKT (Ser473) or anti-AKT (pan) antibody. (B) Immunoblots of whole-cell lysates from S26 cells expressing IL-8 or the empty vector after pretreatment with 20 μM LY294002 for 30min, using anti-phospho-AKT (Ser473) or anti-AKT (pan) antibody. (C) Migratory and invasive abilities of S26 cells expressing IL-8 or the empty vector evaluated by Transwell assay after pretreatment with LY294002. (D) Immunoblots of whole-cell lysates from S26 cells expressing IL-8 or the empty vector 36h after transfection with AKT siRNA or a scrambled siRNA, using anti-phospho-AKT (Ser473) or anti-AKT (pan) antibody. (E) Migratory and invasive abilities of S26 cells expressing IL-8 or the empty vector as evaluated by Transwell assay after transfection with AKT siRNA or the scrambled siRNA. Photomicrographs are at 100×. Bars correspond to mean ± SD of three independent experiments. β-actin served as the loading control.
Paracrine IL-8 stimulation can activate AKT signaling and promote migration and invasion of NPC cells
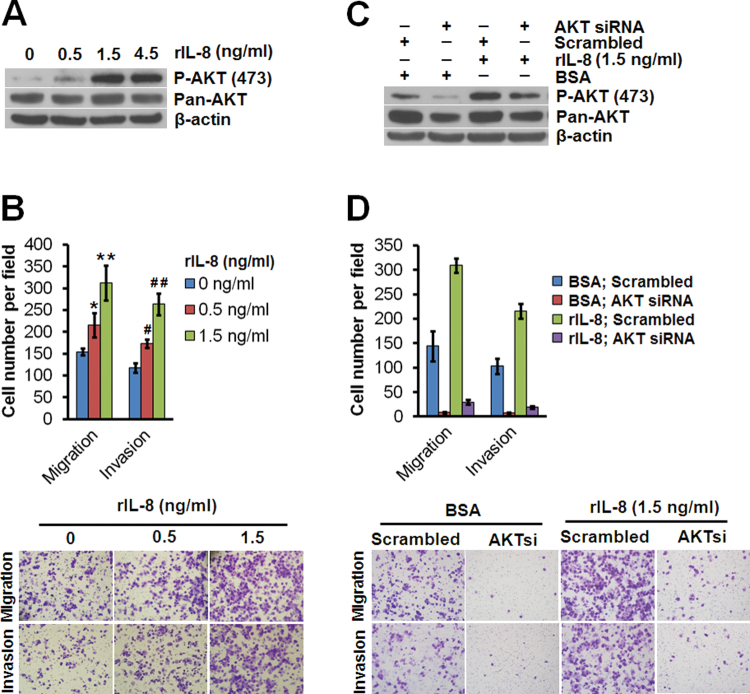
Exogenous stimulation by use of recombinant IL-8 induced AKT phosphorylation in a dose-dependent manner in low-metastasis CNE-2 cells (Figure 5A). The enhancement of migration and invasion of CNE-2 cells by exogenous IL-8 was dose-dependent (Figure 5B). These findings confirmed that a paracrine IL-8 stimulation could also trigger cellular motility in NPC cells. Consistent with the findings in IL-8 autocrine signaling, these biological effects could be abolished by knocking down AKT using siRNA (Figure 5C,5D), suggesting that both the autocrine and paracrine routes of IL-8 signaling similarly activated the AKT pathway.
Fig. 5.
Recombinant IL-8 promotes migration and invasion through activation of AKT in NPC cells. (A) Immunoblots of whole-cell lysates from CNE-2 cells stimulated with recombinant IL-8 (rIL-8) for 30min, using anti-phospho-AKT (Ser473) or anti-AKT (pan) antibody. (B) Migratory and invasive abilities of CNE-2 cells stimulated with various concentrations of rIL-8 as evaluated by Transwell assay. *P = 0.02132, **P = 0.0025, # P = 0.00267, ## P < 0.001, relative to cells without rIL-8 stimulation (Student’s t-test). (C) Immunoblots of whole-cell lysates from CNE-2 cells transfected with AKT siRNA or scrambled siRNA and stimulated with rIL-8 (1.5ng/ml) for 30min at 36h after siRNA transfection, using anti-phospho-AKT (Ser473) or anti-AKT (pan) antibody. (D) Migratory and invasive abilities of CNE-2 cells transfected with AKT siRNA or scrambled siRNA and stimulated with rIL-8 as evaluated by Transwell assay. The photomicrographs are at 100×. Bars correspond to mean ± SD of three independent experiments. β-Actin served as the loading control.
Discussion
Metastasis is the main obstacle in the current clinical management of NPC. Preventing, predicting, and inhibiting NPC metastasis is therefore critical for further improving the survival rate of patients with NPC. In the present study, IL-8 was found to be a strong metastasis promoter for NPC. IL-8 has been proposed to be a potential drug target for several inflammatory diseases (38, 39), as well as for inhibiting tumor angiogenesis (40). Based on the findings in the present study, we believe that IL-8 can also be a potential drug target for preventing and inhibiting NPC metastasis.
IL-8 has been found to have prognostic value in other malignancies, including lung, prostate, gastric, and bladder cancer, and in non-Hodgkin's lymphoma (24, 35, 36, 41–43) However, Liao and colleagues failed to identify IL-8 as an independent prognostic factor for patients with NPC (31). This may be due to a relatively small patient cohort (N = 141) and follow-up quality: only 59.6% (84/141) of the patients had follow-up information, with a mean follow-up time of only 29.2 months). In the present study, we used a cohort of 255 patients; 100% of the patients had follow-up data, and the median follow-up time was 69 months (mean, 65.1 months). Our results show that IL-8 indeed was an independent prognostic factor for OR, DFS, and DMFS of patients with NPC.
The significant prognostic value of IL-8 could be explained by its multifunctional characteristics (11, 44). IL-8 can induce migration of endothelial cells through the activation of the phosphoinositide 3-kinase-Rac1/RhoA pathway (45). In prostate cancer, IL-8 activates the AKT pathway and promotes translational regulation of cyclin D (35). In the present study, NPC cellular motility was induced under IL-8 stimulation, with no alteration in cellular proliferation being induced by either knockdown or overexpression of IL-8 in several NPC cell lines cultured in 10% serum. This suggests that under normal culture conditions, IL-8 mainly promotes the motility of NPC cells without influencing proliferation. Our findings were very different from the observation in melanoma, in which IL-8 can promote cancer cell proliferation (46). The phenotypic disparity induced by IL-8 in different cancer types could be related to more complex signaling mechanisms, including the involvement of Epstein–Barr virus proteins in NPC progression, which can also induce IL-8 expression (28–30).
IL-8 can induce an EMT of normal epithelial cells (47). Our study showed for the first time that IL-8 induces EMT in cancer cells when it promotes NPC metastasis, and these important functions can be achieved by autocrine as well as paracrine signaling of IL-8. The finding of paracine signaling strengthens the hypothesis that detecting circulating IL-8 in patient serum might be useful for prognosis prediction in patients with NPC. Our results also indicated that increased cellular motility caused by IL-8 stimulation can be eliminated by blocking AKT signaling, confirming that AKT is a key signaling pathway by which IL-8 regulates the migratory and invasive abilities of NPC cells. The fact that irradiation can activate AKT (34) further validates our suggestion that blocking the AKT signaling pathway in addition to chemoradiotherapy is a promising therapeutic strategy for reducing the risk of distant metastases in patients with late-stage NPC.
In conclusion, IL-8 is an independent prognostic factor for overall survival, disease-free survival, and distant-metastasis-free survival of patients with NPC. IL-8 is highly expressed in high-metastasis NPC cells and is able to promote NPC cell migration, invasion, and metastasis via the activation of AKT signaling and the induction of EMT. The IL-8 expression level in the primary tumor can therefore be used for prognosis prediction in patients with NPC. Targeting IL-8 signaling is a promising approach for prevention and treatment of NPC metastasis.
Supplementary material
Supplementary Tables 1 and 2 and Figures 1–3 can be found at http://carcin.oxfordjournals.org/
Supplementary Material
Funding
This work was supported by grants from the State Key Program of the National Natural Science Foundation of China (grant no. 81030043); the National High Technology Research and Development Program of China (863 Program; grant no. 20060102A4002); the Major State Basic Research Program (973 Project) of China (grant no. 2006CB910104); the Van Andel Foundation; the National Cancer Center Research Found of Singapore; and the Singapore Millennium Foundation.
Acknowledgement
We thank David Nadziejka, Grand Rapids, Michigan, for critical reading of the manuscript.
References
- 1.Cao, S.M.,, et al. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin. J. Cancer, (2011);30,:114–119. doi: 10.5732/cjc.010.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wee, J.T.,, et al. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin. J. Cancer, (2010);29,:517–526. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad, A.,, et al. Distant metastases of nasopharyngeal carcinoma: a study of 256 male patients. J. Surg. Oncol., (1986);33,:194–197. doi: 10.1002/jso.2930330310. [DOI] [PubMed] [Google Scholar]
- 4.Geara, F.B.,, et al. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol, (1997);43,:53–61. doi: 10.1016/s0167-8140(97)01914-2. [DOI] [PubMed] [Google Scholar]
- 5.Lee, A.W.,, et al. Retrospective analysis of patients with nasopharyngeal carcinoma treated during 1976–1985: survival after local recurrence. Int. J. Radiat. Oncol. Biol. Phys., (1993);26,:773–782. doi: 10.1016/0360-3016(93)90491-d. [DOI] [PubMed] [Google Scholar]
- 6.Huang C.J.,, et al. Patterns of distant metastases in nasopharyngeal carcinoma. Kaohsiung J. Med. Sci., (1996);12,:229–234. [PubMed] [Google Scholar]
- 7.Wei, W.I.,, et al. The management of neck metastases in nasopharyngeal cancer. Curr. Opin. Otolaryngol. Head Neck Surg., (2007);15,:99–102. doi: 10.1097/MOO.0b013e3280148a06. [DOI] [PubMed] [Google Scholar]
- 8.Li, G.P.,, et al. Proteomic profiling between CNE-2 and its strongly metastatic subclone S-18 and functional characterization of HSP27 in metastasis of nasopharyngeal carcinoma. Proteomics, (2011);11,:2911–2920. doi: 10.1002/pmic.201000483. [DOI] [PubMed] [Google Scholar]
- 9.Li X.J.,, et al. Serglycin is a theranostic target in nasopharyngeal carcinoma that promotes metastasis. Cancer Res., (2011);71,:3162–3172. doi: 10.1158/0008-5472.CAN-10-3557. [DOI] [PubMed] [Google Scholar]
- 10.Qian, C.N.,, et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res, (2006);66,:10365–10376. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 11.Waugh, D.J.,, et al. The interleukin-8 pathway in cancer. Clin. Cancer Res., (2008);14,:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 12.Matsushima, K.,, et al. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J. Exp. Med., (1988);167,:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushima, K.,, et al. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem. Immunol., (1992);51,:236–265. [PubMed] [Google Scholar]
- 14.Huang, D.,, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res., (2010);70,:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, S.,, et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am. J. Pathol., (2002);161,:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vegran, F.,, et al. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res., (2011);71,:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Eli, M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology, (1999);67,:12–18. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- 18.Rofstad, E.K.,, et al. Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res., (2000);60,:4932–4938. [PubMed] [Google Scholar]
- 19.Singh, R.K.,, et al. Ultraviolet B irradiation promotes tumorigenic and metastatic properties in primary cutaneous melanoma via induction of interleukin 8. Cancer Res., (1995);55,:3669–3674. [PubMed] [Google Scholar]
- 20.Luca, M.,, et al. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am. J. Pathol., (1997);151,:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue, K.,, et al. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res., (2000);60,:2290–2299. [PubMed] [Google Scholar]
- 22.Karashima, T.,, et al. Nuclear factor-kappaB mediates angiogenesis and metastasis of human bladder cancer through the regulation of interleukin-8. Clin. Cancer Res., (2003);9,:2786–2797. [PubMed] [Google Scholar]
- 23.Shahzad, M.M.,, et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J. Biol. Chem., (2010);285,:35462–35470. doi: 10.1074/jbc.M110.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pine, S.R.,, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J. Natl. Cancer Inst., (2011);103,:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong, J.Q.,, et al. Establishment and characterization of a novel nasopharyngeal carcinoma cell line (SUNE2) from a Cantonese patient. Chin. J. Cancer, (2012);31,:36–44. doi: 10.5732/cjc.011.10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji, M.F.,, et al. Detection of Stage I nasopharyngeal carcinoma by serologic screening and clinical examination. Chin. J. Cancer, (2011);30,:120–123. doi: 10.5732/cjc.010.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, F.Y.,, et al. Detecting plasma Epstein-Barr virus DNA to diagnose postradiation nasopharyngeal skull base lesions in nasopharyngeal carcinoma patients: a prospective study. Chin. J. Cancer, (2012);31,:142–149. doi: 10.5732/cjc.011.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu, M.,, et al. Epstein-Barr virus lytic transactivator Zta enhances chemotactic activity through induction of interleukin-8 in nasopharyngeal carcinoma cells. J. Virol., (2008);82,:3679–3688. doi: 10.1128/JVI.02301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshizaki, T.,, et al. Induction of interleukin-8 by Epstein-Barr virus latent membrane protein-1 and its correlation to angiogenesis in nasopharyngeal carcinoma. Clin. Cancer Res., (2001);7,:1946–1951. [PubMed] [Google Scholar]
- 30.Ren, Q.,, et al. Epstein-Barr virus (EBV) latent membrane protein 1 induces interleukin-8 through the nuclear factor-kappa B signaling pathway in EBV-infected nasopharyngeal carcinoma cell line. Laryngoscope, (2004);114,:855–859. doi: 10.1097/00005537-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Liao, B.,, et al. Macrophage migration inhibitory factor contributes angiogenesis by up-regulating IL-8 and correlates with poor prognosis of patients with primary nasopharyngeal carcinoma. J. Surg. Oncol., (2010);102,:844–851. doi: 10.1002/jso.21728. [DOI] [PubMed] [Google Scholar]
- 32.Xie, L.Q.,, et al. Co-elevated expression of hepatocyte growth factor and Interleukin-8 contributes to poor prognosis of patients with primary nasopharyngeal carcinoma. Oncol. Rep., (2010);23,:141–150. [PubMed] [Google Scholar]
- 33.Scheid, M.P.,, et al. PKB/AKT: functional insights from genetic models. Nat. Rev. Mol. Cell Biol., (2001);2,:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 34.Liu, Y.,, et al. Activation of AKT is associated with metastasis of nasopharyngeal carcinoma. Tumour Biol., (2012);33,:241–245. doi: 10.1007/s13277-011-0272-4. [DOI] [PubMed] [Google Scholar]
- 35.MacManus, C.F.,, et al. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol. Cancer Res., (2007);5,:737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- 36.Araki, S.,, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res., (2007);67,:6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 37.Thiery, J.P.,, et al. Epithelial-mesenchymal transitions in development and disease. Cell, (2009);139,:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Shen, Y.,, et al. Role of CCR2 and IL-8 in acute lung injury: a new mechanism and therapeutic target. Expert Rev. Respir. Med., (2011);5,:107–114. doi: 10.1586/ers.10.80. [DOI] [PubMed] [Google Scholar]
- 39.Skov, L.,, et al. IL-8 as antibody therapeutic target in inflammatory diseases: reduction of clinical activity in palmoplantar pustulosis. J. Immunol., (2008);181,:669–679. doi: 10.4049/jimmunol.181.1.669. [DOI] [PubMed] [Google Scholar]
- 40.Hatfield, K.J.,, et al. Antiangiogenic therapy in acute myelogenous leukemia: targeting of vascular endothelial growth factor and interleukin 8 as possible antileukemic strategies. Curr. Cancer Drug Targets, (2005);5,:229–248. doi: 10.2174/1568009054064651. [DOI] [PubMed] [Google Scholar]
- 41.Kido, S.,, et al. Interleukin 8 and vascular endothelial growth factor – prognostic factors in human gastric carcinomas? Eur. J. Cancer, (2001);37,:1482–1487. doi: 10.1016/s0959-8049(01)00147-2. [DOI] [PubMed] [Google Scholar]
- 42.Shahzad, A.,, et al. Interleukin 8 (IL-8) – a universal biomarker? Int. Arch. Med., (2010);3,:11. doi: 10.1186/1755-7682-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan, A.,, et al. Interleukin-8 messenger ribonucleic acid expression correlates with tumor progression, tumor angiogenesis, patient survival, and timing of relapse in non-small-cell lung cancer. Am. J. Respir. Crit. Care Med., (2000);162,:1957–1963. doi: 10.1164/ajrccm.162.5.2002108. [DOI] [PubMed] [Google Scholar]
- 44.Apostolakis, S.,, et al. Interleukin 8 and cardiovascular disease. Cardiovasc. Res., (2009);84,:353–360. doi: 10.1093/cvr/cvp241. [DOI] [PubMed] [Google Scholar]
- 45.Lai, Y.,, et al. Interleukin-8 induces the endothelial cell migration through the activation of phosphoinositide 3-kinase-Rac1/RhoA pathway. Int. J. Biol. Sci., (2011);7,:782–791. doi: 10.7150/ijbs.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schadendorf,. et al. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J. Immunol., (1994);153,:3360. [PubMed] [Google Scholar]
- 47.Fernando, R.I.,, et al. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res., (2011);71,:5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.