Abstract
The serotonin (5-HT) system is generally considered as a single modulatory system, with broad and diffuse projections. However, accumulating evidence points to the existence of distinct cell groups in the raphe. Here, we review prior evidence for raphe cell heterogeneity, considering different properties of 5-HT neurons, from metabolism to anatomy, and neurochemistry to physiology. We then summarize more recent data in mice and zebrafish that support a genetic diversity of 5-HT neurons, based on differential transcription factor requirements for the acquisition of the 5-HT identity. In both species, PET1 plays a major role in the acquisition and maintenance of 5-HT identity in the hindbrain, although some 5-HT neurons do not require PET1 for their differentiation, indicating the existence of several transcriptional routes to become serotoninergic. In mice, both PET1-dependent and -independent 5-HT neurons are located in the raphe, but have distinct anatomical features, such as the morphology of axon terminals and projection patterns. In zebrafish, all raphe neurons express pet1, but Pet1-independent 5-HT cell groups are present in the forebrain. Overall, these observations support the view that there are a number of distinct 5-HT subsystems, including within the raphe nuclei, with unique genetic programming and functions.
Keywords: development, vesicular glutamate transporter 3, tryptophane hydroxylase, synapse, axon tracing
1. Introduction
Because serotonin (5-HT) innervation is very diffuse and modulatory in nature, all cells in the brain are in one way or another under 5-HT influence. Indeed, 5-HT neurotransmission is implicated in an amazingly vast array of behavioural and physiological states and functions (sleep, mood, appetite, anxiety, neurovegetative control, etc.) [1]. It is common to think of the 5-HT system as a single entity because all 5-HT cells/neurons share common biosynthetic/degradation pathways and act on the same set of receptors. Hence, these neurons are all affected by drugs targeting 5-HT metabolism. However, a growing body of evidence now suggests that there are several 5-HT subsystems in the organism and in the brain in particular. These 5-HT subsystems subserve different functions, some of which can be very local, and have unique cytological and anatomical organizations. It is then likely that a different genetic programming underlies the generation of the diversity present among 5-HT neurons, as will be argued in this review.
The recognition of the existence of different 5-HT subsystems begins outside the brain. Various organs, such as the gut, blood vessels, the thyroid, the pancreas and the mammary gland, produce 5-HT [2–6]. These sources of 5-HT are independently regulated and act locally to control the function of the organ to which they belong. Recently, the placenta was added to the list of organs that synthesize their own 5-HT. In the placenta, serotonin produced in the syncytiotrophoblasts could have a local trophic effect, but could also provide an early developmental source of 5-HT to the embryo before the maturation of the raphe [7,8]. These important observations indicate that the 5-HT phenotype includes multiple different cellular entities and subsystems that serve a specific dedicated function. In the central nervous system (CNS), the situation is less clear. Although the existence of several 5-HT subsystems has been proposed since the first anatomical description of the brainstem cellular 5-HT subgroups in the raphe [9,10], and was further supported by numerous anatomical and functional studies [11,12], it has been difficult to reconcile the evidence derived from multiple technical strategies into one clear view of the anatomical organization of 5-HT systems and to link them to specific functional correlates.
In the present review, we will focus on the CNS of vertebrates, and consider the previous evidence for a heterogeneity of the 5-HT systems, based on different methodological approaches spanning from anatomy to physiology. Then, we will report more recent evidence in mice and zebrafish, illustrating new aspects of the developmental and genetic heterogeneity of central 5-HT neurons.
2. Diversity of serotonin metabolic pathways
A common denominator of all 5-HT cells in the organism is their capacity to produce, take up, store and release 5-HT. These basic neurotransmitter-related functions require a biosynthetic and transport machinery that is common to all 5-HT cells, but with some variants in the proteins involved according to cell types and species.
In mammals, the biosynthetic pathway transforming the essential amino acid tryptophan into 5-HT involves tryptophane hydroxylase (TPH), which comes in two isoforms, TPH1 and TPH2. The raphe neurons of the brain use TPH2 [13,14], although TPH1 expression was detected at certain developmental stages [15]. In most other organs, such as the pineal gland, enterochromaffin cells, mammary gland, islet beta cells of the pancreas, vessel intima and placenta, TPH1 was found to be the dominant isoform [2–7]. However, TPH2 is also expressed by a few cells in the periphery, for instance, in the myenteric 5-HT-containing cells [5]. After biosynthesis, 5-HT is concentrated in intracellular organelles, the synaptic vesicles in neurons, by a transporter, the vesicular monoamine transporter (VMAT), which also exists in two different forms, VMAT2 and VMAT1; VMAT2 is the dominant isoform in the brain, while VMAT1 is essentially expressed in the periphery [16]. In contrast, only one form of the 5-HT transporter (SERT) and of the aromatic l-amino acid decarboxylase (AADC) have been identified. Degradation of 5-HT, an essential part of 5-HT homeostasis, is ensured by monoamine oxidase (MAO), for which there are two isoforms, MAOA and MAOB. MAOA is the main variant responsible for 5-HT degradation in CNS [17], particularly during brain development [18].
In zebrafish, as in other teleost fishes, the serotoninergic phenotype is encoded by a set of genes similar to that in mammals [19]. However, owing to a whole genome duplication event taking place at the base of the teleost radiation, followed by neo-, sub- or non-functionalization of duplicated genes, the zebrafish genome often contains additional copies of genes found in mammals [20,21]. Accordingly, zebrafish teleosts possess three different tph genes (tph1a, tph1b and tph2) [22,23], two sert (serta and sertb, also known as slc6a4a and slc6a4b, respectively) [24,25] and two vmat (vmat1 and vmat2) genes [26]. The mao and aadc genes, however, only exist as single homologues of mammalian genes [26–29]. Interestingly, by pharmacological criteria, the zebrafish Mao resembles the mammalian MAOA, with a higher affinity for 5-HT than for dopamine (DA) or noradrenaline [30]. Expression of some of these 5-HT-related genes in zebrafish has until now been reported only in the CNS, with different patterns according to cell groups (figures 1 and 2). Thus, tph1a and sertb are detectable in the basal forebrain [23,24,31]. In addition, tph1a transcripts are found in cells along the floor plate of the spinal cord, whereas tph1b expression was reported only in a small preoptic cell population during late embryonic stages [23]. In zebrafish as in mammals, tph2 and serta are expressed by the serotoninergic neurons of the raphe nuclei, but they are additionally expressed by neurons in the pretectal area of the forebrain [22,24,31]. All three tph paralogues are detectable in the pineal gland. As expected, all the cell populations expressing either of the Tph-encoding genes also contain 5-HT [27,32]. These characteristics of zebrafish provide a unique possibility to genetically target specific subsets of 5-HT neurons and thereby to relate them to distinct functions.
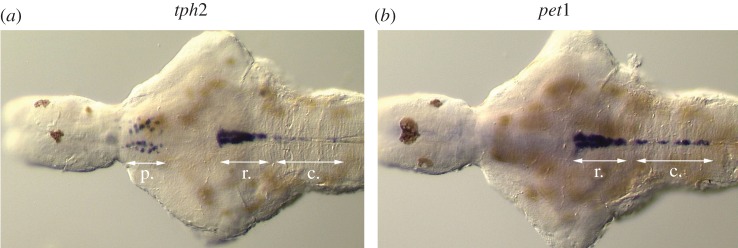
Figure 1.
Transcripts for (a) tph2 and (b) pet1 revealed by in situ hybridization performed on brains dissected from 6-day-old zebrafish. tph2 is present in the rostral (r) and caudal (c) raphe populations, as well as in pretectal (p) neurons and the pineal gland (not shown). In contrast, pet1 is only detectable in the hindbrain populations. Ventral view, anterior to the left. Adapted from [31].
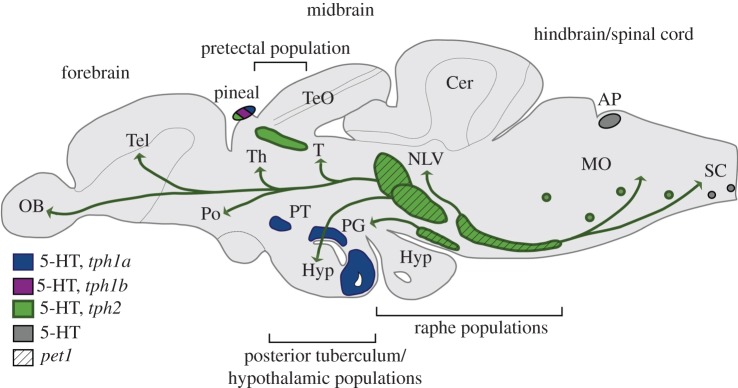
Figure 2.
Schematic drawing illustrating the location of serotoninergic neurons in the adult zebrafish brain (lateral view). 5-HT-immunoreactive cells are also present in the retina (not shown). The main projections originating from the raphe populations are indicated. AP, area postrema; Cer, cerebellum; Hyp, hypothalamus; MO, medulla oblongata; NLV, nucleus lateralis valvulae; OB, olfactory bulb; PG, preglomerular complex, Po, preoptic region; PT, posterior tuberculum; SC, spinal cord; Tel, telencephalon; TeO, tectum opticum; Th, thalamus; V, ventral telencephalic area. Adapted from [19].
Orthologues of all the 5-HT metabolic pathways exist in invertebrates such as Drosophila melanogaster and Caenorhabditis elegans, as recently reviewed by Flames & Hobert [33], and 5-HT immunoreactivity is found in a number of neurons of both species. Furthermore, the 5-HT neurons of both D. melanogaster and C. elegans arise from distinct progenitors, which depend on unique developmental regulatory networks for their differentiation and maturation, and eventually serve very different purposes. Taken together, these findings indicate that, in invertebrate species also, there is a considerable heterogeneity in the genetic programming and functions of the 5-HT neuron population.
In addition to the 5-HT-producing neurons, a large number of cells in the organism can be technically considered as serotoninergic because they store and release 5-HT although they do not produce it. In the periphery, these are the platelets, mastocytes and several neural-crest-derived cells [3,34] or specialized subsets of epithelial cells [35]. In the mammalian brain, a large set of developing neurons in the forebrain (thalamus, limbic cortex, retina, etc.) expresses the 5-HT transporter (SERT) and a combination of VMAT2 or VMAT1, although they do not express any known isoform of the amine biosynthetic enzymes, such as TPH or AADC [36–38]. The expression of the 5-HT-concentrating genes and 5-HT-synthesizing genes can therefore be dissociated during cell differentiation, and are likely to be controlled in part by different transcriptional programmes. For instance, LIM homeobox transcription factor 1, beta (Lmx1b) or FEV (fifth Ewing variant; Pet1), which are transcription factors required for the differentiation of 5-HT neurons in the brainstem (see later text), are not expressed in the brain regions where transient expression of SERT and VMAT2 is observed. Moreover, these transient expression patterns are maintained in Lmx1b or Pet1 knockout mice [39]. The localization and presumed function of these ‘quasi-serotonin’ neurons has been reviewed recently [40,41] and will not be further discussed here.
3. Anatomical diversity of serotonin-producing neurons in the brain
In mammals, all 5-HT cell groups originate embryologically from the hindbrain in rhombomeres (r) 1–3 and 5–7 [42–44]. However, because in the adult brain, the most rostrally located 5-HT neurons (derived from rhombomerere 1) intermix with DA neurons in the midbrain, owing to migration and developmental reorganization, some 5-HT neurons have been inappropriately referred to as mesencephalic. A large majority of 5-HT cells is confined to the hindbrain. These 5-HT neurons form distinct cell groups initially described as the B1–B9 groups. This terminology was coined by Annica Dahlström and Kjell Fuxe in the 1960s, when describing for the first time their anatomical localization, using formaldehyde-induced fluorescence [9]. The B groups referred to a yellow fluorescence that differed from the green fluorescence of the catecholamine cell groups, designated as the A groups. The anatomy of these cell groups was further refined by immunocytochemical analyses using antisera to 5-HT developed by Steinbush and colleagues in the early 1980s [10]. The numbering of the 5-HT cell groups begins caudally (B1, B2, B3) in the medulla and continues rostrally (B4–B9) in the pons and midbrain. Many years later, this terminology is still useful, because it refers to groups of cells with a similar chemical identity, rather than to a specific anatomical structure. Although individual clusters do correspond to specific brainstem nuclei (B1, raphe pallidus; B2, raphe obscurus; B3, raphe magnus; B4, dorsal to prepositus hypoglossi; B5, raphe pontis; B6, caudal part of raphe dorsalis; B7, raphe dorsalis; B8, centralis; B9, the supralemniscal nucleus), none of these nuclei is exclusively serotoninergic. Even in the dorsal raphe, which contains by far the largest number of 5-HT neurons—in the order of 11–15 000 in the rat [45,46]—there are twice as many non-5-HT neurons [45].
These primary anatomical descriptions brought forth the first evidence for a morphological heterogeneity among the raphe neurons. However, contrary to the dopamine system, in which the A8–A14 terminology designates fairly well-defined anatomical subsystems, it has remained difficult to match the B1–B9 cell groups to clearly identifiable anatomo-functional pathways (except perhaps for the most caudal groups implicated in respiratory control).
One of the difficulties in identifying clear anatomo-functional units within the ascending 5-HT pathways derives from the particular geometry of the raphe cell groups, which are bundled into tight clusters along the midline, with a somewhat ‘artificial’ partition by axon tracts. Thus, it is unclear whether the general architecture outlines anatomically separate nuclei. Moreover, while a broad topography of the projections exists, generally the projections have been described as diffuse and highly collateralized [47–49]. Indeed, a single raphe neuron is able to extend axons over very large distances, reaching many different brain regions. Moreover, 5-HT can be released from non-synaptic sites all along the axon (volume transmission [50]).
After several decades of anatomical tracing and functional analyses, some operational subdivisions of the raphe neurons have however emerged, based on the anatomical connections and physiological characteristics. Developmental studies and anatomical tracings showed, for instance, that there are two broad subdivisions of the raphe cell groups into rostral (B6–B9) and caudal cell groups (B1–B5), with projections to the forebrain and to the hindbrain, respectively [51]. Within the rostral domain, further rostrocaudal partitions were made based on retrograde tracing [48]. Schematically, the rostral third projects to the caudate nucleus, the intermediate zone projects to the amygdala and the caudal third to limbic structures, such as the hippocampus [48]. However, this organization only partly matches the more classical subdivision into dorsal (B6, B7) and median (B8, B9) raphe neuronal cell groups [49]. Further studies in rodents and humans [10,52] identified five subregions within the dorsal raphe (DR = B6, B7). These groups were named as follows: the interfascicular nucleus, rostral (DRr), caudal (DRc), ventral (DRV), lateral (DRL, or lateral wings) and dorsal (DRd), the latter being further subdivided into a core (DRDc) and a shell (DRDsh). Besides a clear topographic organization and morphology, the neurons in these different subgroups could be differentiated by their neurochemical and electrophysiological characteristics (see below), and by some general features of their connectivity. For instance, tracing studies showed that the core area of the DR (the DRDc) is characterized by highly collateralized neurons, whereas more peripherally situated areas contain less highly collateralized neurons [47]. For an extensive review of the anatomical organization and connections of these subnuclei see [12].
In non-placental vertebrates (i.e. fish, amphibians, reptiles, birds and monotremes), the general organization of the raphe 5-HT neurons follows a similar plan as in placental mammals. However, non-placentals exhibit a significantly wider distribution of 5-HT immunoreactive cells outside the raphe region. One extreme is represented by jawless and cartilaginous fishes, with prominent 5-HT populations in the forebrain and spinal cord in addition to the hindbrain 5-HT cell groups (reviewed in [19]). However, in most species examined, it is still unclear whether the non-raphe populations are truly serotoninergic or belong to the ‘quasi-serotonin’ neurons discussed earlier. Currently, only a few thorough analyses have been made in the pigeon and zebrafish CNS, in which the more widespread prevalence of 5-HT neurons was correlated to expression of Tph and therefore likely reflects neurons producing 5-HT [19,27,32,53]. In zebrafish, in addition to the 5-HT neurons in the raphe nuclei, 5-HT-containing cells have been identified in the retina, the pretectum, the hypothalamus/posterior tuberculum, the vagal lobes and the spinal cord. Scattered 5-HT cells were also found in the reticular formation and transiently in the preoptic area. Most of these various serotoninergic populations express different tph and sert homologues (see above). Owing to the presence of multiple serotoninergic populations along the anterior/posterior axis, and an intricate and widespread distribution of 5-HT-containing fibres throughout the brain, it has been difficult to separate the serotoninergic innervation originating from the various 5-HT populations. Thus, a direct comparison with the mammalian 5-HT circuits is difficult and the functional relationship between serotoninergic clusters in mammals and zebrafish remains unclear. Nevertheless, it has been assumed that, in teleosts, the rostral and caudal raphe populations innervate most brain and spinal cord areas in a manner similar to that described in mammals [54] and, accordingly, that they play equivalent functional roles. Indeed, 5-HT immunoreactive fibres have been detected in all major areas of the teleost CNS (see [19]).
We isolated the zebrafish ETS-domain transcription factor-encoding gene pet1 [31], which is expressed uniquely in the raphe serotoninergic neurons in zebrafish, and then used the pet1 regulatory elements to generate a transgenic line expressing green fluorescent protein (GFP). This allowed the selective visualization of the raphe 5-HT projections [55]. The analysis of this transgenic line showed that raphe serotoninergic neurons provide a rich innervation to most brain areas, except the optic tectum and cerebellum. Notably, the cerebellum of zebrafish has very few (if any) 5-HT immunoreactive fibres (C. Lillesaar 2009, unpublished data). The optic tectum, unlike in mammals, must receive 5-HT projections from other 5-HT neurons than the raphe. A likely source for these fibres is the pretectal population. Retrograde tracing in the pet1:egfp transgenic line further demonstrated the existence of functional subdivisions within the rostral raphe complex, but less prominent than those described in mammals. One dorsally located population in the rostral raphe projects primarily to the olfactory bulb and the telencephalon, and a second population, containing larger and more ventrally located cells, targets the hypothalamus. A previously overlooked serotoninergic population in the ventrolateral hindbrain of the zebrafish provides a dense innervation to the preglomerular complex. Finally, the caudal raphe population projects locally in the hindbrain and to the spinal cord. This study confirmed that, similar to those of mammals, in zebrafish, projections of the rostral and caudal raphe are restricted to the anterior (forebrain, midbrain) or posterior (hindbrain, spinal cord) domains, respectively. Taken together, these results highlight similarities, but also point out important anatomical differences between zebrafish and mammals with respect to the central serotoninergic systems.
4. Diversity of axonal morphology
A further morphological distinction between the 5-HT neurons was based on the observation of the morphology of 5-HT axon terminals. Careful anatomical examination of the serotoninergic axons in different brain areas identified two types of terminals in rats [56] and primates [57]. In rats, anterograde-tracing experiments showed that dorsal raphe axons displayed fine beaded varicosities, while the medial raphe projections showed large round sparse varicosities. This was matched to the morphology of 5-HT immunostained axons [56]. The distribution of these fibres differed according to the brain regions, and displayed a striking difference in their sensitivity to amphetamine derivatives, 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxymethamphetamine (MDMA), with the largest fibres being more resistant to the effects of toxins than the fine ones. In primates, two types of terminal axons were also described, one with sparse, small, ovoid varicosities (less than 1 µm), and the other with large, spheroidal varicosities (1–5 µm), which are more densely clustered. Axons with large varicosities formed baskets surrounding the soma and the proximal dendrites of some interneurons [57,58].
Ultrastructural analyses of the 5-HT neurons showed yet another level of distinction between the 5-HT axonal projections. This concerned their myelinization and the formation of synapses. While most of the 5-HT axons appeared to be non-myelinated, a sizeable fraction was myelinated, suggesting that ascending 5-HT axons may have different velocities of nerve conduction; the percentage of 5-HT myelinated axons was higher in primates than in rodents [59,60]. Another important ultrastructural distinction among 5-HT axons was the formation of synaptic specializations. A majority of the terminal or ‘en passant’ boutons of 5-HT axons do not form synaptic specializations [61], but a variable proportion of the 5-HT boutons do form synapses, revealed when serial sections are examined. This topic has recently been reviewed in detail by Descarries and co-workers [62]. 5-HT synaptic junctions can be either symmetric (i.e. without a post-synaptic density) or asymmetric (i.e. with a post-synaptic density). In all cases, 5-HT terminals synapse on spine heads or distal dendrites. Evaluations of the proportion of 5-HT terminals forming synapses varies according to the brain structure. Thus, in the cerebral cortex and hippocampus, only 20 per cent of the 5-HT terminals form synaptic junctions, whereas the incidence of synapses rises to 50 per cent in the substantia nigra, and 75 per cent in the basolateral amygdala [62,63].
It is still unclear whether different 5-HT receptors are associated with these different synaptic or non-synaptic terminals. Most of the G-protein-coupled 5-HT receptors are localized outside synaptic zones [64,65]. However, the ion channel receptor 5-HT3, which is essentially present on interneurons, is localized to synapses and can have rapid phasic effects [66]. Furthermore, the 5-HT2A receptor has been found to be localized at post-synaptic densities [67], and could therefore be associated preferentially to synaptic 5-HT junctions. For an extensive review of the subcellular localization of 5-HT receptors, see [68].
5. Electrophysiological diversity of the raphe neurons
Recordings of raphe neurons in the different serotoninergic subnuclei conducted in brain slices from several mammalian species showed some common electrophysiological properties, such as slow, rhythmic activity in spontaneously active cells, broad action potential and large afterhyperpolarization potential [69]. Some variations in firing rate were however noted between the caudal (B3, pallidus) and the rostral (B7 and B8) raphe cell groups [70], as well as differences in inhibitory response to the application of 5-HT1A and 5-HT1B agonists [69,71], which could reflect differences in receptor content or in the downstream coupling of these receptors in distinct parts of the raphe. Moreover, variations were noted in the excitability of the different parts of the dorsal raphe; in particular, there was an increase in the excitability of 5-HT neurons in the lateral wings of the dorsal raphe [72], that could correspond to intrinsic differences between these neurons or between their specific afferents. In vivo juxtacellular recordings of 5-HT or putative 5-HT neurons showed further heterogeneity among raphe neurons, but this time with no particular relationship to a defined raphe cell group. Rather variations in the firing pattern were noted over the sleep–wake cycle [73], or in relationship to hippocampal theta rhythms [74]. For instance, two types of dorsal raphe 5-HT neurons were identified, one type with slow-firing clock-like activity, and another type with a fast-firing pattern highly correlated with theta rhythmic activity [74]. These data reflect an interesting functional diversity of the 5-HT cells, even within one raphe cell group.
This functional diversity was further emphasized by observations of a differential activation of the dorsal raphe neurons under various situations of stress. For instance, whereas fear startle responses increased c-Fos in the most dorsal part of the dorsal raphe (DRD), fear conditioning experiments increased c-Fos expression throughout the dorsal raphe [75]. Similarly, a number of anxiogenic drugs or exposure to social defeat activate the dorsal and caudal parts of the DR [11,76]. These individual functional properties of the raphe neurons could be related to intrinsic differences among the raphe neurons or to separate inputs to the raphe [77], notably from the prefrontal cortex that appears to inhibit the activity of the dorsal raphe neurons [78] and from the locus coeruleus [79,80] that provides a major stimulatory drive.
6. Neurochemical diversity of raphe neurons’ co-transmitter phenotypes
Neurons containing different neuropeptides, gamma aminobutyric acid (GABA) and glutamate have been found to be present in the raphe nuclei. The question of whether these signalling molecules are co-localized with 5-HT has been repeatedly investigated. Co-localization of 5-HT with neuropeptides was shown to be variable according to species and the methods used. For instance, many neuropeptides were reported to be present in the raphe of the rat, such as substance P (SP), thyrotropin-releasing hormone (TRH), leucine-enkephalin (LEU-enk) and methionine-enkephalin (MET-enk), and shown to coexist with 5-HT in the caudal raphe groups, i.e. the raphe magnus, raphe obscurus and raphe pallidus [81]. Corticotropin-releasing factor (CRF) was also identified in a subpopulation of 5-HT neurons that project to the central amygdala [82]. In contrast, an extensive co-localization study, using a panel of 12 different peptides—including the peptides cited earlier (SP, TRH, enkephalins and CRF), revealed no co-localization in the mouse dorsal raphe, although neurons containing these peptides were indeed intermixed with the 5-HT neurons [83]. This indicated that peptide/5-HT co-neurotransmission is not relevant, at least not in mouse dorsal raphe. GABAergic neurons are also abundant in the raphe, although they appear to have a distribution largely complementary to that of 5-HT neurons [71,83,84]. However, glutamate decarboxylase GAD and 5-HT co-localization was reported in several studies [85,86], but representing a very small fraction of the raphe neurons. The other population of raphe neurons is that of the glutamatergic neurons, which may be separated according to the vesicular glutamate transporter subtype they contain—VGLUT1, VGLUT2 or the atypical vesicular glutamate transporter VGLUT3. In the raphe, both VGLUT2- and VGLUT3-positive neurons are found, but no VGLUT1 [85,86]. The VGLUT2-positive neurons are largely distributed around the serotoninergic neurons in the raphe, whereas the distribution of the VGLUT3 neurons largely overlaps that of the 5-HT cells [87–89]. However, not all VGLUT3 neurons are 5-HT, and not all 5-HT neurons contain VGLUT3, depending on the different raphe subnuclei. For example, many 5-HT neurons in the lateral wings do not contain VGLUT3, whereas a high proportion of 5-HT neurons are double-labelled in the DRd [86] (figure 3). Tracing and lesion experiments have shown that the projections of these raphe neuronal subtypes differ [91] (with co-localized projections in the lateral septum, for instance, but not in the olfactory bulb). Further co-localization analyses of VGLUT3 and 5-HT in axon terminals reveal complex patterns of co-localization, with 50 per cent co-localization in the prelimbic cortex and the CA3 field of the hippocampus, but 30 per cent co-localization in the CA1 field of the hippocampus [92].
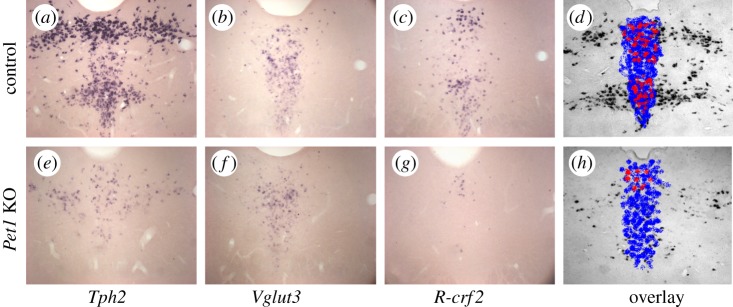
Figure 3.
Transcripts for Tph2, Vglut3 and R-crf2 (receptor for CRF) on consecutive coronal sections through the dorsal raphe of control (a–c) and Pet1 knockout (e–g) mice. An overlay using false colour code is shown in d and h. In control raphe (d), the three transcripts delimit nested domains within the dorsal raphe, outlining in particular the Tph2-positive neurons in the lateral wings, which do not contain Vglut3 or R-crf2. In Pet1 KO raphe, the number of Tph2-positive neurons is reduced by 70%, while Vglut3-containing neurons are reduced by 30%. However, double combining Vglut3 in situ hybridization with TPH immunocytochemistry [90] indicated that most of these remaining Vglut3 neurons are not serotoninergic. Micrographs taken by Vera Kiyasova.
Overall, these studies indicate that the neurochemical architecture of the raphe is complex, with a combination of 5-HT, peptidergic and glutamatergic neurons. The only clear and reproducible co-localization uncovered up to now is that of VGLUT3. However, there is no definitive evidence that glutamate is actually used as a neurotransmitter by these neurons. Rather, the function of VGLUT3 in the 5-HT neurons could be to enhance the efficiency of vesicular packaging, and hence of 5-HT neurotransmission [92].
7. Genetic diversity: revealed in pet1 knockout mice
The molecular cascade leading to the differentiation of the 5-HT raphe neurons has now been largely clarified, and the genetic basis for a heterogeneity along the rostro-caudal axis of the raphe is beginning to emerge. The molecular basis of 5-HT raphe specification has been extensively reviewed recently [33,42,44,93] and will not be detailed here. Briefly, serotoninergic progenitors are produced in the brainstem in different rhombomeres (r1–r3 for the rostral group, r4–r7 for the caudal group) under the influence of a set of secreted factors, including Sonic hedgehog (SHH), Fibroblast growth factor FGF8 and FGF4, which determine their position in the neural tube. Two main transcriptional cascades are involved in the specification of the 5-HT identity, one for the rostral 5-HT cell groups and the other in the caudal 5-HT cell groups. These form transcriptional networks that ultimately converge on factors that act on differentiating neurons as selectors of the monoamine and 5-HT identity. In the brainstem, these factors were identified as LMX1b and PET1 [39,94–96]. Lmx1b is upstream of Pet1 and has broader effects on monoaminergic cells, in particular, in the differentiation of the dopaminergic neurons. Pet1 expression is more limited and quite remarkable by its selectivity to 5-HT neurons of the brainstem. In rodents, the expression of Pet1 precedes the appearance of 5-HT neurons by 24 h [97], and a minimal promoter of Pet1 drives the expression of reporter or other genes selectively to the 5-HT neurons [98]. Moreover, PET1 controls directly a large number of genes that define the 5-HT phenotype during development [94] and in adulthood [95].
In the Pet1 knockout (KO) mouse, a majority of the 5-HT raphe neurons do not develop, and there is a 70 per cent reduction of the raphe neurons in all B1–B9 cell groups, with a downregulation of several key genes of the 5-HT phenotype in residual neurons, suggesting that all raphe 5-HT neurons require PET1 for their full differentiation [94] (figure 3). However, on closer examination of the remaining 5-HT innervation, a very peculiar distribution was noted. Rather than a homogeneous decrease of immunostaining in all brain areas, which was expected based on the homogeneous pattern of cell loss in the raphe nuclei, the remaining 5-HT fibres were concentrated in very specific areas, and completely absent from other areas. For instance, a normally dense innervation was visible in the posterior part of the basolateral nucleus of the amygdala, in the intralaminar thalamic nuclei and in the paraventricular hypothalamic nucleus, with no innervation whatsoever in neighbouring forebrain [90] (figure 4). In most of the areas where 5-HT axon terminals remained in the Pet1 KO, the 5-HT axon varicosities were large, with very few or no fine varicosities. In the cerebral cortex and hippocampus, that contain a majority of 5-HT axons with small varicosities, the density of 5-HT innervation was reduced by 85 per cent, and the residual 5-HT axons in these areas displayed large varicosities. Ultrastructural examination of the remaining 5-HT axon varicosities showed a normal content in small and dense core vesicles, but, more surprisingly, a high incidence of synaptic junctions. The increased frequency of synaptic junctions was confirmed in the three brain areas in which a strong residual 5-HT innervation was noted, the basolateral amygdala, the intralaminar thalamic nuclei and the paraventricular hypothalamic nuclei. This suggested that a particular anatomical subclass of 5-HT raphe neurons, characterized by large varicosities, synapse formation and restricted terminals in limbic brain areas, did not require PET1 for their differentiation.
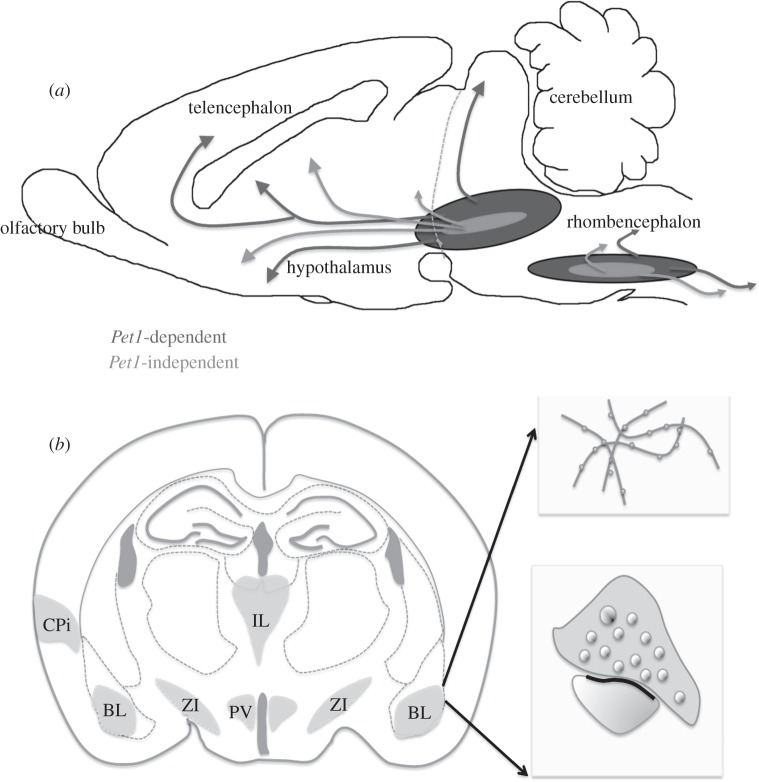
Figure 4.
Schematic illustrating the residual serotonin innervation in the Pet1 KO. (a) The rostral and caudal raphe cell groups send projections to the forebrain and to the brainstem and spinal cord, respectively. In Pet1 KO mice, all 5-HT neurons and projections schematized in dark grey fail to differentiate; they do not acquire a 5-HT phenotype and do not send projections to the hippocampus and cerebral cortex. In contrast, the neurons and projections schematized in light grey differentiate and send topographically appropriate projections. (b) Drawing summarizing the main morphological characteristics of the residual 5-HT innervation in the Pet1 KO. The residual 5-HT terminals target highly selective regions in the forebrain: the basolateral nucleus of the amygdala (BL), paraventricular nucleus of hypothalamus (PV) and mildline thalamic nuclei (IL). In these areas, the residual 5-HT labelled axons display large varicosities (upper insert) and differentiated synaptic junctions (lower insert).
Before reaching this conclusion, we carried out different functional and developmental analyses to rule out possible consequences of the lack of PET1 on the remaining 5-HT neurons. The function of the remaining 5-HT axon terminals in the Pet1 KO was tested by examining the effects of the 5-HT releaser, fenfluramine. One hour after drug exposure, c-Fos became activated in several areas that contain a dense 5-HT innervation, such as the paraventricular hypothalamic nucleus. Moreover, secretion of corticosterone in the blood was triggered. In the Pet1 KO, we observed that c-Fos activation and corticosterone responses were similar to control. Other functional tests, including measures of uptake and release in vitro, showed normal properties of the Pet1 KO terminals. Developmental studies revealed no impairment in the growth of 5-HT fibres in vitro and in vivo; the main ascending 5-HT tracts, notably, were present and normally distributed in the mutants. The topographic organization of the raphe neuron projections appeared to be normal, with raphe projections to the basolateral amygdala arising from the dorsal raphe. However, projections to the hippocampus were reduced in number compared with control, and comprised only non-serotoninergic neurons, indicating that the raphe neurons that failed to differentiate as serotoninergic neurons also failed to reach their normal targets. This interpretation was supported by retrograde tracings from the cerebral cortex, which showed reduced raphe-cortical projections in the Pet1 KO mice [95].
Because of the neurochemical heterogeneity of 5-HT neurons summarized earlier, we investigated in the Pet1 KO the co-localization of Vglut3 and CRF2 receptor gene (R-crf2) in the residual 5-HT raphe neurons (figure 3). Analysis of single labelling from serial sections showed a relative preservation of the Vglut3 labelling in the raphe, and a reduction of the population of neurons expressing R-crf2. However, double-labelling experiments showed that the remaining 5-HT neurons in the Pet1 KO mice did not contain Vglut3. Therefore, the preservation of the Vglut3-expressing neuronal population most likely reflected the preservation of the non-serotonin raphe neurons expressing this transporter [99].
These experiments indicated that the raphe neurons that do not require PET1 for their serotoninergic differentiation normally project to areas such as the basolateral nucleus of the amygdala, where they form functional synapses. In contrast, the more abundant PET1-dependent 5-HT raphe neurons, which normally target the cerebral cortex, hippocampus and striatum, do not differentiate and could remain in an immature state of differentiation, as indicated by their lack of a 5-HT phenotype, lack of projections and electrophysiological characteristics suggesting an immature state.
8. PET1-dependent and -independent serotonin populations in the zebrafish brain
On the basis of expression pattern, it can be assumed that the transcriptional networks controlling raphe 5-HT neuron development in zebrafish are similar to those in mammals, including factors such as NK2 homeobox 2 (Nkx2.2), GATA-binding protein 2 and 3 (Gata2 and 3), and Lmx1b. The importance of the transcription factors Foxa2 and Irx1a for the differentiation of raphe 5-HT neurons has also been demonstrated [100,101]. In zebrafish, pet1 is co-expressed with tph2 in the hindbrain during embryonic, larval and adult stages (figure 2). Zebrafish pet1 expression precedes that of tph2 [31,55], and immunohistochemistry for the mitotic marker phospho-histone H3 shows that zebrafish pet1 is expressed at post-mitotic serotoninergic precursor stages and onwards (G. Stigloher 2008, personal communication). Interestingly, none of the forebrain serotoninergic groups expressed pet1. Considering the key function of Pet1 for the development and maintenance of the full mature 5-HT identity, it can be hypothesized that a paralogous pet1 gene is expressed in the other serotoninergic populations of the zebrafish or that another Ets-domain factor is essential for these populations. However, this factor has not yet been identified.
Little is known regarding the non-raphe 5-HT populations in zebrafish, in terms of requirement of molecules controlling their development. The involvement of Shh, Fgf and wingless-type MMTV integration site family (Wnt) in forebrain development has however been demonstrated [102–105]. As regards 5-HT neurons, it was shown that the transcription factors Fezl [106] and Etv5b (P. Gaspar & C. Lillesaar 2012 unpublished data), as well as the transcription elongation factor Foggy, control their generation in the embryonic stage [107]. The mechanisms of action of these factors remain to be elucidated.
Thus, the generation and maintenance of the neuronal 5-HT identity appear to involve the activation of multiple signalling pathways, and a combination of regulatory factors that is highly complex and variable, both within and between the different 5-HT neuron populations. This possibly reflects the heterogeneity seen among the mature central 5-HT neurons in vertebrates.
9. Concluding remarks
Studies from various fields of investigation bring support to the view that 5-HT-containing cells, in general, and neurons using 5-HT as a transmitter, in particular, constitute a highly heterogeneous population. In the present review, we have described how the neurons vary with respect to metabolic pathways, anatomical characteristics, axonal morphology, neurochemistry, neurotransmission and electrophysiological properties. This plethora of characteristics is likely to be, at least partly, genetically encoded. Indeed, genetic studies in mice and the use of genetically tractable vertebrate model organisms, such as zebrafish, are helping us to decipher the genetic underpinning of the mosaics of neurons, and ultimately shed light on how multiple pathological conditions may affect the function of the central serotoninergic neurons.
For instance, the genetic diversity of central serotonin neurons will be interesting to relate to the regionally localized changes in TPH2 expression that have been observed in the raphe of patients with severe depression [108]; at the other extreme, they may help us to understand how abnormal maturation of raphe neurons could be involved in the pathogenesis of sudden infant death syndrome [109]. Another promising direction of research will be the understanding of the molecular make-up of the diverse serotonin cell groups, with more systematic investigations than those carried out in the past. This should include genome-wide investigations of the composition of the individual raphe cell groups, with transcriptome [110] and proteomic approaches, or single cell analyses of raphe neurons coupled to gene expression analyses [111]. Such investigations have already started to yield some unexpected molecular differences between the rostral and caudal raphe groups [110]. Future research should yield a more refined understanding of why 5-HT neurons are so much alike and yet so different.
Acknowledgements
Funding for this project was from the Agence Nationale de la Recherche (ANR-MNP-neur-032). The research of P.G. is funded by INSERM, Université Paris 6 and the European Commission (FP7-health-2007-A-201714). C.L. was funded by the Fyssen Foundation and is supported by a Chair of Excellence from the Ecole de Neurosciences de Paris (ENP), the Agence Nationale pour la recherche-(ANR-08-CEXC-001-01) and Schlumberger Association (grant DLS/GP/LB090305 to Laure Bally-Cuif). We are indebted to Evan Deneris for the use of the Pet1-knockout mice. Several members in the labs of P.G. and C.L. contributed to the data reviewed here: Laure Bally-Cuif, Adriana Bosco, Sebastian Fernandez, Vera Kiyasova, Jeanne Laine, Aude Muzerelle, William Norton. C.L. would like to express her sincere gratitude to all members of Laure Bally-Cuif's research group. Finally, we thank Andre Sobel for critical reading of the manuscript and Vera Kiyasova for illustrations.
Glossary
- 5-HT
5-hydroxytryptamine, serotonin
- AADC
aromatic l-amino acid decarboxylase
- CNS
central nervous system
- CRF
corticotropin-releasing factor
- DA
dopamine
- DR
dorsal raphe
- DRc
caudal DR
- DRd
dorsal DRd
- DRDc
core DRD
- DRDsh
shell DRDsh
- DRL
lateral DR
- DRr
rostral DR
- DRV
ventral DR
- ETS
E26 family
- FGF
fibroblast growth factor
- GABA
gamma-aminobutyric acid
- GAD
glutamate decarboxylase
- GATA
GATA binding protein, transcription factor
- GFP
green fluorescent protein
- KO
knockout
- LEU-enk
leucine-enkephalin
- LMX1b
LIM homeobox transcription factor 1, beta
- MAO
monoamine oxidase
- MDA
3,4-methylenedioxyamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- MET-enk
methionine-enkephalin
- NKX2.2
NK2 homeobox 2, transcription factor
- PET1
FEV (fifth Ewing variant), transcription factor
- r
rhombomere
- TPH
tryptophane hydroxylase
- TRH
thyrotropin-releasing hormone
- SERT
5-HT transporter, also known as Solute carrier family 6 member 4
- SHH
Sonic hedgehog
- SP
substance P
- VGLUT
vesicular glutamate transporter, also known as Solute carrier family 17
- VMAT
vesicular monoamine transporter, also known as Solute carrier family 18
- Wnt
wingless-type MMTV integration site family
Gene/protein symbols
| species | gene symbol | protein symbol |
|---|---|---|
| Homo sapiens | SHH | SHH |
| Mus musculus, Rattus norvegicus | Shh | SHH |
| Danio rerio | shh | Shh |
References
- 1.Jacobs B. L., Azmitia E. C. 1992. Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229 [DOI] [PubMed] [Google Scholar]
- 2.Gershon M. D. 2000. 5-HT (serotonin) physiology and related drugs. Curr. Opin. Gastroenterol. 16, 113–120 10.1097/00001574-200003000-00004 (doi:10.1097/00001574-200003000-00004) [DOI] [PubMed] [Google Scholar]
- 3.Walther D. J., et al. 2003. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115, 851–862 10.1016/S0092-8674(03)01014-6 (doi:10.1016/S0092-8674(03)01014-6) [DOI] [PubMed] [Google Scholar]
- 4.Eddahibi S., et al. 2006. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113, 1857–1864 10.1161/CIRCULATIONAHA.105.591321 (doi:10.1161/CIRCULATIONAHA.105.591321) [DOI] [PubMed] [Google Scholar]
- 5.Côté F., Fligny C., Bayard E., Launay J., Gershon M., Mallet J., Vodjdani G. 2007. Maternal serotonin is crucial for murine embryonic development. Proc. Natl Acad. Sci. USA 104, 329–334 10.1073/pnas.0606722104 (doi:10.1073/pnas.0606722104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulmann N., et al. 2009. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 7, e1000229. 10.1371/journal.pbio.1000229 (doi:10.1371/journal.pbio.1000229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnin A., Goeden N., Chen K., Wilson M. L., King J., Shih J. C., Blakely R. D., Deneris E. S., Levitt P. 2011. A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350 10.1038/nature09972 (doi:10.1038/nature09972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trowbridge S., Narboux-Nême N., Gaspar P. 2010. Genetic models of serotonin (5-HT) depletion. What do they tell us about the developmental role of 5-HT. Anat. Rec. 294, 1615–1623 10.1002/ar.21248 (doi:10.1002/ar.21248) [DOI] [PubMed] [Google Scholar]
- 9.Dahlström A., Fuxe K. 1964. Localization of monoamines in the lower brain stem. Experientia 20, 398–399 10.1007/BF02147990 (doi:10.1007/BF02147990) [DOI] [PubMed] [Google Scholar]
- 10.Steinbusch H. W. 1981. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6, 557–618 10.1016/0306-4522(81)90146-9 (doi:10.1016/0306-4522(81)90146-9) [DOI] [PubMed] [Google Scholar]
- 11.Abrams J. K., Johnson P. L., Hollis J. H., Lowry C. A. 2004. Anatomic and functional topography of the dorsal raphe nucleus. Ann. NY Acad. Sci. 1018, 46–57 10.1196/annals.1296.005 (doi:10.1196/annals.1296.005) [DOI] [PubMed] [Google Scholar]
- 12.Hale M. W., Lowry C. A. 2011. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology 213, 243–264 10.1007/s00213-010-2089-z (doi:10.1007/s00213-010-2089-z) [DOI] [PubMed] [Google Scholar]
- 13.Gutknecht L., Kriegebaum C., Waider J., Schmitt A., Lesch K. P. 2009. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur. Neuropsychopharmacol. 19, 266–282 10.1016/j.euroneuro.2008.12.005 (doi:10.1016/j.euroneuro.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 14.Alenina N., et al. 2009. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl Acad. Sci. USA 106, 10 332–10 337 10.1073/pnas.0810793106 (doi:10.1073/pnas.0810793106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K., et al. 2006. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. J. Neurosci. 26, 530–534 10.1523/JNEUROSCI.1835-05.2006 (doi:10.1523/JNEUROSCI.1835-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson J. D., Schafer M. K., Bonner T. I., Eiden L. E., Weihe E. 1996. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc. Natl Acad. Sci. USA 93, 5166–5171 10.1073/pnas.93.10.5166 (doi:10.1073/pnas.93.10.5166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih J. C., Wu J. B., Chen K. 2011. Transcriptional regulation and multiple functions of MAO genes. J. Neural Transm. 118, 979–986 10.1007/s00702-010-0562-9 (doi:10.1007/s00702-010-0562-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitalis T., Alvarez C., Chen K., Shih J. C., Gaspar P., Cases O. 2003. Developmental expression pattern of monoamine oxidases in sensory organs and neural crest derivatives. J. Comp. Neurol. 464, 392–403 10.1002/cne.10804 (doi:10.1002/cne.10804) [DOI] [PubMed] [Google Scholar]
- 19.Lillesaar C. 2011. The serotonergic system in fish. J. Chem. Neuroanat. 41, 294–308 10.1016/j.jchemneu.2011.05.009 (doi:10.1016/j.jchemneu.2011.05.009) [DOI] [PubMed] [Google Scholar]
- 20.Furutani-Seiki M., Wittbrodt J. 2004. Medaka and zebrafish, an evolutionary twin study. Mech. Dev. 121, 629–637 10.1016/j.mod.2004.05.010 (doi:10.1016/j.mod.2004.05.010) [DOI] [PubMed] [Google Scholar]
- 21.Meyer A., Schartl M. 1999. Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr. Opin. Cell Biol. 11, 699–704 10.1016/S0955-0674(99)00039-3 (doi:10.1016/S0955-0674(99)00039-3) [DOI] [PubMed] [Google Scholar]
- 22.Teraoka H., et al. 2004. Hedgehog and Fgf signaling pathways regulate the development of tphR-expressing serotonergic raphe neurons in zebrafish embryos. J. Neurobiol. 60, 275–288 10.1002/neu.20023 (doi:10.1002/neu.20023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellipanni G., Rink E., Bally-Cuif L. 2002. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Mech. Dev. 119(Suppl. 1), S215–S220 10.1016/S0925-4773(03)00119-9 (doi:10.1016/S0925-4773(03)00119-9) [DOI] [PubMed] [Google Scholar]
- 24.Norton W. H., Folchert A., Bally-Cuif L. 2008. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J. Comp. Neurol. 511, 521–542 10.1002/cne.21831 (doi:10.1002/cne.21831) [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Takai R., Yoshioka H., Shirabe K. 2006. Characterization and expression of serotonin transporter genes in zebrafish. Tohoku J. Exp. Med. 208, 717–774 10.1620/tjem.208.267 (doi:10.1620/tjem.208.267) [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto K., Vernier P. 2011. The evolution of dopamine systems in chordates. Front. Neuroanat. 5, 2–21 10.3389/fnana.2011.00021 (doi:10.3389/fnana.2011.00021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaslin J., Panula P. 2001. Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio). J. Comp. Neurol. 440, 342–377 10.1002/cne.1390 (doi:10.1002/cne.1390) [DOI] [PubMed] [Google Scholar]
- 28.Anichtchik O., Sallinen V., Peitsaro N., Panula P. 2006. Distinct structure and activity of monoamine oxidase in the brain of zebrafish (Danio rerio). J. Comp. Neurol. 498, 593–610 10.1002/cne.21057 (doi:10.1002/cne.21057) [DOI] [PubMed] [Google Scholar]
- 29.Filippi A., Mahler J., Schweitzer J., Driever W. 2010. Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J. Comp. Neurol. 518, 423–438 10.1002/cne.22213 (doi:10.1002/cne.22213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallinen V., Sundvik M., Reenila I., Peitsaro N., Khrustalyov D., Anichtchik O., Toleikyte G., Kaslin J., Panula P. 2009. Hyperserotonergic phenotype after monoamine oxidase inhibition in larval zebrafish. J. Neurochem. 109, 403–415 10.1111/j.1471-4159.2009.05986.x (doi:10.1111/j.1471-4159.2009.05986.x) [DOI] [PubMed] [Google Scholar]
- 31.Lillesaar C., Tannhauser B., Stigloher C., Kremmer E., Bally-Cuif L. 2007. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev. Dyn. 236, 1072–1084 10.1002/dvdy.21095 (doi:10.1002/dvdy.21095) [DOI] [PubMed] [Google Scholar]
- 32.McLean D. L., Fetcho J. R. 2004. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J. Comp. Neurol. 480, 38–56 10.1002/cne.20280 (doi:10.1002/cne.20280) [DOI] [PubMed] [Google Scholar]
- 33.Flames N., Hobert O. 2011. Transcriptional control of the terminal fate of monoaminergic neurons. Annu. Rev. Neurosci. 34, 153–184 10.1146/annurev-neuro-061010-113824 (doi:10.1146/annurev-neuro-061010-113824) [DOI] [PubMed] [Google Scholar]
- 34.Hansson S. R., Mezey E., Hoffman B. J. 1999. Serotonin transporter messenger RNA expression in neural crest-derived structures and sensory pathways of the developing rat embryo. Neuroscience 89, 243–265 10.1016/S0306-4522(98)00281-4 (doi:10.1016/S0306-4522(98)00281-4) [DOI] [PubMed] [Google Scholar]
- 35.Jonz M. G., Fearon I. M., Nurse C. A. 2004. Neuroepithelial oxygen chemoreceptors of the zebrafish gill. J. Physiol. 560, 737–752 10.1113/jphysiol.2004.069294 (doi:10.1113/jphysiol.2004.069294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebrand C., Cases O., Adelbrecht C., Doye A., Alvarez C., El Mestikawy S., Seif I., Gaspar P. 1996. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron 17, 823–835 10.1016/S0896-6273(00)80215-9 (doi:10.1016/S0896-6273(00)80215-9) [DOI] [PubMed] [Google Scholar]
- 37.Lebrand C., Cases O., Wehrle R., Blakely R. D., Edwards R. H., Gaspar P. 1998. Transient developmental expression of monoamine transporters in the rodent forebrain. J. Comp. Neurol. 401, 506–524 (doi:10.1002/(SICI)1096-9861(19981130)401:4<506::AID-CNE5>3.0.CO;2-#) [DOI] [PubMed] [Google Scholar]
- 38.Hansson S. R., Mezey E., Hoffman B. J. 1998. Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience 83, 1185–1201 10.1016/S0306-4522(97)00444-2 (doi:10.1016/S0306-4522(97)00444-2) [DOI] [PubMed] [Google Scholar]
- 39.Cheng L., Chen C., Luo P., Tan M., Qiu M., Johnson R., Ma Q. 2003. Lmx1b, Pet-1, and Nkx2. 2 coordinately specify serotonergic neurotransmitter phenotype. J. Neurosci. 23, 9961–9967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narboux-Neme N., Pavone L. M., Avallone L., Zhuang X., Gaspar P. 2008. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs). Neuropharmacology 55, 994–1005 10.1016/j.neuropharm.2008.08.020 (doi:10.1016/j.neuropharm.2008.08.020) [DOI] [PubMed] [Google Scholar]
- 41.Homberg J. R., Schubert D., Gaspar P. 2010. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol. Sci. 31, 60–65 10.1016/j.tips.2009.11.003 (doi:10.1016/j.tips.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 42.Cordes S. P. 2005. Molecular genetics of the early development of hindbrain serotonergic neurons. Clin. Genet. 68, 487–494 10.1111/j.1399-0004.2005.00534.x (doi:10.1111/j.1399-0004.2005.00534.x) [DOI] [PubMed] [Google Scholar]
- 43.Jensen P., Farago A. F., Awatramani R. B., Scott M. M., Deneris E. S., Dymecki S. M. 2008. Redefining the serotonergic system by genetic lineage. Nat. Neurosci. 11, 417–419 10.1038/nn2050 (doi:10.1038/nn2050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiyasova V., Gaspar P. 2012. Serotonin raphe neurons from specification to axon guidance. Eur. J. Neurosci. 34, 1553–1563 10.1111/j.1460-9568.2011.07910.x (doi:10.1111/j.1460-9568.2011.07910.x) [DOI] [PubMed] [Google Scholar]
- 45.Descarries L., Watkins K. C., Garcia S., Beaudet A. 1982. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J. Comp. Neurol. 207, 239–254 10.1002/cne.902070305 (doi:10.1002/cne.902070305) [DOI] [PubMed] [Google Scholar]
- 46.Vertes R. P., Crane A. M. 1997. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J. Comp. Neurol. 378, 411–424 (doi:10.1002/(SICI)1096-9861(19970217)378:3<411::AID-CNE8> 3.0.CO;2-6) [DOI] [PubMed] [Google Scholar]
- 47.Fallon J. H., Loughlin S. E. 1982. Monoamine innervation of the forebrain: collateralization. Brain Res. Bull. 9, 295–307 10.1016/0361-9230(82)90143-5 (doi:10.1016/0361-9230(82)90143-5) [DOI] [PubMed] [Google Scholar]
- 48.Imai H., Steindler D. A., Kitai S. T. 1986. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J. Comp. Neurol. 243, 363–380 10.1002/cne.902430307 (doi:10.1002/cne.902430307) [DOI] [PubMed] [Google Scholar]
- 49.Vertes R. P., Fortin W. J., Crane A. M. 1999. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 407, 555–582 (doi:10.1002/(SICI)1096-9861(19990517)407:4<555::AID-CNE7>3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 50.Fuxe K., Dahlström A. B., Jonsson G., Marcellino D., Guescini M., Dam M., Manger P., Agnati L. 2010. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 90, 82–100 10.1016/j.pneurobio.2009.10.012 (doi:10.1016/j.pneurobio.2009.10.012) [DOI] [PubMed] [Google Scholar]
- 51.Lidov H., Molliver M. 1982. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res. Bull. 9, 559–604 10.1016/0361-9230(82)90164-2 (doi:10.1016/0361-9230(82)90164-2) [DOI] [PubMed] [Google Scholar]
- 52.Baker K. G., Halliday G. M., Tork I. 1990. Cytoarchitecture of the human dorsal raphe nucleus. J. Comp. Neurol. 301, 147–161 10.1002/cne.903010202 (doi:10.1002/cne.903010202) [DOI] [PubMed] [Google Scholar]
- 53.Meneghelli C., Rocha N. H., Mengatto V., Hoeller A. A., Santos T. S., Lino-de-Oliveira C., Marino-Neto J. 2009. Distribution of tryptophan hydroxylase-immunoreactive neurons in the brainstem and diencephalon of the pigeon (Columba livia). J. Chem. Neuroanat. 38, 34–46 10.1016/j.jchemneu.2009.03.007 (doi:10.1016/j.jchemneu.2009.03.007) [DOI] [PubMed] [Google Scholar]
- 54.Ekström P., Veen T. V. 1984. Distribution of 5-hydroxytryptamine (serotonin) in the brain of the teleost Gasterosteus aculeatus. J. Comp. Neurol. 226, 307–320 10.1002/cne.902260302 (doi:10.1002/cne.902260302) [DOI] [PubMed] [Google Scholar]
- 55.Lillesaar C., Stigloher C., Tannhäuser B., Wullimann M. F., Bally-Cuif L. 2009. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1expression. J. Comp. Neurol. 512, 158–182 10.1002/cne.21887 (doi:10.1002/cne.21887) [DOI] [PubMed] [Google Scholar]
- 56.Kosofsky B. E., Molliver M. E. 1987. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse 1, 153–168 10.1002/syn.890010204 (doi:10.1002/syn.890010204) [DOI] [PubMed] [Google Scholar]
- 57.Hornung J. P., Fritschy J. M., Tork I. 1990. Distribution of two morphologically distinct subsets of serotoninergic axons in the cerebral cortex of the marmoset. J. Comp. Neurol. 297, 165–181 10.1002/cne.902970202 (doi:10.1002/cne.902970202) [DOI] [PubMed] [Google Scholar]
- 58.Hornung J. P., Celio M. R. 1992. The selective innervation by serotoninergic axons of calbindin-containing interneurons in the neocortex and hippocampus of the marmoset. J. Comp. Neurol. 320, 457–467 10.1002/cne.903200404 (doi:10.1002/cne.903200404) [DOI] [PubMed] [Google Scholar]
- 59.Azmitia E. C., Gannon P. 1983. The ultrastructural localization of serotonin immunoreactivity in myelinated and unmyelinated axons within the medial forebrain bundle of rat and monkey. J. Neurosci. 3, 2083–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Honda T., Semba K. 1994. Serotonergic synaptic input to cholinergic neurons in the rat mesopontine tegmentum. Brain Res. 647, 299–306 10.1016/0006-8993(94)91329-3 (doi:10.1016/0006-8993(94)91329-3) [DOI] [PubMed] [Google Scholar]
- 61.Beaudet A., Descarries L. 1981. The fine structure of central serotonin neurons. J. Physiol. (Paris) 77, 193–203 [PubMed] [Google Scholar]
- 62.Descarries L., Riad M., Parent M. 2010. Ultrastructure of the serotonin innervation in the mammalian central nervous system. In Handbook of the behavioural neurobiology of serotonin (eds Müller C. P., Jacobs B. L.), pp. 65–101 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 63.Muller J. F., Mascagni F., McDonald A. J. 2007. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J. Comp. Neurol. 505, 314–335 10.1002/cne.21486 (doi:10.1002/cne.21486) [DOI] [PubMed] [Google Scholar]
- 64.Riad M., Garcia S., Watkins K. C., Jodoin N., Doucet E., Langlois X., el Mestikawy S., Hamon M., Descarries L. 2000. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 417, 181–194 (doi:10.1002/(SICI)1096-9861(20000207)417:2<181::AID-CNE4>3.0.CO;2-A) [DOI] [PubMed] [Google Scholar]
- 65.Cornea-Hébert V., Riad M., Wu C., Singh S. K., Descarries L. 1999. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J. Comp. Neurol. 409, 187–209 (doi:10.1002/(SICI)1096-9861(19990628)409:2<187::AID-CNE2>3.0.CO;2-P) [DOI] [PubMed] [Google Scholar]
- 66.Varga V., Losonczy A., Zemelman B., Borhegyi Z., Nyiri G., Domonkos A., Hangya B., Holderith N., Magee J., Freund T. 2009. Fast synaptic subcortical control of hippocampal circuits. Science 326, 449. 10.1126/science.1178307 (doi:10.1126/science.1178307) [DOI] [PubMed] [Google Scholar]
- 67.Becamel C., Gavarini S., Chanrion B., Alonso G., Galeotti N., Dumuis A., Bockaert J., Marin P. 2004. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J. Biol. Chem. 279, 20 257–20 266 10.1074/jbc.M312106200 (doi:10.1074/jbc.M312106200) [DOI] [PubMed] [Google Scholar]
- 68.Descarries L., Cornea-Hébert V., Riad M. 2006. Cellular and subcellular localization of serotonin receptors in the central nervous system. In The serotonin receptors: from molecular pharmacology to human therapeutics (ed. Roth B. L.), pp. 277–317 Totowa, NJ: Humana Press [Google Scholar]
- 69.Beck S. G., Pan Y.-Z., Akanawa A. C., Kirby L. G. 2004. Median and dorsal raphe neurons are not electrophysiologically identical. J. Neurophysiol. 91, 994–1005 10.1152/jn.00744.2003 (doi:10.1152/jn.00744.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trulson M. E., Frederickson C. J. 1987. A comparison of the electrophysiological and pharmacological properties of serotonin-containing neurons in the nucleus raphe dorsalis, raphe medianus and raphe pallidus recorded from mouse brain slices in vitro: role of autoreceptors. J. Neurophysiol. 18, 179–190 [DOI] [PubMed] [Google Scholar]
- 71.Calizo L., Akanwa A., Ma X., Pan Y. Z., Lemos J., Craige C., Heemstra L., Beck S. 2011. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61, 524–543 10.1016/j.neuropharm.2011.04.008 (doi:10.1016/j.neuropharm.2011.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crawford L. K., Craige C. P., Beck S. G. 2010. Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. J. Neurophysiol. 103, 2652–2663 10.1152/jn.01132.2009 (doi:10.1152/jn.01132.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urbain N., Creamer K., Debonnel G. 2006. Electrophysiological diversity of the dorsal raphe cells across the sleep–wake cycle of the rat. J. Physiol. 573, 679–695 10.1113/jphysiol.2006.108514 (doi:10.1113/jphysiol.2006.108514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kocsis B., Varga V., Dahan L., Sik A. 2006. Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc. Natl Acad. Sci. USA 2006, 1059–1064 10.1073/pnas.0508360103 (doi:10.1073/pnas.0508360103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spannuth B. M., Hale M. W., Evans A. K., Lukkes J. L., Campeau S., Lowry C. A. 2011. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience 179, 104–119 10.1016/j.neuroscience.2011.01.042 (doi:10.1016/j.neuroscience.2011.01.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gardner K. L., Thrivikraman K. V., Lightman S. L., Plotsky P. M., Lowry C. A. 2005. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience 136, 181–191 10.1016/j.neuroscience.2005.07.042 (doi:10.1016/j.neuroscience.2005.07.042) [DOI] [PubMed] [Google Scholar]
- 77.Peyron C., Petit J., Rampon C., Jouvet M., Luppi P. 1997. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82, 443–468 10.1016/S0306-4522(97)00268-6 (doi:10.1016/S0306-4522(97)00268-6) [DOI] [PubMed] [Google Scholar]
- 78.Hajos M., Richards C. D., Szekely A. D., Sharp T. 1998. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience 87, 95–108 10.1016/S0306-4522(98)00157-2 (doi:10.1016/S0306-4522(98)00157-2) [DOI] [PubMed] [Google Scholar]
- 79.Peyron C., Luppi P. H., Fort P., Rampon C., Jouvet M. 1996. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J. Comp. Neurol. 364, 402–413 (doi:10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- 80.Kim M. A., Lee H. S., Lee B. Y., Waterhouse B. D. 2004. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 1026, 56–67 10.1016/j.brainres.2004.08.022 (doi:10.1016/j.brainres.2004.08.022) [DOI] [PubMed] [Google Scholar]
- 81.Kachidian P., Poulat P., Marlier L., Privat A. 1991. Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. J. Neurosci. Res. 30, 521–530 10.1002/jnr.490300309 (doi:10.1002/jnr.490300309) [DOI] [PubMed] [Google Scholar]
- 82.Commons K., Connolley K., Valentino R. 2003. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 28, 206–215 10.1038/sj.npp.1300045 (doi:10.1038/sj.npp.1300045) [DOI] [PubMed] [Google Scholar]
- 83.Fu W., Le Maitre E., Fabre V., Bernard J. F., David Xu Z. Q., Hökfelt T. 2010. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J. Comp. Neurol. 518, 3464–3494 10.1002/cne.22407 (doi:10.1002/cne.22407) [DOI] [PubMed] [Google Scholar]
- 84.Day H. E., Greenwood B. N., Hammack S. E., Watkins L. R., Fleshner M., Maier S. F., Campeau S. 2004. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J. Comp. Neurol. 474, 364–378 10.1002/cne.20138 (doi:10.1002/cne.20138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herzog E., Gilchrist J., Gras C., Muzerelle A., Ravassard P., Giros B., Gaspar P., El Mestikawy S. 2004. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience 123, 983–1002 10.1016/j.neuroscience.2003.10.039 (doi:10.1016/j.neuroscience.2003.10.039) [DOI] [PubMed] [Google Scholar]
- 86.Hioki H., Nakamura H., Ma Y., Konno M., Hayakawa T., Nakamura K., Fujiyama F., Kaneko T. 2009. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J. Comp. Neurol. 518, 668–686 10.1002/cne.22237 (doi:10.1002/cne.22237) [DOI] [PubMed] [Google Scholar]
- 87.Gras C., Herzog E., Bellenchi G. C., Bernard V., Ravassard P., Pohl M., Gasnier B., Giros B., El Mestikawy S. 2002. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J. Neurosci. 22, 5442–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fremeau R. T., Jr, et al. 2002. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl Acad. Sci. USA 99, 14 488–14 493 10.1073/pnas.222546799 (doi:10.1073/pnas.222546799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hioki H., Fujiyama F., Nakamura K., Wu S., Matsuda W., Kaneko T. 2004. Chemically specific circuit composed of vesicular glutamate transporter 3-and preprotachykinin B-producing interneurons in the rat neocortex. Cereb. Cortex 14, 1266–1275 10.1093/cercor/bhh088 (doi:10.1093/cercor/bhh088) [DOI] [PubMed] [Google Scholar]
- 90.Kiyasova V., Fernandez S. P., Laine J., Stankovski L., Muzerelle A., Doly S., Gaspar P. 2011. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. 31, 2756–2768 10.1523/JNEUROSCI.4080-10.2011 (doi:10.1523/JNEUROSCI.4080-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shutoh F., Ina A., Yoshida S., Konno J., Hisano S. 2008. Two distinct subtypes of serotonergic fibers classified by co-expression with vesicular glutamate transporter 3 in rat forebrain. Neurosci. Lett. 432, 132–136 10.1016/j.neulet.2007.12.050 (doi:10.1016/j.neulet.2007.12.050) [DOI] [PubMed] [Google Scholar]
- 92.Amilhon B., et al. 2010. VGLUT3 (Vesicular Glutamate Transporter Type 3) contribution to the regulation of serotonergic transmission and anxiety. J. Neurosci. 30, 2198–2210 10.1523/JNEUROSCI.5196-09.2010 (doi:10.1523/JNEUROSCI.5196-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scott M. M., Deneris E. S. 2005. Making and breaking serotonin neurons and autism. Int. J. Dev. Neurosci. 23, 277–285 10.1016/j.ijdevneu.2004.05.012 (doi:10.1016/j.ijdevneu.2004.05.012) [DOI] [PubMed] [Google Scholar]
- 94.Hendricks T., et al. 2003. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233–247 10.1016/S0896-6273(02)01167-4 (doi:10.1016/S0896-6273(02)01167-4) [DOI] [PubMed] [Google Scholar]
- 95.Liu C., Maejima T., Wyler S. C., Casadesus G., Herlitze S., Deneris E. S. 2010. Pet-1 is required across different stages of life to regulate serotonergic function. Nat. Neurosci. 13, 1190–1198 10.1038/nn.2623 (doi:10.1038/nn.2623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song N. N., Xiu J. B., Huang Y., Chen J. Y., Zhang L., Gutknecht L., Lesch K. P., Li H., Ding Y. Q. 2011. Adult raphe-specific deletion of Lmx1b leads to central serotonin deficiency. PLoS ONE 6, e15998. 10.1371/journal.pone.0015998 (doi:10.1371/journal.pone.0015998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hendricks T., Francis N., Fyodorov D., Deneris E. 1999. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J. Neurosci. 19, 10 348–10 356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott M. M., et al. 2005. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc. Natl Acad. Sci. USA 102, 16 472–16 477 10.1073/pnas.0504510102 (doi:10.1073/pnas.0504510102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jackson J., Bland B. H., Antle M. C. 2009. Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse 63, 31–41 10.1002/syn.20581 (doi:10.1002/syn.20581) [DOI] [PubMed] [Google Scholar]
- 100.Cheng C. W., Yan C. H., Choy S. W., Hui M. N., Hui C. C., Cheng S. H. 2007. Zebrafish homologue irx1a is required for the differentiation of serotonergic neurons. Dev. Dyn. 236, 2661–2667 10.1002/dvdy.21272 (doi:10.1002/dvdy.21272) [DOI] [PubMed] [Google Scholar]
- 101.Norton W. H., et al. 2005. Monorail/Foxa2 regulates floorplate differentiation and specification of oligodendrocytes, serotonergic raphe neurones and cranial motoneurones. Development 132, 645–658 10.1242/dev.01611 (doi:10.1242/dev.01611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scholpp S., Wolf O., Brand M., Lumsden A. 2006. Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon. Development 133, 855–864 10.1242/dev.02248 (doi:10.1242/dev.02248) [DOI] [PubMed] [Google Scholar]
- 103.Wang X., Lee J. E., Dorsky R. I. 2009. Identification of Wnt-responsive cells in the zebrafish hypothalamus. Zebrafish 6, 49–58 10.1089/zeb.2008.0570 (doi:10.1089/zeb.2008.0570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walshe J., Mason I. 2003. Unique and combinatorial functions of Fgf3 and Fgf8 during zebrafish forebrain development. Development 130, 4337–4349 10.1242/dev.00660 (doi:10.1242/dev.00660) [DOI] [PubMed] [Google Scholar]
- 105.Kapsimali M., Caneparo L., Houart C., Wilson S. W. 2004. Inhibition of Wnt/Axin/beta-catenin pathway activity promotes ventral CNS midline tissue to adopt hypothalamic rather than floorplate identity. Development 131, 5923–5933 10.1242/dev.01453 (doi:10.1242/dev.01453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rink E., Guo S. 2004. The too few mutant selectively affects subgroups of monoaminergic neurons in the zebrafish forebrain. Neuroscience 127, 147–154 10.1016/j.neuroscience.2004.05.004 (doi:10.1016/j.neuroscience.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 107.Guo S., Yamaguchi Y., Schilbach S., Wada T., Lee J., Goddard A., French D., Handa H., Rosenthal A. 2000. A regulator of transcriptional elongation controls vertebrate neuronal development. Nature 408, 366–369 10.1038/35042590 (doi:10.1038/35042590) [DOI] [PubMed] [Google Scholar]
- 108.Bach-Mizrachi H., Underwood M. D., Tin A., Ellis S. P., Mann J. J., Arango V. 2008. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol. Psychiatry 13, 507–513 10.1038/sj.mp.4002143 (doi:10.1038/sj.mp.4002143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kinney H., Richerson G., Dymecki S., Darnall R., Nattie E. 2009. The brainstem and serotonin in the sudden infant death syndrome. Ann. Rev. Pathol. Mechan. Dis. 4, 517–550 10.1146/annurev.pathol.4.110807.092322 (doi:10.1146/annurev.pathol.4.110807.092322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wylie C. J., Hendricks T. J., Zhang B., Wang L., Lu P., Leahy P., Fox S., Maeno H., Deneris E. S. 2010. Distinct transcriptomes define rostral and caudal serotonin neurons. J. Neurosci. 30, 670–684 10.1523/JNEUROSCI.4656-09.2010 (doi:10.1523/JNEUROSCI.4656-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fernandez S. P., Gaspar P. 2012. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology 62, 144–154 10.1016/j.neuropharm.2011.08.049 (doi:10.1016/j.neuropharm.2011.08.049) [DOI] [PubMed] [Google Scholar]