Abstract
Aggression, which comprises multi-faceted traits ranging from negative emotionality to antisocial behaviour, is influenced by an interaction of biological, psychological and social variables. Failure in social adjustment, aggressiveness and violence represent the most detrimental long-term outcome of neurodevelopmental disorders. With the exception of brain-specific tryptophan hydroxylase-2 (Tph2), which generates serotonin (5-HT) in raphe neurons, the contribution of gene variation to aggression-related behaviour in genetically modified mouse models has been previously appraised (Lesch 2005 Novartis Found Symp. 268, 111–140; Lesch & Merschdorf 2000 Behav. Sci. Law 18, 581–604). Genetic inactivation of Tph2 function in mice led to the identification of phenotypic changes, ranging from growth retardation and late-onset obesity, to enhanced conditioned fear response, increased aggression and depression-like behaviour. This spectrum of consequences, which are amplified by stress-related epigenetic interactions, are attributable to deficient brain 5-HT synthesis during development and adulthood. Human data relating altered TPH2 function to personality traits of negative emotionality and neurodevelopmental disorders characterized by deficits in cognitive control and emotion regulation are based on genetic association and are therefore not as robust as the experimental mouse results. Mouse models in conjunction with approaches focusing on TPH2 variants in humans provide unexpected views of 5-HT's role in brain development and in disorders related to negative emotionality, aggression and antisocial behaviour.
Keywords: tryptophan hydroxylase (TPH2), cognition, emotion, impulsivity, aggression, violence
1. Introduction
Negative emotionality, aggression and antisociality are complex temperamental traits and social behaviours that arise out of multiple causes involving biological and psychological dynamisms and social forces, and different forms of emotional behaviour may each result from different biopsychosocial pathways. The societal implications of aggressiveness, which results in numerous facets of aggressive behaviour and ranges from the establishment of hierarchies and dominance to antisocial behaviour and delinquency, have been examined with preclinical and clinical frameworks. Developmentally inappropriate conduct, aggressiveness and failure in social adjustment represent the most detrimental and harmful long-term outcome of a wide spectrum of neurodevelopmental disorders characterized by deficits in cognitive control and emotion regulation.
In both humans and animals, the term aggression comprises a variety of behaviours that are heterogeneous for clinical phenomenology and neurobiological features. While the impact of complex cultural variables on behaviour impedes simple extrapolation of animal phenotypes to human traits, clinical observation, experimental paradigms in the laboratory and cluster/factor-analytic statistics have been used in attempts to subdivide aggression. On the basis of different approaches, human aggression may be differentiated into several subtypes depending on the presence or absence of causes or motivation, nature of trigger, characteristics of mediators, form of manifestation, direction and function (for review, see [1]). The dichotomy between an impulsive–reactive–hostile–affective subtype and a controlled–proactive–instrumental–predatory subtype has emerged as the most promising construct of qualitatively distinct subtypes of human aggression [2]. In animal models, violence is defined as a form of escalated aggressive behaviour that is expressed out of context and inhibitory control, with a loss of adaptive function in social communication [3,4]. Individual differences in the temperamental traits of impulsivity and aggressiveness, and the ultimate behavioural consequences (such as distinct types of aggression, violence and self-injurious behaviour, including suicidality and addiction) are relatively enduring and continuously distributed as well as substantially heritable, and therefore are likely to result from additive or non-additive interaction of multiple genetic variations with environmental influences. This possibility has encouraged many investigators to apply dimensional approaches to behavioural genetics [5].
The brain serotonin (5-hydroxytryptamine, 5-HT) system originates from the raphe of the mammalian brainstem, where serotonergic neurons are clustered into nine nuclei numbered B1–9 on a rostrocaudal axis [6,7]. These clusters are subdivided into a rostral and a caudal part, with the rostral subdivision comprising the caudal linear nucleus, the dorsal raphe nucleus (DR: B6, B7) and the median raphe nucleus (MnR: B9, B8 and B5). These groups of serotonergic neurons project primarily into the forebrain where they innervate virtually all regions (e.g. cerebral cortex, amygdala, hippocampus, basal ganglia, thalamus and hypothalamus), thus mediating perception, cognition, emotional states, circadian rhythms, food intake and reproduction. The caudal portion, which projects mainly to the spinal cord and cerebellum, consists of nuclei termed as raphe pallidus (B1), raphe obscurus (B2) and raphe magnus (B3). This subsystem is involved in motor activity, pain control and regulation of the autonomic nervous system.
Tph2 is the key enzyme in the synthesis of neuronal 5-HT [8–10] and catalyses the hydroxylation of tryptophan (Trp) to 5-hydroxytryptophan (5-HTP), which is directly transformed to 5-HT by the amino acid decarboxylase (AADC). Tph2 is specifically expressed in the serotonergic neurons of the brainstem raphe complex and is exclusively responsible for the 5-HT synthesis within the brain, whereas Tph1 is the peripheral isoform [11]. The gene encoding TPH2 is located on human chromosome 12q21.1 and was mapped to chromosome 10D1 in the mouse, respectively.
A wide spectrum of different human behavioural traits as well as neurodevelopmental and neuropsychiatric disorders have been linked to TPH2 variation. Reduced TPH2 expression and function resulting from common variants in the gene's transcriptional control region is associated with anxiety-, depression- and aggression-related personality traits and moderates emotion-related neurocircuitry in various species ranging from humans to non-human primates and rodents (for review, see [12]. Similarly, these regulatory and other structural variants (in the non-coding and coding regions, respectively) seem to have a role in neurodevelopmental and psychiatric conditions such as depression, bipolar disorder, suicide, anxiety disorders (especially obsessive–compulsive disorder), substance-use disorders and attention-deficit/hyperactivity disorder (ADHD). In addition, therapeutic responses and side effects following treatment with selective serotonin-reuptake inhibitors (SSRIs) and other compounds have been found to be associated with TPH2 variants [13]. Although many of these findings have been replicated, uncertainties remain about the biological foundation for the associations.
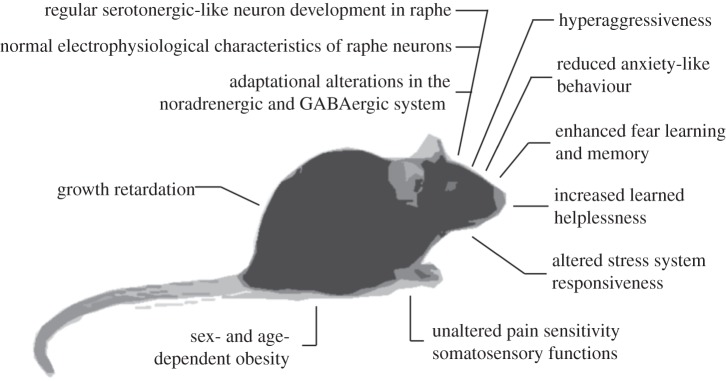
To explore the question of what traits or neuropsychiatric disorders are attributable to TPH2 dysfunction across the lifespan, mice with targeted inactivation of Tph2 were generated [14], revealing a remarkable phenotypic pleiotropy (figures 1 and 2; table 1).
Figure 1.
Overview of the major pleiotropic central and peripheral phenotypes in Tph2 knockout mice
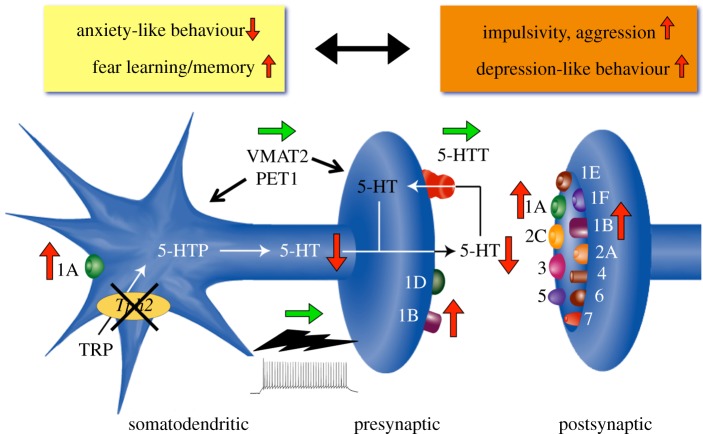
Figure 2.
Behavioural phenotype in Tph2 knockout mice in relation to adaptive changes at the 5-HT neuron level. 5-HTT, 5-HT transporter; VMAT2, vesicular monoamine transporter-2; PET1, 5-HT neuron-specific transcription factors; TRP, tryptophan; 5-HTP, 5-hydroxytryptamine; 1A, 5-HT1A receptor. Upward-facing arrows, increased; downward-facing arrows, decreased; right-facing arrows, unchanged.
Table 1.
Phenotypic changes in Tph2 knockout mice and other genetic models of brain 5-HT deficiency. Upward-facing arrows, increased; downward-facing arrows, decreased; right-facing arrows, unchanged. CMS, chronic mild stress; KO, knockout; cKO, conditional knockout; EPM, elevated plus-maze; FST, forced swim test; TST, tail suspension test; 5-Htt/Sert, 5-HT transporter; Vmat2, vesicular monoamine transporter-2; Pet1, Lmx1b, 5-HT neuron-specific transcription factors; NE, norepinephrine; DA, dopamine; GABA, gamma-butyric acid; Trp, tryptophan; 5-HTP, 5-hydroxytryptamine; 8-OH-DPAT, 5-HT1A receptor agonist; ht, hypothermic response.
| Tph2+/− | Tph2−/− | Tph2−/− and CMS | other mouse models of brain 5-HT deficiency: Lmx1b cKO, L;a Pet1 KO, P;b Tph2 R439H mutant, R/Hc | |
|---|---|---|---|---|
| growth/body weight, obesity | ↑ | ↓ | L→; P→ | |
| aggression (male) | ↑ | ↑↑ | P↑; R/H↑ | |
| anxiety-like behaviour (EPM) | → | ↓ | → | L↑; P↑→↓ |
| conditioned fear response | → | ↑ | ↑↑ | L↑; P↑ |
| depression-like behaviour (FST) | → | ↑ | → | P→; R/H↑TST |
| somatosensory sensitivity (tactile, thermal, pain) | → | → | ||
| mRNA expression of 5-HT neuron-specific marker | ||||
| 5-Htt/Sert | → | → | L↓; P↓ | |
| Vmat2 | → | → | L↓; P↓ | |
| Pet1 | → | → | L→ | |
| neurotransmitter concentration | ||||
| 5-HT/5-HIAA | ↓ | ↓↓↓ | L↓; R/H↓ | |
| NE | ↓ | ↓ | L→ | |
| DA | → | → | L→ | |
| monoaminergic neurons | ||||
| 5-HT | → | → | L↓; P↓; R/H↓ | |
| NE | → | ↓ | R/H→ | |
| DA | → | → | R/H→ | |
| GABAergic system (GABA concentration/GABAergic interneurons) | ||||
| frontal cortex | ↓ | → | ||
| hippocampus | →/→ | ↑/→ | ||
| amygdala | ↑/→ | →/↓ | ||
| 5-HT receptor expression/function | ||||
| 5-HT1A | → | ↑↑ | R/H→/↓ht | |
| 5-HT1B | → | ↑↑ | ||
| electrophysiology (5-HT neuron firing rate) | ||||
| baseline | → | → | ||
| Trp | ↓ | → | ||
| 5-HTP | ↓ | |||
| 8-OH-DPAT | ↓ | ↓ | ||
Alternative genetic-engineering tools were used in modifying brain 5-HT synthesis, which resulted in consequences that were largely consistent with those of studies on Tph2 knockout (KO) mice [16,17,19,21–23] (table 1). Many of these effects can now be understood on the basis of specific developmental, neurochemical, receptor signalling and other molecular consequences of Tph2 inactivation. In this overview, we describe the diversity of phenotypes in these mice and discuss the underlying mechanisms. We also provide some thoughts on the relevance of these observations to human neurobiology, behaviour and epigenetic interactions, as well as neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behaviour.
2. TPH2 in personality traits of negative emotionality and in disorders of cognitive control and emotion regulation
Human variants of TPH2 have been investigated for association with personality and behavioural traits as well as with various clinical cohorts characterized by emotion dysregulation. While traits of emotionality are persistent and continuously distributed dimensions of normal human personality, pathological manifestations of cognition and emotion regulation are ubiquitous in a wide spectrum of psychiatric conditions. Variance in personality traits, including those related to failure in cognitive control and emotion regulation, such as anxiety, depression and aggression, is thought to be generated by a complex interaction of environmental factors with a number of gene products involving brain structures and circuits such as the 5-HT system. Several single nucleotide polymorphisms (SNPs) in and downstream of the transcriptional control region of TPH2 showed association with personality traits as well as cluster B and cluster C personality disorders [24]. Cluster B comprises antisocial, borderline and narcissistic personality disorders (dramatic, emotional or erratic cluster), and cluster C consists of avoidant-, dependent- and obsessive-compulsive personality disorders (anxious or fearful cluster).
Functional magnetic resonance imaging (fMRI) provides evidence that acute tryptophan depletion, which results in a transient reduction in brain 5-HT (for review, see [25] and references therein), as well as a single, potentially functional, variant in the upstream regulatory region of TPH2, bias the responsiveness of the amygdala in a face-processing task involving assessment of angry and fearful faces [26,27], indicating that allelic variation of TPH2 function may contribute to individual variability in stress responsivity and anxiety in humans. Moreover, Tph2 polymorphisms predict brain serotonin synthesis in the orbitofrontal cortex in humans estimated in vivo using positron emission tomography and α-[11C]methyl-l-tryptophan trapping [28]. There is also emerging evidence from psychophysiological studies that TPH2 variation influences 5-HT synthesis in the brain and thus modulates emotional processing. Startle responses to intense noise bursts in individuals viewing pictures of negative, positive or neutral valence showed an interaction between TPH2 genotype, sex and age [29]. Two genes of the 5-HT signalling pathway, TPH2 and 5-HTT/SERT, encoding the 5-HT transporter were demonstrated to exert additive effects using event-related potentials for the early posterior negativity in a passive emotional picture perception task and fMRI in a complementary cognitive-affective task [30,31]. The additive effect in the MRI paradigm was more pronounced for visuospatial than for verbal stimuli, and more robust for negatively than for positively valenced stimuli, whereas fMRI effects were strong in the putamen, albeit also observed in the amygdala at a less stringent threshold, and in other cortical regions. These findings indicate an additive effect of two critical genes in the serotonergic regulation of neural processing of affective stimuli, and identify the putamen as a subdivision of the striatum as a critical site where interactive gene-by-gene regulation takes place. TPH2 variants were found to be associated with function of the prefrontal cortex during a response inhibition task in adult patients with ADHD, suggesting that deficient cognitive control involves a mechanism relevant to the pathophysiology of ADHD [32]. Taken together, these findings link potentially functional TPH2 variants to personality traits related to negative emotionality as well as to categorical cluster B and C personality disorders and confirm TPH2 as a susceptibility and/or modifier gene for disorders characterized by emotion dysregulation.
In line with this notion, SNP and haplotype analyses of TPH2 revealed evidence for association of TPH2 variants with depression, suicide and bipolar affective disorder, although inconsistent findings were also reported (for review, see [12]). Investigation of TPH2 expression in the brainstem of depressed patients who had committed suicide demonstrated increases in TPH2 mRNA [33,34] and protein [35–37] within the DR with evidence for specificity in distinct subdivisions. Increased TPH2 expression in depressed patients could result from both rare and frequent variants, their epistatic interaction among themselves (gene-by-gene interaction, G × G) and their interaction with early life experiences, acute stressful life events or chronic environmental adversity (gene-by-environment interaction, G × E), all of which can alter 5-HT neurotransmission and have been implicated in determining susceptibility to depression and a spectrum of co-morbid disorders, such as alcohol dependence [38].
Finally, allelic variation of TPH2 function appears to influence the risk of a variety of neuropsychiatric disorders such as ADHD and obsessive–compulsive disorder (OCD), clinical entities commonly associated with difficulties to control emotions and with a high co-morbidity of depression [39,40]. Transmission disequilibrium of potentially functional variants in the transcriptional control or in the coding region of TPH2 in ADHD [41–43], and preferential transmission of a haplotype of TPH2 in early-onset OCD [44] were reported. However, common variants in the TPH2 region did not seem to be associated with adult ADHD in a large European sample [45].
3. Tph2 mutant mice
(a). Behavioural phenotypes
Various approaches have been used to experimentally alter Tph2 expression and function in mice, including the constitutive Tph2 KO reviewed here [46] (figures 1 and 2; table 1). Commonly used inbred mouse strains were found to be homozygous for the Tph2 1473G allele (e.g. BALB/c; DBA/2), resulting in 40–70% reduction in 5-HT synthesis and a 40 per cent decrease in 5-HT concentrations in frontal cortex and striatum when compared with mice homozygous for the 1473C allele (e.g. C57Bl/6; 129S1/SvJ) [47]. The strains with a less-active version of Tph2 show lower aggression and increased anxiety-like behaviour [48]. In order to clarify that these behaviours are specifically mediated by the C1473G SNP and not by other strain-specific genetic background effects, the 1473G was crossed into mouse strains that naturally express the other Tph2 allele; however, this approach yielded contradictory results [49,50]. C57Bl/6 mice homozygous for the 1473G allele as well as BALB/c mice show reduced 5-HT synthesis rates, but 5-HT tissue concentrations remain unchanged, which indicates that altered 5-HT levels and behaviour in BALB/c mice are likely to be induced by other strain-dependent factors rather than by the Tph2 G1473C SNP. On the other hand, this finding suggests that the 5-HT system is able to compensate for reduced 5-HT synthesis, which is in line with the data derived from mice carrying the human TPH2 loss-of-function R439H mutation and displaying a 50 per cent reduction in extracellular 5-HT in various brain regions [19,20].
Mice with targeted inactivation of Pet1 [17] and Lmx1b [16,51], coding for transcription factors involved in the specification of 5-HT neurons, were also generated. Both represent modification functionally upstream of the specification process rather than a selective inactivation of neuronal 5-HT synthesis. In Pet1 KO mice, 5-HT deficiency is incomplete with approximately 30 per cent of the 5-HT neurons developing and persisting in various raphe nuclei. While mice with a constitutive Lmx1b inactivation are not viable, in conditional Lmx1b KO (cKO) mice, in which the gene deletion is driven specifically in 5-HT neurons, these neurons are generated but fail to differentiate and survive. In contrast, in Tph2−/− mice, raphe neurons and their projections, although devoid of Tph2 and 5-HT, are morphologically and functionally preserved [11,14].
(i). Impulsivity and aggression
Defensive aggression-like behaviour of a male resident towards male intruders is an ethologically determined response to territorial threats. Overwhelming evidence links 5-HT to impulsive and aggressive behaviour as the primary determinant of aggression control [1]. Several regions of the frontal and cingulate cortices, amygdala, septum, hypothalamus and periaqueductal grey matter are among the best documented to be involved in the neural circuitry of aggression. Serotonergic fibres extensively project to each of these regions and it is well established that both aggressiveness and increased impulsivity are associated with brain 5-HT deficiency. In the resident-intruder paradigm, Tph2−/− males exhibit up to 10-fold more defensive aggressive behaviour, particularly increased impulsivity reflected by decreased latency of the first attack, number of attacks and duration of fighting than controls [46]. Chronic unpredictable stress further aggravates these traits. The impulsive and hyperaggressive behaviour of Tph2−/− mice resembles the increased defensiveness reported for Pet1 KO [17] and Tph2 R439H mutants [19]. Acute treatment with 5-HT1A and 5-HT1B receptor agonists (or 5-HT2A/2C antagonists) via their inhibitory action on neurotransmission (pre-synaptically or post-synaptically) was reported to reduce aggressive behaviour, and it was suggested that low 5-HT levels in the brain are associated with maladaptive forms of excessive violence rather than with natural defensiveness [3]. Because 5-HT deficiency is likely to result in impaired inhibition of engagement and sustainment of aggressive behaviour, it may explain that Tph2−/− mice display exaggerated aggressive behaviour as a consequence of the failure of 5-HT-mediated inhibitory control, thus rendering these mice inept to acquire the abilities of social adjustment.
(ii). Anxiety-like behaviour and conditioned fear response
Functional variants in TPH2 are associated with anxiety-related, harm-avoidant and other personality traits of negative emotionality as well as with various clinical cohorts with neuropsychiatric disorders characterized by emotional dysregulation. Likewise, Tph2 KO mice exhibit altered anxiety-like and conditioned fear response-related behaviours in a sex-dependent mode. Although female Tph2−/− mice decreased anxiety-like behaviour on the elevated plus-maze (EPM), which tests conflict-based exploration of aversive environment, genotype effects were not significant in males. Sex differences have previously been observed in mouse models with a range of genetic lesions impacting 5-HT system development and function [1,52].
Similar to Tph2 null mutant mice, Lmx1b cKO mice exhibit reduced innate anxiety-like behaviour [15], whereas for Pet1 KO mice, anxiogenic as well as anxiolytic effects were reported [17,18,53]. Selective lesion studies of ascending serotonergic pathways by neurotoxins resulted in varying degrees of anxiolytic effects dependent on experimental condition and site of injection [54–57]. As an integral part of the neural circuitry of stress responsivity, the 5-HT system has generally been viewed to exert modulatory functions. In addition, the findings derived from mouse models deficient in brain 5-HT provide evidence that 5-HT also moderates neurobiological consequences of environmental adversity, enhances appraisal of threats as well as behavioural expression of innate anxiety and fear responses, thereby permanently encoding the impact of stressful experience.
Despite reduced innate anxiety-like behaviour, Tph2 KO mice display gene dose- and sex-dependent increases in fear acquisition and memory in both cue and context trials compared with controls [46]. After chronic mild stress (CMS) experience, Tph2−/− mice are insensitive to stress-induced increases in locomotor activity and resilient to stress-induced anxiety-like behaviour, but increases in fear responses are intensified. Lmx1b cKO and Pet1 KO mice also show a marked increase in freezing following fear learning [15,18]. These findings suggest that 5-HT is critical for the inhibition of exaggerated fear acquisition via neural circuits involving amygdala and connected structures such as hippocampus, medial prefrontal cortex (mPFC), hypothalamus, bed nucleus of the stria terminalis (BNST) and brainstem nuclei, including the raphe complex and locus coeruleus (LC) [58].
While anxiety and fear are assumed to be separate dimensions within a spatial continuum, it appears paradoxical only at first sight that central 5-HT deficiency leads to a dissociation of conditioned fear from innate anxiety. On the one hand, anxiety-like behaviour elicited by the EPM test corresponds to more diffuse and generalized anxiety in anticipation of potential distant danger (instinctive fear of predators) not yet identifiable and to which an escape exists by returning to the closed arm (conflict exploration versus risk). On the other hand, during the conditioning process, the animal has actually received an uncontrollable aversive stimulus and has to face instant threat from which no escape is possible. As these two experiences are distinct regarding the anxiogenic circumstances and the neural circuits involved, opposing effects of 5-HT on general anxiety and learned fear seem nevertheless plausible [59,60]. Involvement of different pathways is also indicated by differential pharmacological modulation [61]. An alternative model describing complementary effects of the central nucleus of the amygdala (CeA) and the BNST on potentiated fear (e.g. post-traumatic or panic disorder) and sustained non-associated fear (e.g. generalized anxiety disorder), respectively, was proposed, with low anxiety being associated with potentiated fear responses [62].
The amygdala is central to emotion processing and modulation of fear-related behaviour, ranging from innate anxiety to conditioned fear acquisition and retention [63]. The lateral amygdala (LA) is richly innervated by 5-HT fibres and serves as the perceptive interface, as it receives multi-modal, early sensory information from the thalamus and cortical regions [64,65] and, together with the basolateral nucleus of the amygdala (BLA), is the principal unit where fearful memory is generated and stored. Anxiety- and fear-related input is then processed towards specific downstream pathways to express appropriate behavioural responses. As an early step, the CeA is known to be the output unit for freezing behaviour in fear conditioning, while the BNST would be the effector station for sustained non-associative anxiety [62]. The LA is a cortex-like structure composed of projecting glutamatergic pyramidal cells and gamma-aminobutyric acid (GABA)ergic interneurons, which receive modulatory 5-HT projections [66] (also see §3d). Given the behavioural profile displayed by Tph2−/− mice, low innate anxiety but high fear-conditioning, it may be assumed that the basal activity or the encoding of fear-associated stimuli in amygdala are altered by 5-HT deficiency. Recording of spontaneous activity revealed hypoactivity of glutamatergic pyramidal neurons in Tph2−/− mice as an electrophysiological correlate of reduced innate anxiety-like behaviour, possibly mediated via a reduced activation of the BNST (Araragi et al. manuscript in preparation).
In contrast, evoked response following cortical fibre stimulation revealed increased efficiency of the cortical-amygdaloidal pathway in Tph2−/− mice, which appears to represent a neurophysiological correlate of their exaggerated fear learning and memory in fear conditioning via an over-activation of the CeA and its downstream neural circuit. Fear conditioning was previously shown to be a molecular process increasing synaptic efficacy on LA neuron dendrites as a result of input from cortical and/or thalamic fibres conveying the unconditioned (US) and conditioned stimulus (CS), commonly called long-term potentiation (LTP; reviewed in [67]). LTP in the LA appears to be a critical mechanism for storing memories of the association between the CS and US [68,69]. LTP formation may be enhanced when at least one of the involved pathways, thalamic or cortical, is more sensitive, as demonstrated in Tph2−/− mice. Together with previous reports [70], the view is supported that 5-HT deficiency within the LA leads to a reduced activation of GABAergic interneurons [71], which in turn results in insufficient inhibition of glutamatergic projecting neurons and failure to delimit exaggerated fear responses. Taken together, it may be concluded that 5-HT mediates distal aversive stimuli, but is not essential for the integration of proximal or physical aversive stimuli, thus differentially regulating distinct context- and neural-pathways-dependent forms of fear or anxiety.
(iii). Depression-like behaviour
Although depression-like behaviour is challenging to model in mice, the tail suspension test (TST) and the forced swim test (FST) are widely used to validate antidepressant drugs. Tph2−/− mice exhibited more behavioural despair as reflected by the onset and duration of immobility when facing a life-threatening inescapable situation, thus confirming the notion of a link between 5-HT deficiency and depression-like behaviour [46]. Contrasting results between FST and TST were reported for a different line of Tph2−/− mice [23], while Pet1 KO mice did not display more behavioural despair [53], and Tph2 R439H mutant mice showed increased immobility in the TST [19]. Because extreme 5-HT deficiency also leads to a reduction in brain norepinephrine (NE; see §3c), it is possible that the latter contributes to the observed phenotype. Remarkably, 5-HT deficiency-driven depression-like behaviour was reversed by CMS, resulting in an essentially rescued phenotype, whereas no effect of CMS was seen in controls. Although this possibility cannot be completely ruled out, it is rather unlikely that the increase in active struggle of Tph2−/− mice is merely due to a CMS-induced locomotor activation, higher impulsivity or cognitive flexibility, because other behavioural paradigms failed to provide evidence for an alteration of these traits. Taken together, these findings indicate that CMS rescues behavioural consequences of emotionality in 5-HT-deficient mice, thus increasing adaptive capacity, and thus resilience, to the deleterious effects of Tph2 inactivation.
Reduction of immobility in the FST following CMS is uncommon but has been observed in previous studies using for example, parachloroamphetamine (PCA)-induced partial lesioning of 5-HT fibres followed by chronic variable stress [72]. Moreover, work in rodents by other investigators demonstrated that the experience of controllable stress may improve coping during subsequent stressful episodes by activating the mPFC, which in turn inhibits DR-mediated adverse effects of stress [73–75]. While CMS is not typically controllable stress, its ‘real life’ quality for a mouse living in a hostile, predator-infested habitat may induce similar effects in conjunction with DR malfunction owing to the lack of 5-HT synthesis [76]. Alternatively, predictable CMS appears to improve mood by increasing adult neurogenesis [77]. Multiple adaptive mechanisms along various developmental trajectories are therefore likely to be operative in Tph2−/− mice to modify brain function and responses to environment adversity. From a clinical point of view, it appears rather counterintuitive that 5-HT deficiency at the same time results in anxiolytic effect and in depression-like behaviour. Nevertheless, corresponding models similar to those of the 5-HT1A KO mouse emulate the phenotype of Tph2−/− mice with increased anxiety and reduced depressive-like behaviour [78]. In depressed patients, depression is frequently accompanied by symptoms of anxiety, which are ameliorated by compounds targeting 5-HT (and NE) neurotransmission.
Anxiety disorders are frequently associated with co-morbid depression. However, ethologically relevant behaviour in mice and symptoms in depressed patients are far from being homologous. In humans, depression is complicated by conscious cognitive and emotional re-evaluation and by projections into the future, which can be anticipated as dark, hopeless and anxiety-provoking. Moreover, species-typical symptoms, such as guilt, suicidality, projection and introjection, cannot be modelled in rodents. In humans, cognitive appraisal modulates brain responses to emotional stimuli and carries the potential to counteract both genetic and environmental susceptibility factors. In this context, the regulatory role of the prefrontal cortex in controlling limbic structures is critical. Thus, a simplified, more manageable and versatile model such as the Tph2 KO mouse may help deciphering basic mechanisms and neuronal circuits involved in this dual role of 5-HT likely to be operant in humans as well. Provided that diagnostic tools allow distinction of different symptoms, the form of anxiety co-morbid with depression might be of different nature and aetiology than those of core anxiety disorders, such as generalized anxiety disorder, phobias, post-traumatic disorder and panic disorder. The dual 5-HT hypothesis further elaborated by Graeff and associates [79] describes distinct neural circuits emerging from the DR and MnR, respectively promoting and preventing anxiety and depression. While complex emotional states cannot be reduced to imbalance within a single neurotransmitter-specific circuitry, the lack of 5-HT in both DR and MnR in stress-naive Tph2−/− mice is in accordance with this dual model. Taken together, these findings suggest that it is clinically relevant to understand the neural circuitry and adaptive mechanisms that mediate the amelioration of depressive symptoms by mild stressors of an enriched environment.
(b). Specification of raphe neurons lacking 5-HT synthesis
Several in vitro studies showed a morphogenic effect of 5-HT on proliferation, differentiation, migration and survival of neural cells [80–82]. During ontogeny, 5-HT appears long before maturation of raphe serotonergic neurons, suggesting a fundamental role in embryonic and brain development. Whereas in vivo studies generally underscore this notion, conditional Lmx1b KO mice, which are largely deficient in central serotonergic neurons, are viable without apparent developmental abnormalities [16,51,83]. Likewise, neuroanatomical alterations were not observed in the brain of Tph2−/− mice (11,14). Conserved particularly is the expression of genes specifying a serotonergic phenotype in raphe neurons lacking 5-HT synthesis. The serotonin transporter (5-Htt/Sert) is present on the soma of raphe neurons as well as on their fibres and terminals in various projection areas, although they have lost the capacity to synthesize and release 5-HT. The 5-HT neuron-specific transcription factor Pet1, the vesicular monoamine transporter-2, as well as the somatodendritic autoreceptors 5-HT1A are expressed by raphe cells displaying a 5-HT neuron-like morphological phenotype.
In brainstem sections of Tph2−/− mice, the 5-HT-devoid neurons retain the slow, tonic pattern of firing typical of serotonergic neurons (1–2 spikes/s) demonstrating that endogenous 5-HT is not required to mediate these electrophysiological properties. Similarly, the neurons preserve the sensitivity of their pacemaker rhythm to the inhibitory effect of pharmacological 5-HT1A receptor activation. Moreover, these neurons lack sensitivity to the inhibitory effect of Trp, confirming complete loss of Tph functionality and 5-HT-synthesis capacity. When the Tph2-dependent rate-limiting step is bypassed by supplementation with the intermediary 5-HTP, the synthesis of 5-HT is re-established as reflected by inhibition of firing activity. Because 5-HTP is selectively taken up by serotonergic neurons in the DR and converted into 5-HT by AADC [84], serotonergic-like neurons of Tph2−/− mice are sensitive to endogenous 5-HT and their lack of response to Trp is not due to dysfunctional 5-HT1A autoreceptor signalling (also see §3e). In brainstem slices from Tph2+/− mice, serotonergic neurons responded to Trp as well as the 5-HT1A receptor agonist 8-OH-DPAT, with a decrease in firing rate similar to that observed in wild-type mice, suggesting that a gene dose-dependent reduction in 5-HT synthesis does not result in functional changes in the 5-HT system at baseline.
While genetic inactivation of the upstream transcription factors Lmx1b and Pet1 compromises the development of the majority of 5-HT neurons [16,17,51], it is concluded that intrinsic 5-HT production is neither essential for the development maintenance and survival of serotonergic neurons, nor for the molecular specification of a serotonergic-like phenotype. This further suggests that the developmental role of 5-HT in the maturation/differentiation of the 5-HT system itself has been overvalued, and the notion of an autoregulation of 5-HT system development [6,7] may need to be addressed from a new standpoint. It remains to be elucidated in detail whether subtle impairment in neurite outgrowth, axonal guidance/target finding and altered dendritic arborization occurs and whether these neurons use neuropeptides and/or other monoamines with low affinity for the 5-Htt/Sert as physiological or ‘borrowed’ neurotransmitters in establishing function and connectivity. Together, functional serotonergic-like neurons in Tph2 KO mice have lost the capacity to synthesize 5-HT from Trp but not from 5-HTP, which in vivo may originate from peripheral sources and could contribute to the remaining traces of 5-HT in the brain of Tph2−/− mice.
(c). Monoamine transmitters and neurons
In Tph2−/− mutants, 5-HT concentrations are markedly reduced across all brain regions and virtually absent from the serotonergic neuron-containing raphe region, with only traces detectable by HPLC, thus verifying that 5-HT synthesis within neurons depends on the activity of the Tph2 isoform [46]. While perfusion of brain with removal of the residual blood in capillaries resulted in minimal amounts of 5-HT in the rostral raphe region at the lower detection limit (less than 1.2%), it remains possible that a small number of blood components with high 5-HT content, such as platelets or mastocytes, remain trapped in capillaries of brain tissue [14]. Very low brain 5-HT levels were also detected in other Tph2−/− mice [21] as well as in Tph1/Tph2−/− double KO mice [23]. In addition, we previously showed that Tph1 is not upregulated in Tph2−/− brain, indicating that Tph1-driven 5-HT synthesis can be ruled out in the brain [11]. However, there are several alternative explanations: (i) HPLC does not detect 5-HT but a closely related compound with the same retention time, a possibility to be resolved by mass spectrometry, (ii) the immediate 5-HT precursor (5-HTP) produced by peripheral Tph1 crosses the blood-brain barrier and could be transformed into 5-HT because AADC is ubiquitously expressed, (iii) other enzymes, such as phenylalanine hydroxylase, or as yet unknown enzymes, use tryptophan as substrate and produce 5-HT, and (iv) alternative metabolic pathways are able to produce 5-HT as end- or by-product. Of note, 5-hydroxindoleacetic acid (5-HIAA) is more reduced or even undetectable than 5-HT itself, suggesting that either the metabolic pathway of 5-HT is inhibited, with monoamine oxidase A (MAOA) activity specifically downregulated in 5-HT neurons, or the 5-HT-like traces do not represent 5-HT but another compound degraded via another pathway. Taken together, the deficiency in 5-HT is so extreme that complete dysruption of 5-HT neurotransmission in Tph2−/− brain is likely despite the presence of neurons with 5-HT cell-like specification.
While dopamine concentrations are reduced only in hippocampus, 5-HT deficiency is accompanied by a consistent reduction in NE across brain regions. Furthermore, Tph2−/− mice exhibited a reduced number of tyrosine hydoxylase (TH)-expressing cells in the LC, which can partly explain the lower NE content in its projection areas using unbiased stereological assessment [46]. The LC is extensively innervated by Sert-positive fibres containing 5-HT in controls and devoid of 5-HT in Tph2−/− mice. It is conceivable that the absence of trophic effect of 5-HT in Tph2−/− mice impacts development or survival of NE-specific neurons. Alternatively, the absence of 5-HT release may inhibit expression and activity of TH in NE neurons, presumably by indirect input from inhibitory GABAergic or excitatory glutamatergic neurons (also see §3d), expression and activity of TH in NE neurons. Several studies reported that chronic treatment with the SSRI fluoxetine induces an increase in TH gene expression in the LC [85,86]. Conversely, 5-HT deficiency may thus downregulate TH activity in the LC, eventually reducing NE biosynthesis.
TH is also present along NE fibres projecting towards target areas and regulation terminally is likely because the DR does not seem to exert a direct inhibitory influence on the release of NE in the LC [87]. While 5-HT and NE fibres with synaptic varicosities co-localize in forebrain regions, a feedback loop, involving alpha2-adrenergic receptors on 5-HT fibres and 5-HT3 receptors on NE fibres, allows a reciprocal regulation of the release of both neurotransmitters by which 5-HT3 receptors stimulate the synaptic release of NE [88]. The stimulation of the neurotransmitter release is accompanied by an activation of its synthesis, whereas the lack of stimulating effect by 5-HT on NE fibres reduces TH activity and thus NE synthesis. Overall, the findings confirm that monoaminergic systems are interdependent and subject to concomitant regulation in behaviour and psychopathology.
(d). Gamma-aminobutyric acid and interneurons
Morphogenic effects of 5-HT impact migration, differentiation and survival of GABAergic interneurons [80–82] and 5-HT influences GABAergic cell migration via 5-HT6 receptors during late embryonic stages [89], thus assisting their integration into cortical networks [90]. The BLA is fundamentally involved in the regulation of fear and anxiety [91] and is densely innervated by serotonergic fibres from the DR nucleus [92]. Within the BLA, parvalbumine (PV)/GABAergic neurons specifically express 5-HT2A receptors [93] and tightly control glutamatergic output neurons by perisomatic inhibition [94], whereas 5-HT2C receptors are expressed on other interneuron subtypes [95]. Furthermore, anxiogenic compounds have been shown to recruit GABAergic interneurons, including the PV-specific subpopulation in the BLA probably via serotonergic input from the DR [96]. The dorsal hippocampus was shown to be critically involved in context-dependent learning processes [97–99]. While the dorsal and ventral hippocampus are interconnected, they represent functionally separate subdivisions integrated in different neuronal networks, thus mediating diverse behaviours [100]. Furthermore, the distribution of different interneuron subtypes (including the PV-specific population) was shown to be distinct for both subregions of the hippocampus [101].
Elimination of 5-HT synthesis does not appear to affect GABA concentrations in whole-brain tissue [21], but measurement in different brain regions of Tph2 KO mice in combination with unbiased stereological assessment of GABAergic cell subpopulations in the hippocampus and amygdala revealed differential alterations [102]. While hippocampal GABA concentrations were increased in Tph2−/− mice, GABA was increased in heterozygous Tph2+/− mice in the amygdala compared with Tph2−/− and wild-type control mice but opposite in prefrontal cortex. This was accompanied by altered cell density of GABAergic interneurons within the BLA and of PV-specific GABAergic interneurons in the CA3 region of the dorsal hippocampus.
Increased GABAergic transmission in the BLA has been associated with reduced anxiety-related behaviour [103], whereas mice deficient for GAD65 display 50 per cent reduced GABA concentrations in the amygdala and exhibit an anxiety-like phenotype [104]. In contrast, increased GABA transmission was shown in a mouse model of increased trait anxiety [105]. Tph2−/− mice exhibited an altered anxiety-related phenotype with a dissociation of innate anxiety-like behaviour and conditioned fear responses but unchanged GABA concentrations. In contrast, Tph2+/− mice showed an intermediate behavioural phenotype compared with Tph2−/− and wild-type animals. This may be due to a counterbalancing effect of an impaired inhibition of the PFC indicated by reduced GABA concentrations in Tph2+/− and of glutamate concentrations specifically increased in PFC of Tph2−/− mice. Altered function of the PFC controlling other subcortical structures of the limbic system [106]—possibly as a consequence of increased activation of intercalated neurons residing at the boundary of the BLA to the central nucleus of the amygdala [107]—is likely to result in an increase in the frequency of inhibitory post-synaptic potentials in BLA output neurons. Recently, mice expressing the R439H TPH2 form were found to display increased cortical 5-HT2A receptor expression due to diminished concentrations of 5-HT [20]. Tph2+/− mice possess 20–30% reduced 5-HT concentrations in the rostral raphe but unaffected frontal cortex 5-HT concentrations. Distinct 5-HT receptor expression and activation by pyramidal cells and interneuron subtypes may lead to a disturbed control of network activity through altered gamma oscillations [108]. Therefore, elevated GABA concentrations in the Tph2+/− mice may either be directly triggered by the impact of 5-HT deficiency on network activity or represent a consequence of compensatory mechanisms during development increasing expression and activation of 5-HT1A and 5-HT2A/C receptors. Reduced 5-HT concentrations in Tph2+/− mice seem to be sufficient to develop normal numbers of interneurons within the BLA.
In Tph2−/− mice, the overall number and density of GABAergic interneurons within the BLA were decreased [102]. Possibly as an outcome of impaired proliferation, this may represent a mechanism to cope with an imbalance of GABAergic transmission during ontogeny, and to maintain synchronous oscillatory activity, which has been shown to be important for fear learning and memory [94,109]. As PV-specific neurons were unaffected, other interneuron subpopulations might account for the decreased density of interneurons [110] and altered GABA concentrations [111]. However, because total cell numbers in the BLA remain unchanged, other populations such as glial cells or glutamatergic neurons within the BLA may be increased to account for unaffected total cell numbers. Tph2−/− mice also displayed elevated concentrations of GABA in the hippocampus, with a trend towards reduced PV-specific neuron numbers in the CA3 region of the dorsal hippocampus, whereas volume, total number and density of interneurons remain unaffected. Selective activation of MnR 5-HT neurons has been reported to directly activate dorsal hippocampal interneurons [112], leading to an overall inhibition of the hippocampal formation. Dense innervations of the MnR originating beaded serotonergic axons with large spherical varicosities and fine DR axons can be found in the dorsal hippocampus [113,114]. Serotonergic fibres innervate different subpopulations of interneurons in the hippocampus acting in concert with cholinergic fibres in regulating the hippocampal processing of information [115]. GABAergic and PV-specific fast-spiking interneurons have been shown to be important for synchronous oscillatory activity of the hippocampus, which correlates with behaviour and is important for synaptic plasticity [116–118]. Therefore, reduced densities of PV-specific interneurons may represent a plausible mechanism to compensate for altered hippocampal GABA metabolism and/or disturbed synchronous oscillatory activity induced by a lack of 5-HT during development and adulthood. On the basis of the involvement of the dorsal subdivision of the hippocampus in learning and memory processes, these findings may reflect increased conditioned fear responses in Tph2−/− mice and lead to a better insight into the mechanism of how early-life 5-HT deficiency impacts the development of anxiety-related disorders.
(e). Adaptive 5-HT receptor regulation
The density of 5-HT1A and 5-HT1B receptors and their coupling G-proteins are increased across several brain regions of male Tph2−/− mice, particularly in terminal fields of the frontal cortex and septum employing quantitative autoradiography and stimulated [35S]GTP-γ-S binding [46]. This finding can be explained by two mechanisms, which may be operational independently or act in concert. The opposite phenomenon was observed in mouse models characterized by robust increases in extracellular 5-HT in the brain such as monoamine oxidase A (Maoa) null mutant mice where 5-HT1A and 5-HT1B receptors are desensitized and downregulated [119,120] and, to a lesser extent in a brain-region-specific manner, in 5-Htt KO mice [121]. Moreover, 5-HT1A receptors are downregulated in patients with depression and anxiety disorders as well as during SSRI treatment [122–124]. Sensitization and upregulation of 5-HT1A and 5-HT1B receptors in 5-HT-deficient mice may therefore be due to a direct cellular mechanism compensating for reduced 5-HT ligand availability by an increased Htr1a and Htr1b gene expression. An alternative explanation rests on evidence for a reciprocal regulation of hypothalamic-pituitary-adrenal (HPA) axis activity and 5-HT1A receptor function. Studies in animal models demonstrated that chronic stress-induced corticosterone secretion results in a downregulation of 5-HT1A [125,126] mediated by transcriptional repression of the Htr1a gene promoter via differential activation of intranuclear glucocorticoid and mineralocorticoid receptors [127–130]. Taken together, these data suggest that this regulatory loop also relates the low corticosterone levels to increased 5-HT1A expression in Tph2−/− mice: either low corticosterone level induces the expression of 5-Htr1a or 5-HT deficiency leads to increased expression, which is resistant to repression due to low corticosterone levels. Finally, although our electrophysiological data confirm that 5-HT1A are functional in the raphe as autoreceptors and thus probably also in the other brain regions as heteroreceptors, it remains to be elucidated whether the 5-HT traces remaining in regions where 5-HT1A is upregulated, such as the hippocampus, is sufficient to hyperpolarize neural cells and mediate serotonergic signalling. This possibility is however not supported by the increased aggressive behaviour observed in Tph2−/− males (see §3a).
(f). Growth, body weight and obesity across the lifespan
5-HT is implicated in the regulation of metabolic pathways influencing somatic growth, food intake and body weight. The overall life expectancy was not reduced by central 5-HT deficiency. While reduced weight gain was observed in Tph2−/− females during the first 24 weeks, this growth retardation persisted in male Tph2−/− mice throughout the lifespan. Hypomorphism was already observed during the early developmental period. This reduction in body weight may result from altered regulation at different levels, including reduced food intake, implicating impaired perception of energy needs and satiety, increased metabolic activity and energy expenditure or lower storage, implicating dysregulated glucose turnover, lipid and protein metabolic cycles or altered thermoregulation. Beyond the age of six months, an obesity phenotype emerges in female heterozygous Tph2+/− mice and becomes more exaggerated throughout life, with accumulation of fat tissue stored in the abdominal and pericardial cavity.
Following Tph2 inactivation, growth retardation but normalization after weaning (with normal weight four months of age) was also observed by Alenina et al. [21]. In contrast, it was reported that Tph2−/− mice display a reduced fat pad and size and that, at one and a half and three months, they display both reduced food intake and increased metabolism linked to altered leptin regulation [131]. These observations are unexpected in the face of reports that 5-HT or drugs increasing its release are anorexigenic via hypothalamic actions [132], reduced meal size [132], decreased body weight [133,134] and increased energy expenditure [135]. While the low body weight in Tph2−/− mice contrasts data and conclusions from other investigators, the observation of age-related obesity, as reflected by excess fat storage particularly in Tph2+/− females exhibiting reduced brain 5-HT, concurs. 5-HT transporter null mutant mice (5-Htt/Sert−/−), which display increased synaptic 5-HT but a reduced synthesis and total 5-HT brain concentrations in the face of decreased locomotor activity, also develop obesity and type 2 diabetes in adulthood, on the basis of a metabolic syndrome with insulin resistance [121]. Taken together, the gene dose- and sex-dependent divergence of body weight and fat storage in Tph2 KO mice supports the notion of a nonlinear dual effect of central 5-HT on somatic development, long-term body weight regulation and metabolic homeostasis via different pathways and endocrine systems.
4. TPH2, hypothalamic–pituitary–adrenal system and environmental adversity
Although converging evidence links exposure to stressful life events with increased risk for disorders of emotion regulation, there is significant individual variability in vulnerability to environmental cues, and the environmentally moderated penetrance of genetic variation is thought to play a major role in determining who will either develop disease or will remain resilient to it [38]. Research on genetic factors in the aetiology of these disorders has been complicated by a mysterious discrepancy between high heritability estimates and a scarcity of replicable gene-disorder associations. One explanation for this incongruity is that at least some specific gene effects are conditional on environmental cues, i.e. G × E interaction is present. Numerous studies in rodents reported that environmental adversity including early-life experience (e.g. prenatal stress, maternal neglect/separation) and psychosocial stress throughout the life cycle (e.g. subordinate rank, repeated social defeat) have persistent effects across the lifespan on 5-HT and its metabolite 5-HIAA, as well as on 5-HT receptor subtype expression and function in specific brain regions [136–139]. Studies of G × E interaction using non-human primates and genetically modified mice suggest that particularly adverse early-life experience impact sensitivity to stress-induced alterations in serotonergic neurotransmission later in life [38].
Early work already pointed towards environmental adversity as an important determinant of Tph expression and serotonergic neurotransmission. Acute stress increases Tph (presumably Tph2 isoform) activity in the DR [140] and a stress-induced rise in activity due to phosphorylation of the enzyme has been observed [141]. Inescapable randomly presented sound stress resulted in a transient phosphorylation-dependent rise in enzymatic activity of Tph in the MnR nucleus, whereas chronic sound stress has been shown to induce sustained and phosphatase-resistant increases in Tph activity, providing initial evidence that increases in Tph activity following chronic stress are mediated by increased Tph expression [142]. Repeated immobilization stress leads to increased Tph mRNA and protein concentrations in the DR and MnR nuclei [143]. While stress-mediated changes in Tph mRNA expression following immobilization stress are insensitive to adrenalectomy, chronic dexamethasone treatment of adrenalectomized female and male rats increases Tph mRNA in the pineal gland and decreases Tph mRNA expression in the midbrain raphe complex [144–146].
While it has been reported that Tph2 mRNA is downregulated by synthetic glucocorticoids and modulation of Tph2 expression by long-term antidepressant treatment is dependent on the glucocorticoid status or acute stressors in the murine DR [141,146–150], studies suggest that Tph1 mRNA (which may be present in the DR in extremely small quantities) and Tph protein but not Tph2 mRNA are upregulated by stress, suggesting resistance of the Tph2 isoform expression to stressful stimuli and apparent compensation by Tph1 isoform upregulation [151]. Another meticulously executed study investigated the differential pattern of isoform-specific expression showing that restraint stress for one week induced a 2.5-fold upregulation of Tph1 mRNA in DR with no change in two alternatively spliced Tph2 mRNA species. Therefore, it seems that stress-related mechanisms carry the potential to alter Tph1 and Tph2 mRNA expression, but the increases in Tph2 function may be dependent on the developmental period (e.g. prenatal, adolescence, adulthood), nature and intensity of the cue (e.g. acute, repetitive, chronic), the time course following exposure or context of stressful experience. The recent discovery of alternative splicing in conjunction with RNA editing in the coding region of TPH2 in humans but not in mice adds another level of complexity and demands careful re-examination of previously reported expression data [152].
Although few studies have evaluated the effects of stress-related stimuli on the patterns of gene expression in morpho-functional detail, initial studies support the hypothesis that stress-related stimuli may differentially alter patterns of Tph2 expression in specific subdivisions of the raphe complex, including the DR nucleus, which comprises clusters of neurons with unique cytoarchitectonic characteristics and gene expression patterns. Chronic infusions of the stress- and anxiety-related peptide CRF increased the ratio of Tph2 mRNA expression in the central core region of the dorsal part of the DR, which, among others, extends serotonergic terminals to both the central and basolateral amygdala as well as the medial PFC, whereas Sert mRNA expression was decreased in the midrostrocaudal part of the DR nucleus, which contains many amygdala-projecting neurons [145,153]. In a model of maladaptive stress responsivity, mice deficient in CRF receptor-2 failed to show robust stress-mediated adaptations, including elevations in Tph2 expression and increases in anti-apoptotic factors [154]. Emerging evidence that different subsets of serotonergic neurons project to neural cicuits, which process cognition and emotion and thus integrate physiological responses to environmental cues, will lead to a better understanding of the functional characteristics of specific 5-HT signalling subsystems underlying the pathophysiology of disorders of emotion regulation.
Nevertheless, a role of epigenetic programming in the regulation of Tph2 mRNA expression in specific subdivisions of the rat DR appears likely, and preliminary studies looking at G × E interaction in the non-human primate model have started to provide a useful insight into the neurobiological underpinnings of enhanced Tph2 mRNA and protein expression described in patients with depression, the regional specificity of these effects and their mechanistic consequences. In rhesus monkeys, SNP variants and related haplotypes in both the gene's 5′-flanking transcriptional control region and 3′-UTR with profound in vitro effects on Tph2 expression were demonstrated to influence central 5-HT turnover, HPA axis function and self-injurious behaviour [155,156]. Moreover, investigation of genetic and environmental effects at the Tph2 locus in rhesus monkeys revealed that the functional A2051C polymorphism in rhTph2 is associated with CSF 5-HIAA concentrations, morning plasma cortisol levels and cortisol response to ACTH challenge, whereas the effects on the afternoon cortisol level, plasma ACTH level, dexamethasone suppression of urinary cortisol excretion and aggressive behaviours were dependent on adverse rearing experience.
The neural and molecular mechanisms by which environmental adversity in early life moderates 5-HT system function and thus increases disease risk in adulthood is not known, but may include epigenetic programming of gene expression during (brain) development, which can either be disruptive (maladaptive) or (neuro)plastic in terms of instantly or predictively adaptive [38]. These molecular mechanisms and associated epigenetic markers, such as genome-wide gene expression, DNA methylation, and chromatin modification profiles, are dynamic and reversible and may also provide powerful targets for intervention strategies. Therefore, more insights into the exact role of epigenetic regulation in the process of neurodevelopmental programming contributes to the establishment of early diagnosis and the design of innovative treatments targeting mechanisms of resilience. Together, the results from non-human primate and mouse studies support the G × E interaction hypothesis [38] by showing that allelic variation of Tph2 function is associated with a vulnerability to adversity across the lifespan, leading to multiple unfavourable outcomes resembling emotional disorders. Identifying the molecular mechanisms underlying epigenetic programming by adverse environment in animal models amenable to genetic manipulation or with similar genetic variation is likely to help our understanding of the individual differences in resilience to stress.
5. Conclusion and outlook
Converging lines of evidence suggest that variation in the transcriptional regulation of 5-HT signalling-related genes and in the activity of their respective gene products, i.e. proteins, plays a critical role in synaptic plasticity of a multitude of neuronal networks, thus setting the stage for expression of complex traits and their associated behaviours throughout development and adult life. Moreover, genetic variation in genes moderating 5-HT system function, in conjunction with other rare and common variants of the genetic background and with inadequate adaptive responses to environmental stressors, is also likely to contribute to inappropriate impulsivity and aggression-related behaviour emerging from compromised brain development and from highly efficient neuroadaptive processes across the life cycle.
Negative emotionality, increased impulsivity and hostility, resulting in aggressive, violent and antisocial behaviour, are not infrequent, the expression of which must be carefully controlled to ensure the success of individuals, small groups and large societies, especially within the evolutionarily recent framework extending from the development of agriculture, to urbanization/ industrialization, to rapid population growth and globalization.
Genetic mechanisms are not the only pathway that leads to individual differences in personality dimensions, behaviour and psychopathology. Complex traits are most likely to be generated by a complicated interaction of environmental and experiential factors with a large number of genes. Even pivotal regulatory proteins of neurotransmission, such as receptors, transporters and modifying enzymes, will individually have only modest impact, while noise from epigenetic as well as non-genetic mechanisms genuinely obstruct identification of relevant genes. Although methods for the detection of G × E interaction in the behavioural genetics of aggression and violence are still in the process of attaining sophistication, the most relevant consequence of gene identification for personality and behavioural traits may be that it will provide the tools required to systematically clarify the effects of epigenetic programming [76,157–162].
On the basis of the remarkable progress in technologies that allow the alteration or elimination of individual genes to create unique animal models, gene modification strategies are likely to continue to increase our knowledge about which gene products are involved in emotion- and aggression-related traits. However, because a missing or dysfunctional gene might affect many developmental processes throughout ontogeny, and compensatory mechanisms may be activated in KO mice, behavioural data from mice with targeted gene deletions should be interpreted with caution. It is evident that many neurotransmitters and their receptors are expressed at early periods of neural development, and it is widely appreciated that they participate in the structural organization of the brain. The increasing versatility of conditional KO strategies, in which a gene-of-interest can be inactivated tissue-specifically any time during ontogeny, are therefore likely to avoid these imperfections associated with phenotypic data from constitutive KOs.
Despite the usefulness of the gene targeting strategy in identifying specific pathways that may be involved in aggression, this approach is limited to known candidate genes. Owing to the complexity in the expression of aggressive behaviour, it is impossible to predict which genes contribute to the variability of this trait in different populations. The current state-of-the-art of this field illustrates how progress in behavioural genetics might be accelerated by closer integration of neuroscience and genetic approaches and a dimensional, quantitative approach to behavioural phenotypes.
In the present epigenomic era, modest advances in behavioural genetics are contrasted by giant leaps in genome-wide screening approaches, leading to the identification of a remarkable number of intriguingly complicated genetic and epigenetic mechanisms. Novel conceptual paradigms and technical progress have facilitated investigations into the connection between genes of the 5-HT signalling pathway, (social) cognition and emotionality: (i) generation of mouse models allowing time- and cell-specific alteration of gene function in different subdivisions of the brain 5-HT system which project to neural units fine-tuning the cognitive–emotional interface, (ii) validation of G × E models in non-human primates and rodents, (iii) functional neuroimaging of 5-HT-specific neurocircuits in humans, (iv) rapidly evolving sequencing technologies in the quest for low-frequent and rare genetic variants with high effect size but variable penetrance, and (v) inclusion of a more extensive phenotypic spectrum (e.g. sex-specific differences, higher cognitive functions, social skills, resilience, etc.). These elaborations continue to enable a more profound understanding of the integration of physiological responses to environmental adversity and the pathophysiology of disorders of emotion regulation.
Acknowledgements
The work by the authors is supported by the DFG (KFO 125, SFB 581/B9, SFB TRR 58/A1 and A5), BMBF (IZKF Wuerzburg, 01KS9603) and the EC (NEWMOOD LSHM-CT-2003-503474).
References
- 1.Lesch K. P., Merschdorf U. 2000. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav. Sci. Law 18, 581–604 (doi:10.1002/1099-0798(200010)18:5<581::AID-BSL411>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 2.Vitiello B., Stoff D. M. 1997. Subtypes of aggression and their relevance to child psychiatry. J. Am. Acad. Child Adolesc. Psychiatry 36, 307–315 10.1097/00004583-199703000-00008 (doi:10.1097/00004583-199703000-00008) [DOI] [PubMed] [Google Scholar]
- 3.de Boer S. F., Caramaschi D., Natarajan D., Koolhaas J. M. 2009. The vicious cycle towards violence: focus on the negative feedback mechanisms of brain serotonin neurotransmission. Front Behav. Neurosci. 3, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miczek K. A., de Almeida R. M., Kravitz E. A., Rissman E. F., de Boer S. F., Raine A. 2007. Neurobiology of escalated aggression and violence. J. Neurosci. 27, 11 803–11 806 10.1523/JNEUROSCI.3500-07.2007 (doi:10.1523/JNEUROSCI.3500-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plomin R., Owen M. J., McGuffin P. 1994. The genetic basis of complex human behaviors. Science 264, 1733–1739 10.1126/science.8209254 (doi:10.1126/science.8209254) [DOI] [PubMed] [Google Scholar]
- 6.Dahlstrom A., Fuxe K. 1964. Localization of monoamines in the lower brain stem. Experientia 20, 398–399 10.1007/BF02147990 (doi:10.1007/BF02147990) [DOI] [PubMed] [Google Scholar]
- 7.Azmitia E. C., Whitaker-Azmitia P. M. 1997. Development and adult plasticity of serotonergic neurons and their target cells. In Serotonergic neurons and 5-HT receptors in the C.N.S. (eds Baumgarten H. G., Göthert M.), pp. 1–39 Berlin, Germany: Springer [Google Scholar]
- 8.Cote F., et al. 2003. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl Acad. Sci. USA 100, 13 525–13 530 10.1073/pnas.2233056100 (doi:10.1073/pnas.2233056100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walther D. J., et al. 2003. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76. 10.1126/science.1078197 (doi:10.1126/science.1078197) [DOI] [PubMed] [Google Scholar]
- 10.Walther D. J., Bader M. 2003. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 66, 1673–1680 10.1016/S0006-2952(03)00556-2 (doi:10.1016/S0006-2952(03)00556-2) [DOI] [PubMed] [Google Scholar]
- 11.Gutknecht L., Kriegebaum C., Waider J., Schmitt A., Lesch K. P. 2009. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur. Neuropsychopharmacol. 19, 266–282 10.1016/j.euroneuro.2008.12.005 (doi:10.1016/j.euroneuro.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 12.Waider J., Araragi N., Gutknecht L., Lesch K. P. 2011. Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive control and emotion regulation: a perspective. Psychoneuroendocrinology 36, 393–405 [DOI] [PubMed] [Google Scholar]
- 13.Serretti A., Chiesa A., Porcelli S., Han C., Patkar A. A., Lee S. J., Park M. H., Pae C. U. 2011. Influence of TPH2 variants on diagnosis and response to treatment in patients with major depression, bipolar disorder and schizophrenia. Psychiatry Res. 189, 26–32 [DOI] [PubMed] [Google Scholar]
- 14.Gutknecht L., Waider J., Kraft S., Kriegebaum C., Holtmann B., Reif A., Schmitt A., Lesch K.-P. 2008. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural Transm. A 115, 1127–1132 10.1007/s00702-008-0096-6 (doi:10.1007/s00702-008-0096-6) [DOI] [PubMed] [Google Scholar]
- 15.Dai J. X., et al. 2008. Enhanced contextual fear memory in central serotonin-deficient mice. Proc. Natl Acad. Sci. USA 105, 11 981–11 986 10.1073/pnas.0801329105 (doi:10.1073/pnas.0801329105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song N. N., et al. 2011. Adult raphe-specific deletion of Lmx1b leads to central serotonin deficiency. PLoS ONE 6, e15998. 10.1371/journal.pone.0015998 (doi:10.1371/journal.pone.0015998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendricks T. J., et al. 2003. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233–247 10.1016/S0896-6273(02)01167-4 (doi:10.1016/S0896-6273(02)01167-4) [DOI] [PubMed] [Google Scholar]
- 18.Kiyasova V., Fernandez S. P., Laine J., Stankovski L., Muzerelle A., Doly S., Gaspar P. 2011. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. 31, 2756–2768 10.1523/JNEUROSCI.4080-10.2011 (doi:10.1523/JNEUROSCI.4080-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaulieu J. M., Zhang X., Rodriguiz R. M., Sotnikova T. D., Cools M. J., Wetsel W. C., Gainetdinov R. R., Caron M. G. 2008. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc. Natl Acad. Sci. USA 105, 1333–1338 10.1073/pnas.0711496105 (doi:10.1073/pnas.0711496105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen J. P., Siesser W. B., Sachs B. D., Peterson S., Cools M. J., Setola V., Folgering J. H., Flik G., Caron M. G. 2011. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol. Psychiatry. 10.1038/mp.2011.50 (doi:10.1038/mp.2011.50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alenina N., et al. 2009. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl Acad. Sci. USA 106, 10 332–10 337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kriegebaum C., Song N. N., Gutknecht L., Huang Y., Schmitt A., Reif A., Ding Y. Q., Lesch K. P. 2010. Brain-specific conditional and time-specific inducible Tph2 knockout mice possess normal serotonergic gene expression in the absence of serotonin during adult life. Neurochem. Int. 57, 512–517 [DOI] [PubMed] [Google Scholar]
- 23.Savelieva K. V., Zhao S., Pogorelov V. M., Rajan I., Yang Q., Cullinan E., Lanthorn T. H., Bartolomucci A. 2008. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE 3, e3301. 10.1371/journal.pone.0003301 (doi:10.1371/journal.pone.0003301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutknecht L., et al. 2007. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int. J. Neuropsychopharmacol. 10, 309–320 10.1017/S1461145706007437 (doi:10.1017/S1461145706007437) [DOI] [PubMed] [Google Scholar]
- 25.Homberg J. R., Lesch K. P. 2011. Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry 69, 513–519 10.1016/j.biopsych.2010.09.024 (doi:10.1016/j.biopsych.2010.09.024) [DOI] [PubMed] [Google Scholar]
- 26.Brown S. M., et al. 2005. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol. Psychiatry 10, 884–888, 805 (doi:10.1038/sj.mp.4001716) [DOI] [PubMed] [Google Scholar]
- 27.Canli T., Congdon E., Gutknecht L., Constable R. T., Lesch K. P. 2005. Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. J. Neural Transm. 112, 1479–1485 10.1007/s00702-005-0391-4 (doi:10.1007/s00702-005-0391-4) [DOI] [PubMed] [Google Scholar]
- 28.Booij L., Turecki G., Leyton M., Gravel P., Lopez De Lara C., Diksic M., Benkelfat C. 2011. Tryptophan hydroxylase(2) gene polymorphisms predict brain serotonin synthesis in the orbitofrontal cortex in humans. Mol Psychiatry. 10.1038/mp.2011.79 (doi:10.1038/mp.2011.79) [DOI] [PubMed] [Google Scholar]
- 29.Armbruster D., Mueller A., Strobel A., Kirschbaum C., Lesch K. P., Brocke B. 2011. Influence of functional tryptophan hydroxylase 2 gene variation and sex on the startle response in children, young adults, and older adults. Biol. Psychol. 83, 214–221 10.1016/j.biopsycho.2009.12.010 (doi:10.1016/j.biopsycho.2009.12.010) [DOI] [PubMed] [Google Scholar]
- 30.Canli T., Congdon E., Todd Constable R., Lesch K. P. 2008. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on neural correlates of affective processing. Biol. Psychol. 79, 118–125 [DOI] [PubMed] [Google Scholar]
- 31.Herrmann M. J., et al. 2007. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on emotional processing. Cereb. Cortex 17, 1160–1163 10.1093/cercor/bhl026 (doi:10.1093/cercor/bhl026) [DOI] [PubMed] [Google Scholar]
- 32.Baehne C. G., Ehlis A. C., Plichta M. M., Conzelmann A., Pauli P., Jacob C., Gutknecht L, Lesch K.-P, Fallgatter A. J. 2009. Tph2 gene variants modulate response control processes in adult ADHD patients and healthy individuals. Mol. Psychiatry 14, 1032–1039 10.1038/mp.2008.39 (doi:10.1038/mp.2008.39) [DOI] [PubMed] [Google Scholar]
- 33.Bach-Mizrachi H., Underwood M. D., Kassir S. A., Bakalian M. J., Sibille E., Tamir H., Mann J. J., Arango V. 2006. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology 31, 814–824 10.1038/sj.npp.1300897 (doi:10.1038/sj.npp.1300897) [DOI] [PubMed] [Google Scholar]
- 34.Bach-Mizrachi H., Underwood M. D., Tin A., Ellis S. P., Mann J. J., Arango V. 2008. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol. Psychiatry 13, 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boldrini M., Underwood M. D., Mann J. J., Arango V. 2005. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 1041, 19–28 10.1016/j.brainres.2005.01.083 (doi:10.1016/j.brainres.2005.01.083) [DOI] [PubMed] [Google Scholar]
- 36.Bonkale W. L., Turecki G., Austin M. C. 2006. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse 60, 81–85 10.1002/syn.20278 (doi:10.1002/syn.20278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underwood M. D., Arango V., Bakalian M. J., Ruggiero D. A., Mann J. J. 1999. Dorsal raphe nucleus serotonergic neurons innervate the rostral ventrolateral medulla in rat. Brain Res. 824, 45–55 10.1016/S0006-8993(99)01181-6 (doi:10.1016/S0006-8993(99)01181-6) [DOI] [PubMed] [Google Scholar]
- 38.Lesch K. P. 2011. When the serotonin transporter gene meets adversity: the contribution of animal models to understanding epigenetic mechanisms in affective disorders and resilience. Curr. Top. Behav. Neurosci. 7, 251–280 10.1007/7854_2010_109 (doi:10.1007/7854_2010_109) [DOI] [PubMed] [Google Scholar]
- 39.Feinberg N. A., et al. 2010. Proang compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35, 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob C. P., et al. 2007. Co-morbidity of adult attention-deficit/hyperactivity disorder with focus on personality traits and related disorders in a tertiary referral centre. Eur. Arch. Psychiatry Clin. Neurosci. 257, 309–317 [DOI] [PubMed] [Google Scholar]
- 41.McKinney J., Johansson S., Halmoy A., Dramsdahl M., Winge I., Knappskog P. M., Haavik J. 2008. A loss-of-function mutation in tryptophan hydroxylase 2 segregating with attention-deficit/hyperactivity disorder. Mol. Psychiatry 13, 365–367 10.1038/sj.mp.4002152 (doi:10.1038/sj.mp.4002152) [DOI] [PubMed] [Google Scholar]
- 42.McKinney J. A., Turel B., Winge I., Knappskog P. M., Haavik J. 2009. Functional properties of missense variants of human tryptophan hydroxylase 2. Hum. Mutat. 30, 787–794 10.1002/humu.20956 (doi:10.1002/humu.20956) [DOI] [PubMed] [Google Scholar]
- 43.Walitza S., et al. 2005. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in attention-deficit/hyperactivity disorder. Mol. Psychiatry. 10, 1126–1132 10.1038/sj.mp.4001734 (doi:10.1038/sj.mp.4001734) [DOI] [PubMed] [Google Scholar]
- 44.Mossner R., et al. 2006. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in children and adolescents with obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 9, 437–442 10.1017/S1461145705005997 (doi:10.1017/S1461145705005997) [DOI] [PubMed] [Google Scholar]
- 45.Johansson S., et al. 2010. Common variants in the TPH1 and TPH2 regions are not associated with persistent ADHD in a combined sample of 1,636 adult cases and 1,923 controls from four European populations. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 1008–1015 [DOI] [PubMed] [Google Scholar]
- 46.Gutknecht L., et al. Brain serotonin deficiency promotes resilience to chronic stress via sex-specific adaptive mechanisms. Submitted.
- 47.Zhang X., Beaulieu J. M., Sotnikova T. D., Gainetdinov R. R., Caron M. G. 2004. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305, 217. 10.1126/science.1097540 (doi:10.1126/science.1097540) [DOI] [PubMed] [Google Scholar]
- 48.Osipova D. V., Kulikov A. V., Popova N. K. 2009. C1473G polymorphism in mouse tph2 gene is linked to tryptophan hydroxylase-2 activity in the brain, intermale aggression, and depressive-like behavior in the forced swim test. J. Neurosci. Res. 87, 1168–1174 10.1002/jnr.21928 (doi:10.1002/jnr.21928) [DOI] [PubMed] [Google Scholar]
- 49.Siesser W. B., Zhang X., Jacobsen J. P., Sotnikova T. D., Gainetdinov R. R., Caron M. G. 2010. Tryptophan hydroxylase 2 genotype determines brain serotonin synthesis but not tissue content in C57Bl/6 and BALB/c congenic mice. Neurosci. Lett. 481, 6–11 10.1016/j.neulet.2010.06.035 (doi:10.1016/j.neulet.2010.06.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenner K., Qadri F., Bert B., Voigt J. P., Bader M. 2008. The mTPH2 C1473G single nucleotide polymorphism is not responsible for behavioural differences between mouse strains. Neurosci. Lett. 431, 21–25 10.1016/j.neulet.2007.11.012 (doi:10.1016/j.neulet.2007.11.012) [DOI] [PubMed] [Google Scholar]
- 51.Ding Y. Q., Marklund U., Yuan W., Yin J., Wegman L., Ericson J., Deneris E., Johnson R. L, Chen Z.-F. 2003. Lmx1b is essential for the development of serotonergic neurons. Nat. Neurosci. 6, 933–938 10.1038/nn1104 (doi:10.1038/nn1104) [DOI] [PubMed] [Google Scholar]
- 52.Lesch K. P. 2005. Serotonergic gene inactivation in mice: models for anxiety and aggression? Novartis Found Symp. 268, 111–140 [PubMed] [Google Scholar]
- 53.Schaefer T. L., Vorhees C. V., Williams M. T. 2009. Mouse plasmacytoma-expressed transcript 1 knock out induced 5-HT disruption results in a lack of cognitive deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. Neuroscience 164, 1431–1443 10.1016/j.neuroscience.2009.09.059 (doi:10.1016/j.neuroscience.2009.09.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrade T. G., Graeff F. G. 2001. Effect of electrolytic and neurotoxic lesions of the median raphe nucleus on anxiety and stress. Pharmacol. Biochem. Behav. 70, 1–14 10.1016/S0091-3057(01)00512-3 (doi:10.1016/S0091-3057(01)00512-3) [DOI] [PubMed] [Google Scholar]
- 55.Sziray N., Kuki Z., Nagy K. M., Marko B., Kompagne H., Levay G. 2010. Effects of single and simultaneous lesions of serotonergic and noradrenergic pathways on open-space and bright-space anxiety-like behavior in two animal models. Behav. Brain Res. 209, 93–98 10.1016/j.bbr.2010.01.019 (doi:10.1016/j.bbr.2010.01.019) [DOI] [PubMed] [Google Scholar]
- 56.Netto S. M., Silveira R., Coimbra N. C., Joca S. R., Guimaraes F. S. 2002. Anxiogenic effect of median raphe nucleus lesion in stressed rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 26, 1135–1141 10.1016/S0278-5846(02)00248-8 (doi:10.1016/S0278-5846(02)00248-8) [DOI] [PubMed] [Google Scholar]
- 57.Rex A., Thomas H., Hortnagl H., Voits M., Fink H. 2003. Behavioural and microdialysis study after neurotoxic lesion of the dorsal raphe nucleus in rats. Pharmacol. Biochem. Behav. 74, 587–593 10.1016/S0091-3057(02)01043-2 (doi:10.1016/S0091-3057(02)01043-2) [DOI] [PubMed] [Google Scholar]
- 58.McNally G. P., Johansen J. P., Blair H. T. 2011. Placing prediction into the fear circuit. Trends Neurosci. 34, 283–292 10.1016/j.tins.2011.03.005 (doi:10.1016/j.tins.2011.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graeff F. G., Viana M. B., Mora P. O. 1996. Opposed regulation by dorsal raphe nucleus 5-HT pathways of two types of fear in the elevated T-maze. Pharmacol. Biochem. Behav. 53, 171–177 10.1016/0091-3057(95)02012-8 (doi:10.1016/0091-3057(95)02012-8) [DOI] [PubMed] [Google Scholar]
- 60.Graeff F. G., Zangrossi H., Jr 2010. The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Central Nervous System Agents Med. Chem. 10, 207–217 [DOI] [PubMed] [Google Scholar]
- 61.Blanchard D. C., Griebel G., Blanchard R. J. 2003. The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur. J. Pharmacol. 463, 97–116 10.1016/S0014-2999(03)01276-7 (doi:10.1016/S0014-2999(03)01276-7) [DOI] [PubMed] [Google Scholar]
- 62.Walker D. L., Miles L. A., Davis M. 2009. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1291–1308 10.1016/j.pnpbp.2009.06.022 (doi:10.1016/j.pnpbp.2009.06.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis M., Shi C. 2000. The amygdala. Curr. Biol. 10, R131. 10.1016/S0960-9822(00)00345-6 (doi:10.1016/S0960-9822(00)00345-6) [DOI] [PubMed] [Google Scholar]
- 64.McDonald A. J. 1998. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 55, 257–332 10.1016/S0301-0082(98)00003-3 (doi:10.1016/S0301-0082(98)00003-3) [DOI] [PubMed] [Google Scholar]
- 65.Turner B. H., Herkenham M. 1991. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J. Comp. Neurol. 313, 295–325 10.1002/cne.903130208 (doi:10.1002/cne.903130208) [DOI] [PubMed] [Google Scholar]
- 66.Muller J. F., Mascagni F., McDonald A. J. 2007. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J. Comp. Neurol. 505, 314–335 10.1002/cne.21486 (doi:10.1002/cne.21486) [DOI] [PubMed] [Google Scholar]
- 67.Sah P., Westbrook R. F., Luthi A. 2008. Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Ann. N. Y. Acad. Sci. 1129, 88–95 10.1196/annals.1417.020 (doi:10.1196/annals.1417.020) [DOI] [PubMed] [Google Scholar]
- 68.Blair H. T., Schafe G. E., Bauer E. P., Rodrigues S. M., LeDoux J. E. 2001. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn. Mem. 8, 229–242 10.1101/lm.30901 (doi:10.1101/lm.30901) [DOI] [PubMed] [Google Scholar]
- 69.Schafe G. E., Nader K., Blair H. T., LeDoux J. E. 2001. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 24, 540–546 10.1016/S0166-2236(00)01969-X (doi:10.1016/S0166-2236(00)01969-X) [DOI] [PubMed] [Google Scholar]
- 70.Ehrlich I., Humeau Y., Grenier F., Ciocchi S., Herry C., Luthi A. 2009. Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771 10.1016/j.neuron.2009.05.026 (doi:10.1016/j.neuron.2009.05.026) [DOI] [PubMed] [Google Scholar]
- 71.Stutzmann G. E., LeDoux J. E. 1999. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J. Neurosci. 19, RC8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harro J., Tonissaar M., Eller M., Kask A., Orel L. 2001. Chronic variable stress and partial 5-HT denervation by parachloroamphetamine treatment in the rat: effects on behavior and monoamine neurochemistry. Brain Res. 899, 227–239 10.1016/S0006-8993(01)02256-9 (doi:10.1016/S0006-8993(01)02256-9) [DOI] [PubMed] [Google Scholar]
- 73.Amat J., Paul E., Watkins L. R., Maier S. F. 2008. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience 154, 1178–1186 10.1016/j.neuroscience.2008.04.005 (doi:10.1016/j.neuroscience.2008.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baratta M. V., Zarza C. M., Gomez D. M., Campeau S., Watkins L. R., Maier S. F. 2009. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur. J. Neurosci. 30, 1111–1116 10.1111/j.1460-9568.2009.06867.x (doi:10.1111/j.1460-9568.2009.06867.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christianson J. P., Paul E. D., Irani M., Thompson B. M., Kubala K. H., Yirmiya R., Watkins L. R., Maier S. F. 2008. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav. Brain Res. 193, 87–93 10.1016/j.bbr.2008.04.024 (doi:10.1016/j.bbr.2008.04.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heiming R. S., Jansen F., Lewejohann L., Kaiser S., Schmitt A., Lesch K. P., Sachser N. 2009. Living in a dangerous world: the shaping of behavioral profile by early environment and 5-HTT genotype. Front Behav. Neurosci. 3, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parihar V. K., Hattiangady B., Kuruba R., Shuai B., Shetty A. K. 2011. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol. Psychiatry 16, 171–183 10.1038/mp.2009.130 (doi:10.1038/mp.2009.130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gross C., Hen R. 2004. The developmental origins of anxiety. Nat. Rev. Neurosci. 5, 545–552 10.1038/nrn1429 (doi:10.1038/nrn1429) [DOI] [PubMed] [Google Scholar]
- 79.Graeff F. G., Guimaraes F. S., De Andrade T. G., Deakin J. F. 1996. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 54, 129–141 10.1016/0091-3057(95)02135-3 (doi:10.1016/0091-3057(95)02135-3) [DOI] [PubMed] [Google Scholar]
- 80.Di Pino G., Moessner R., Lesch P., Lauder J., Persico A. 2004. Roles for serotonin in neurodevelopment: more than just neural transmission. Curr. Neuropharmacol. 2, 403–418 10.2174/1570159043359495 (doi:10.2174/1570159043359495) [DOI] [Google Scholar]
- 81.Gaspar P., Cases O., Maroteaux L. 2003. The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012 10.1038/nrn1256 (doi:10.1038/nrn1256) [DOI] [PubMed] [Google Scholar]
- 82.Bonnin A., Torii M., Wang L., Rakic P., Levitt P. 2007. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat. Neurosci. 10, 588–597 10.1038/nn1896 (doi:10.1038/nn1896) [DOI] [PubMed] [Google Scholar]
- 83.Zhao Z. Q., Scott M., Chiechio S., Wang J. S., Renner K. J., Gereau R. W. T., Johnson R. L., Deneris E. S., Chen Z.-F. 2006. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J. Neurosci. 26, 12 781–12 788 10.1523/JNEUROSCI.4143-06.2006 (doi:10.1523/JNEUROSCI.4143-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lynn-Bullock C. P., Welshhans K., Pallas S. L., Katz P. S. 2004. The effect of oral 5-HTP administration on 5-HTP and 5-HT immunoreactivity in monoaminergic brain regions of rats. J. Chem. Neuroanat. 27, 129–138 10.1016/j.jchemneu.2004.02.003 (doi:10.1016/j.jchemneu.2004.02.003) [DOI] [PubMed] [Google Scholar]
- 85.Brady L. S., Gold P. W., Herkenham M., Lynn A. B., Whitfield H. J., Jr 1992. The antidepressants fluoxetine, idazoxan and phenelzine alter corticotropin-releasing hormone and tyrosine hydroxylase mRNA levels in rat brain: therapeutic implications. Brain Res. 572, 117–125 10.1016/0006-8993(92)90459-M (doi:10.1016/0006-8993(92)90459-M) [DOI] [PubMed] [Google Scholar]
- 86.Heydendael W., Jacobson L. 2010. Widespread hypothalamic–pituitary–adrenocortical axis-relevant and mood-relevant effects of chronic fluoxetine treatment on glucocorticoid receptor gene expression in mice. Eur. J. Neurosci. 31, 892–902 10.1111/j.1460-9568.2010.07131.x (doi:10.1111/j.1460-9568.2010.07131.x) [DOI] [PubMed] [Google Scholar]
- 87.Pudovkina O. L., Cremers T. I., Westerink B. H. 2002. The interaction between the locus coeruleus and dorsal raphe nucleus studied with dual-probe microdialysis. Eur. J. Pharmacol. 445, 37–42 10.1016/S0014-2999(02)01663-1 (doi:10.1016/S0014-2999(02)01663-1) [DOI] [PubMed] [Google Scholar]
- 88.Mongeau R., Blier P., de Montigny C. 1997. The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res. Brain Res. Rev. 23, 145–195 10.1016/S0165-0173(96)00017-3 (doi:10.1016/S0165-0173(96)00017-3) [DOI] [PubMed] [Google Scholar]
- 89.Riccio O., Potter G., Walzer C., Vallet P., Szabo G., Vutskits L., Kiss J. Z., Dayer A. G. 2009. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol. Psychiatry 14, 280–290 10.1038/mp.2008.89 (doi:10.1038/mp.2008.89) [DOI] [PubMed] [Google Scholar]
- 90.Vitalis T., Cases O., Passemard S., Callebert J., Parnavelas J. G. 2007. Embryonic depletion of serotonin affects cortical development. Eur. J. Neurosci. 26, 331–344 10.1111/j.1460-9568.2007.05661.x (doi:10.1111/j.1460-9568.2007.05661.x) [DOI] [PubMed] [Google Scholar]
- 91.Pape H. C., Pare D. 2010. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463 10.1152/physrev.00037.2009 (doi:10.1152/physrev.00037.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Molliver M. E. 1987. Serotonergic neuronal systems: what their anatomic organization tells us about function. J. Clin. Psychopharmacol. 7(Suppl. 6), 3S–23S [PubMed] [Google Scholar]
- 93.McDonald A. J., Mascagni F. 2007. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience 146, 306–320 10.1016/j.neuroscience.2007.01.047 (doi:10.1016/j.neuroscience.2007.01.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muller J. F., Mascagni F., McDonald A. J. 2005. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J. Neurosci. 25, 7366–7376 10.1523/JNEUROSCI.0899-05.2005 (doi:10.1523/JNEUROSCI.0899-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wright D. E., Seroogy K. B., Lundgren K. H., Davis B. M., Jennes L. 1995. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 351, 357–373 10.1002/cne.903510304 (doi:10.1002/cne.903510304) [DOI] [PubMed] [Google Scholar]
- 96.Hale M. W., Johnson P. L., Westerman A. M., Abrams J. K., Shekhar A., Lowry C. A. 2010. Multiple anxiogenic drugs recruit a parvalbumin-containing subpopulation of GABAergic interneurons in the basolateral amygdala. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1285–1293 10.1016/j.pnpbp.2010.07.012 (doi:10.1016/j.pnpbp.2010.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chowdhury N., Quinn J. J., Fanselow M. S. 2005. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav. Neurosci. 119, 1396–1402 10.1037/0735-7044.119.5.1396 (doi:10.1037/0735-7044.119.5.1396) [DOI] [PubMed] [Google Scholar]
- 98.Fendt M., Fanselow M. S., Koch M. 2005. Lesions of the dorsal hippocampus block trace fear conditioned potentiation of startle. Behav. Neurosci. 119, 834–838 10.1037/0735-7044.119.3.834 (doi:10.1037/0735-7044.119.3.834) [DOI] [PubMed] [Google Scholar]
- 99.Quinn J. J., Wied H. M., Ma Q. D., Tinsley M. R., Fanselow M. S. 2008. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus 18, 640–654 10.1002/hipo.20424 (doi:10.1002/hipo.20424) [DOI] [PubMed] [Google Scholar]
- 100.Fanselow M. S., Dong H. W. 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19 10.1016/j.neuron.2009.11.031 (doi:10.1016/j.neuron.2009.11.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jinno S., Kosaka T. 2010. Stereological estimation of numerical densities of glutamatergic principal neurons in the mouse hippocampus. Hippocampus 20, 829–840 [DOI] [PubMed] [Google Scholar]
- 102.Waider J., Proft F., Langlhofer G., Lesch K. P., Gutknecht L.GABA concentration and interneuron subpopulations are differentially altered by brain serotonin deficiency in tryptophan hydroxylase-2 (Tph2) knockout mice. Submitted. [DOI] [PubMed]
- 103.Davis M., Rainnie D., Cassell M. 1994. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 17, 208–214 10.1016/0166-2236(94)90106-6 (doi:10.1016/0166-2236(94)90106-6) [DOI] [PubMed] [Google Scholar]
- 104.Stork O., Ji F.-Y., Kaneko K., Stork S., Yoshinobu Y., Moriya T., Shibata S., Obata K. 2000. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 865, 45–58 10.1016/S0006-8993(00)02206-X (doi:10.1016/S0006-8993(00)02206-X) [DOI] [PubMed] [Google Scholar]
- 105.Tasan R. O., Bukovac A., Peterschmitt Y. N., Sartori S. B., Landgraf R., Singewald N., Sperk G. 2011. Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience 183, 71–80 10.1016/j.neuroscience.2011.03.051 (doi:10.1016/j.neuroscience.2011.03.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Del Arco A., Mora F. 2009. Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J. Neural Transm. 116, 941–952 10.1007/s00702-009-0243-8 (doi:10.1007/s00702-009-0243-8) [DOI] [PubMed] [Google Scholar]
- 107.Likhtik E., Popa D., Apergis-Schoute J., Fidacaro G. A., Pare D. 2008. Amygdala intercalated neurons are required for expression of fear extinction. Nature 454, 642–645 10.1038/nature07167 (doi:10.1038/nature07167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Puig M. V., Watakabe A., Ushimaru M., Yamamori T., Kawaguchi Y. 2010. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J. Neurosci. 30, 2211–2222 10.1523/JNEUROSCI.3335-09.2010 (doi:10.1523/JNEUROSCI.3335-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freund T. F., Buzsaki G. 1996. Interneurons of the hippocampus. Hippocampus 6, 347–470 (doi:10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 110.Spampanato J., Polepalli J., Sah P. 2011. Interneurons in the basolateral amygdala. Neuropharmacology 60, 765–773 10.1016/j.neuropharm.2010.11.006 (doi:10.1016/j.neuropharm.2010.11.006) [DOI] [PubMed] [Google Scholar]
- 111.Sosulina L., Graebenitz S., Pape H. C. 2010. GABAergic interneurons in the mouse lateral amygdala: a classification study. J. Neurophysiol. 104, 617–626 10.1152/jn.00207.2010 (doi:10.1152/jn.00207.2010) [DOI] [PubMed] [Google Scholar]
- 112.Varga V., et al. 2009. Fast synaptic subcortical control of hippocampal circuits. Science 326, 449–453 10.1126/science.1178307 (doi:10.1126/science.1178307) [DOI] [PubMed] [Google Scholar]
- 113.Hensler J. G. 2006. Serotonergic modulation of the limbic system. Neurosci. Biobehav. Rev. 30, 203–214 10.1016/j.neubiorev.2005.06.007 (doi:10.1016/j.neubiorev.2005.06.007) [DOI] [PubMed] [Google Scholar]
- 114.Mamounas L. A., Mullen C. A., O'Hearn E., Molliver M. E. 1991. Dual serotoninergic projections to forebrain in the rat: morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J. Comp. Neurol. 314, 558–586 10.1002/cne.903140312 (doi:10.1002/cne.903140312) [DOI] [PubMed] [Google Scholar]
- 115.Gulyas A. I., Acsady L., Freund T. F. 1999. Structural basis of the cholinergic and serotonergic modulation of GABAergic neurons in the hippocampus. Neurochem. Int. 34, 359–372 10.1016/S0197-0186(99)00041-8 (doi:10.1016/S0197-0186(99)00041-8) [DOI] [PubMed] [Google Scholar]
- 116.Buzsaki G., Draguhn A. 2004. Neuronal oscillations in cortical networks. Science 304, 1926–1929 10.1126/science.1099745 (doi:10.1126/science.1099745) [DOI] [PubMed] [Google Scholar]
- 117.Klausberger T. 2009. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur. J. Neurosci. 30, 947–957 10.1111/j.1460-9568.2009.06913.x (doi:10.1111/j.1460-9568.2009.06913.x) [DOI] [PubMed] [Google Scholar]
- 118.Klausberger T., Marton L. F., O'Neill J., Huck J. H., Dalezios Y., Fuentealba P., Suen W. Y., Papp E., Kaneko T. 2005. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J. Neurosci. 25, 9782–9793 10.1523/JNEUROSCI.3269-05.2005 (doi:10.1523/JNEUROSCI.3269-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Evrard A., et al. 2002. Altered regulation of the 5-HT system in the brain of MAO-A knock-out mice. Eur. J. Neurosci. 215, 841–851 10.1046/j.1460-9568.2002.01917.x (doi:10.1046/j.1460-9568.2002.01917.x) [DOI] [PubMed] [Google Scholar]
- 120.Lanoir J., Hilaire G., Seif I. 2006. Reduced density of functional 5-HT1A receptors in the brain, medulla and spinal cord of monoamine oxidase: a knockout mouse neonates. J. Comp. Neurol. 495, 607–623 10.1002/cne.20916 (doi:10.1002/cne.20916) [DOI] [PubMed] [Google Scholar]
- 121.Murphy D. L., Lesch K. P. 2008. Targeting the murine serotonin transporter: insights into human neurobiology. Nat. Rev. Neurosci. 9, 85–96 10.1038/nrn2284 (doi:10.1038/nrn2284) [DOI] [PubMed] [Google Scholar]
- 122.Lesch K. P., Wiesmann M., Hoh A., Muller T., Disselkamp-Tietze J., Osterheider M., Schulte H. M. 1992. 5-HT1A receptor–effector system responsivity in panic disorder. Psychopharmacology (Berl.) 106, 111–117 10.1007/BF02253597 (doi:10.1007/BF02253597) [DOI] [PubMed] [Google Scholar]
- 123.Lesch K. P., Hoh A., Schulte H. M., Osterheider M., Muller T. 1991. Long-term fluoxetine treatment decreases 5-HT1A receptor responsivity in obsessive-compulsive disorder. Psychopharmacology (Berl.) 105, 415–420 10.1007/BF02244438 (doi:10.1007/BF02244438) [DOI] [PubMed] [Google Scholar]
- 124.Lesch K. P., Mayer S., Disselkamp-Tietze J., Hoh A., Wiesmann M., Osterheider M., Schulte H. M. 1990. 5-HT1A receptor responsivity in unipolar depression. Evaluation of ipsapirone-induced ACTH and cortisol secretion in patients and controls. Biol. Psychiatry 28, 620–628 10.1016/0006-3223(90)90400-V (doi:10.1016/0006-3223(90)90400-V) [DOI] [PubMed] [Google Scholar]
- 125.Fernandes C., McKittrick C. R., File S. E., McEwen B. S. 1997. Decreased 5-HT1A and increased 5-HT2A receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology 22, 477–491 10.1016/S0306-4530(97)00052-8 (doi:10.1016/S0306-4530(97)00052-8) [DOI] [PubMed] [Google Scholar]
- 126.Lopez J. F., Chalmers D. T., Little K. Y., Watson S. J. 1998. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol. Psychiatry 43, 547–573 10.1016/S0006-3223(97)00484-8 (doi:10.1016/S0006-3223(97)00484-8) [DOI] [PubMed] [Google Scholar]
- 127.Farisse J., Hery F., Barden N., Hery M., Boulenguez P. 2000. Central 5-HT(1) and 5-HT(2) binding sites in transgenic mice with reduced glucocorticoid receptor number. Brain Res. 862, 145–153 10.1016/S0006-8993(00)02104-1 (doi:10.1016/S0006-8993(00)02104-1) [DOI] [PubMed] [Google Scholar]
- 128.Meijer O. C., de Kloet E. R. 1998. Corticosterone and serotonergic neurotransmission in the hippocampus: functional implications of central corticosteroid receptor diversity. Crit. Rev. Neurobiol. 12, 1–20 [PubMed] [Google Scholar]
- 129.Meijer O. C., Williamson A., Dallman M. F., Pearce D. 2000. Transcriptional repression of the 5-HT1A receptor promoter by corticosterone via mineralocorticoid receptors depends on the cellular context. J. Neuroendocrinol. 12, 245–254 10.1046/j.1365-2826.2000.00445.x (doi:10.1046/j.1365-2826.2000.00445.x) [DOI] [PubMed] [Google Scholar]
- 130.Ou X. M., Storring J. M., Kushwaha N., Albert P. R. 2001. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J. Biol. Chem. 276, 14 299–14 307 [DOI] [PubMed] [Google Scholar]
- 131.Yadav V. K., et al. 2009. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138, 976–989 10.1016/j.cell.2009.06.051 (doi:10.1016/j.cell.2009.06.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leibowitz S. F., Alexander J. T. 1998. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol. Psychiatry 44, 851–864 10.1016/S0006-3223(98)00186-3 (doi:10.1016/S0006-3223(98)00186-3) [DOI] [PubMed] [Google Scholar]
- 133.Curzon G. 1990. Serotonin and appetite. Ann. N. Y. Acad. Sci. 600, 521–530 (discussion 30–1) 10.1111/j.1749-6632.1990.tb16907.x (doi:10.1111/j.1749-6632.1990.tb16907.x) [DOI] [PubMed] [Google Scholar]
- 134.Meguid M. M., Fetissov S. O., Varma M., Sato T., Zhang L., Laviano A., Rossi-Fanelli F. 2000. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 16, 843–857 10.1016/S0899-9007(00)00449-4 (doi:10.1016/S0899-9007(00)00449-4) [DOI] [PubMed] [Google Scholar]
- 135.Bross R., Hoffer L. J. 1995. Fluoxetine increases resting energy expenditure and basal body temperature in humans. Am. J. Clin. Nutr. 61, 1020–1025 [DOI] [PubMed] [Google Scholar]
- 136.van Riel E., Meijer O. C., Steenbergen P. J., Joels M. 2003. Chronic unpredictable stress causes attenuation of serotonin responses in cornu ammonis 1 pyramidal neurons. Neuroscience 120, 649–658 10.1016/S0306-4522(03)00355-5 (doi:10.1016/S0306-4522(03)00355-5) [DOI] [PubMed] [Google Scholar]
- 137.Gartside S. E., Johnson D. A., Leitch M. M., Troakes C., Ingram C. D. 2003. Early life adversity programs changes in central 5-HT neuronal function in adulthood. Eur. J. Neurosci. 17, 2401–2408 10.1046/j.1460-9568.2003.02668.x (doi:10.1046/j.1460-9568.2003.02668.x) [DOI] [PubMed] [Google Scholar]
- 138.Van Den Bogaert A., et al. 2006. Association of brain-specific tryptophan hydroxylase, TPH2, with unipolar and bipolar disorder in a Northern Swedish, isolated population. Arch. Gen. Psychiatry 63, 1103–1110 10.1001/archpsyc.63.10.1103 (doi:10.1001/archpsyc.63.10.1103) [DOI] [PubMed] [Google Scholar]
- 139.Arborelius L., Eklund M. B. 2007. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience 145, 738–750 10.1016/j.neuroscience.2006.12.007 (doi:10.1016/j.neuroscience.2006.12.007) [DOI] [PubMed] [Google Scholar]
- 140.Azmitia E. C., Jr, McEwen B. S. 1976. Early response of rat brain tryptophan hydroxylase activity to cycloheximide, puromycin and corticosterone. J. Neurochem. 27, 773–778 10.1111/j.1471-4159.1976.tb10407.x (doi:10.1111/j.1471-4159.1976.tb10407.x) [DOI] [PubMed] [Google Scholar]
- 141.Singh V. B., Corley K. C., Phan T. H., Boadle-Biber M. C. 1990. Increases in the activity of tryptophan hydroxylase from rat cortex and midbrain in response to acute or repeated sound stress are blocked by adrenalectomy and restored by dexamethasone treatment. Brain Res. 516, 66–76 10.1016/0006-8993(90)90898-L (doi:10.1016/0006-8993(90)90898-L) [DOI] [PubMed] [Google Scholar]
- 142.Boadle-Biber M. C., Corley K. C., Graves L., Phan T. H., Rosecrans J. 1989. Increase in the activity of tryptophan hydroxylase from cortex and midbrain of male Fischer 344 rats in response to acute or repeated sound stress. Brain Res. 482, 306–316 10.1016/0006-8993(89)91193-1 (doi:10.1016/0006-8993(89)91193-1) [DOI] [PubMed] [Google Scholar]
- 143.Chamas F. M., Underwood M. D., Arango V., Serova L., Kassir S. A., Mann J. J., Sabban E. L. 2004. Immobilization stress elevates tryptophan hydroxylase mRNA and protein in the rat raphe nuclei. Biol. Psychiatry 55, 278–283 10.1016/S0006-3223(03)00788-1 (doi:10.1016/S0006-3223(03)00788-1) [DOI] [PubMed] [Google Scholar]
- 144.Clark M. S., Russo A. F. 1997. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res. Mol. Brain Res. 48, 346–354 10.1016/S0169-328X(97)00106-X (doi:10.1016/S0169-328X(97)00106-X) [DOI] [PubMed] [Google Scholar]
- 145.Clark J. A., Flick R. B., Pai L. Y., Szalayova I., Key S., Conley R. K., Deutch A. Y., Hutson P. H., Mezey E. 2008. Glucocorticoid modulation of tryptophan hydroxylase-2 protein in raphe nuclei and 5-hydroxytryptophan concentrations in frontal cortex of C57/Bl6 mice. Mol. Psychiatry 13, 498–506 10.1038/sj.mp.4002041 (doi:10.1038/sj.mp.4002041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Clark J. A., Pai L. Y., Flick R. B., Rohrer S. P. 2005. Differential hormonal regulation of tryptophan hydroxylase-2 mRNA in the murine dorsal raphe nucleus. Biol. Psychiatry 57, 943–946 10.1016/j.biopsych.2005.01.013 (doi:10.1016/j.biopsych.2005.01.013) [DOI] [PubMed] [Google Scholar]
- 147.Heydendael W., Jacobson L. 2008. Differential effects of imipramine and phenelzine on corticosteroid receptor gene expression in mouse brain: potential relevance to antidepressant response. Brain Res. 1238, 93–107 10.1016/j.brainres.2008.08.018 (doi:10.1016/j.brainres.2008.08.018) [DOI] [PubMed] [Google Scholar]
- 148.Shishkina G. T., Kalinina T. S., Dygalo N. N. 2007. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience 150, 404–412 10.1016/j.neuroscience.2007.09.017 (doi:10.1016/j.neuroscience.2007.09.017) [DOI] [PubMed] [Google Scholar]
- 149.Dygalo N. N., Shishkina G. T., Kalinina T. S., Yudina A. M., Ovchinnikova E. S. 2006. Effect of repeated treatment with fluoxetine on tryptophan hydroxylase-2 gene expression in the rat brainstem. Pharmacol. Biochem. Behav. 85, 220–227 10.1016/j.pbb.2006.08.004 (doi:10.1016/j.pbb.2006.08.004) [DOI] [PubMed] [Google Scholar]
- 150.Abumaria N., Rygula R., Hiemke C., Fuchs E., Havemann-Reinecke U., Ruther E., Flügge G. 2007. Effect of chronic citalopram on serotonin-related and stress-regulated genes in the dorsal raphe nucleus of the rat. Eur. Neuropsychopharmacol. 17, 417–429 10.1016/j.euroneuro.2006.08.009 (doi:10.1016/j.euroneuro.2006.08.009) [DOI] [PubMed] [Google Scholar]
- 151.Abumaria N., Ribic A., Anacker C., Fuchs E., Flugge G. 2008. Stress upregulates TPH1 but not TPH2 mRNA in the rat dorsal raphe nucleus: identification of two TPH2 mRNA splice variants. Cell Mol. Neurobiol. 28, 331–342 10.1007/s10571-007-9259-5 (doi:10.1007/s10571-007-9259-5) [DOI] [PubMed] [Google Scholar]
- 152.Grohmann M., et al. 2010. Alternative splicing and extensive RNA editing of human TPH2 transcripts. PLoS ONE 5, e8956. 10.1371/journal.pone.0008956 (doi:10.1371/journal.pone.0008956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Clark M. S., McDevitt R. A., Neumaier J. F. 2006. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J. Comp. Neurol. 498, 611–623 10.1002/cne.21073 (doi:10.1002/cne.21073) [DOI] [PubMed] [Google Scholar]
- 154.McEuen J. G., Beck S. G., Bale T. L. 2008. Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. J. Neurosci. 28, 8169–8177 10.1523/JNEUROSCI.0004-08.2008 (doi:10.1523/JNEUROSCI.0004-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen G. L., Novak M. A., Meyer J. S., Kelly B. J., Vallender E. J., Miller G. M. 2010. TPH2 5′- and 3′-regulatory polymorphisms are differentially associated with HPA axis function and self-injurious behavior in rhesus monkeys. Genes Brain Behav. 9, 335–347 10.1111/j.1601-183X.2010.00564.x (doi:10.1111/j.1601-183X.2010.00564.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen G. L., Novak M. A., Meyer J. S., Kelly B. J., Vallender E. J., Miller G. M. 2010. The effect of rearing experience and TPH2 genotype on HPA axis function and aggression in rhesus monkeys: a retrospective analysis. Horm. Behav. 57, 184–191 10.1016/j.yhbeh.2009.10.012 (doi:10.1016/j.yhbeh.2009.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bartolomucci A., Carola V., Pascucci T., Puglisi-Allegra S., Cabib S., Lesch K. P., Parmigiani S., Palanza P., Gross C. 2010. Increased vulnerability to psychosocial stress in heterozygous serotonin transporter knockout mice. Dis. Model Mech. 3, 459–470 10.1242/dmm.004614 (doi:10.1242/dmm.004614) [DOI] [PubMed] [Google Scholar]
- 158.Carola V., Frazzetto G., Pascucci T., Audero E., Puglisi-Allegra S., Cabib S., Lesch K. P., Gross C. 2008. Identifying molecular substrates in a mouse model of the serotonin transporter x environment risk factor for anxiety and depression. Biol. Psychiatry 63, 840–846 10.1016/j.biopsych.2007.08.013 (doi:10.1016/j.biopsych.2007.08.013) [DOI] [PubMed] [Google Scholar]
- 159.Jansen F., Heiming R. S., Kloke V., Kaiser S., Palme R., Lesch K. P., Sachser N. 2011. Away game or home match: the influence of venue and serotonin transporter genotype on the display of offensive aggression. Behav. Brain Res. 219, 291–301 10.1016/j.bbr.2011.01.029 (doi:10.1016/j.bbr.2011.01.029) [DOI] [PubMed] [Google Scholar]
- 160.Jansen F., Heiming R. S., Lewejohann L., Touma C., Palme R., Schmitt A., Lesch K. P., Sachser N. 2010. Modulation of behavioural profile and stress response by 5-HTT genotype and social experience in adulthood. Behav. Brain Res. 207, 21–29 10.1016/j.bbr.2009.09.033 (doi:10.1016/j.bbr.2009.09.033) [DOI] [PubMed] [Google Scholar]
- 161.Kloke V., Jansen F., Heiming R. S., Palme R., Lesch K. P., Sachser N. 2011. The winner and loser effect, serotonin transporter genotype, and the display of offensive aggression. Physiol. Behav. 103, 565–574 10.1016/j.physbeh.2011.04.021 (doi:10.1016/j.physbeh.2011.04.021) [DOI] [PubMed] [Google Scholar]
- 162.Van den Hove, et al. 2011. Sex-specific effects of prenatal stress in 5-HTT deficient mice: towards molecular mechanisms of gene × environment interactions. PLoS ONE 6, e22715. 10.1371/journal.pone.0022715 (doi:10.1371/journal.pone.0022715) [DOI] [PMC free article] [PubMed] [Google Scholar]