Abstract
Glutathione (GSH) is a non-protein thiol compound which has been repeatedly reported to play an important role in plant responses during biotic stresses. However, our knowledge of glutathione-related molecular mechanisms underlying plant defense responses still remains limited. We first discovered that the Arabidopsis thaliana phytoalexin deficient 2-1 (pad2-1) mutant was linked to glutathione deficiency since the mutation was identified in the GSH1 gene encoding the first enzyme of glutathione biosynthesis: Glutamate Cysteine Ligase (GCL). Interestingly, this glutathione-deficient mutant pad2-1 also displays a high susceptibility to a wide range of invaders. We recently reported that the glutathione deficiency in pad2-1 is directly related to a low content of GCL protein. In parallel, we highlighted that the altered redox potential in pad2-1 upregulates the oxidative-stress marker genes GR1, GSTF6 and RbohD during infection with the hemibiotrophic oomycete Phytophthora brassicae. Moreover, the impairment of early signaling events such as plasma membrane depolarization, production of nitric oxide and reactive oxygen species also correlates with the reduced hypersensitive response (HR) observed during P. brassicae infection. Concerning the impaired salicylic acid (SA)-dependent pathway in pad2-1, our results indicated that transcripts of IsoChorismate Synthase1 (ICS1, a main enzyme of SA biosynthesis) do not accumulate in response to pathogen. In this review, we integrate previous knowledge and recent discoveries about pad2-1 to better understand the involvement of glutathione in the pad2-1 pleiotropic phenotype observed during biotic stresses.
Keywords: Phytophthora brassicae, pad2-1 mutant, Arabidopsis thaliana, defence response, glutathione, redox environment
Glutathione (γ-glutamylcysteinylglycine) is an abundant and ubiquitous low-molecular-weight thiol in all aerobic organisms. The presence of cysteine confers its biological properties mainly as antioxidant function through its involvement in cell redox homeostasis. It has been largely reported for more than 20 years to play an essential role in many cellular processes as development, growth or environmental response in plants.1 Although the involvement of glutathione in plant responses to pathogens has been described, underlying molecular mechanisms still remain to be discovered. Using forward genetic approaches, we are now able to carry out thorough research on the role of glutathione in cell processes. Several mutants in Arabidopsis thaliana have been identified to contain low glutathione amounts compared with wild type and are related to mutations in the GSH1 gene (At4g23100) encoding Glutamate Cysteine Ligase (GCL), the first enzyme of glutathione biosynthesis. These glutathione-deficient mutants display a large range of phenotypes such as altered development, enhanced abiotic stress sensitivity as well as pathogen susceptibility: - rml1 (root-meristemless1; ~3% of wild-type GSH; abortion in plant development2); - rax1-1 (regulator of APX2 1-1; ~40% of wild-type GSH; sensitivity to high light3); - zir1 (zinc tolerance induced by iron 1; ~15% of wild-type GSH; deficiency in Fe-mediated Zn tolerance4); - cad2-1 (cadmium-sensitive 2-1; ~30% of wild-type GSH; sensitivity to heavy metals and moderate susceptibility to pathogens5,6); and -pad2-1 (phytoalexin-deficient 2-1; ~20% of wild-type GSH; susceptibility to pathogens6; and deficiency in Fe-mediated Zn tolerance4). Of these, the pad2-1 mutant was defined as the best candidate to highlight the key role of glutathione in signaling processes leading to plant resistance to pathogens. Indeed, the low content of glutathione in pad2-1 confers an enhanced susceptibility to various fungal, bacterial and oomycete pathogens (Botrytis cinerea, Alternaria brassicicola, Pseudomonas syringae, Phytophthora brassicae) as well as insect herbivores (Spodoptera littoralis).6-15 Supporting this phenotype in pad2-1, many studies reported deficiencies in defense responses as (1) the low accumulation of antimicrobial defense compounds camalexin and indole glucosinolates, and (2) the altered salicylic acid (SA)-dependent pathway with a low accumulation of SA correlated with an impaired PR-1 gene expression. All these responses are altered in pad2-1 under P. brassicae infection and lead to plant hyper-susceptibility.10,15By using P. brassicae pathogen or oligogalacturonide elicitor, we showed that glutathione deficiency of pad2-1 affects most cellular events including oxidative stress-related events, early signaling events, defense gene expression and hypersensitive response (HR). Here, we present these new data which improve the understanding of the role of glutathione in plant defense mechanisms. We propose a summary figure to establish the link between the glutathione deficiency and the susceptibility of pad2-1 to pathogens, especially P. brassicae (Fig. 1).
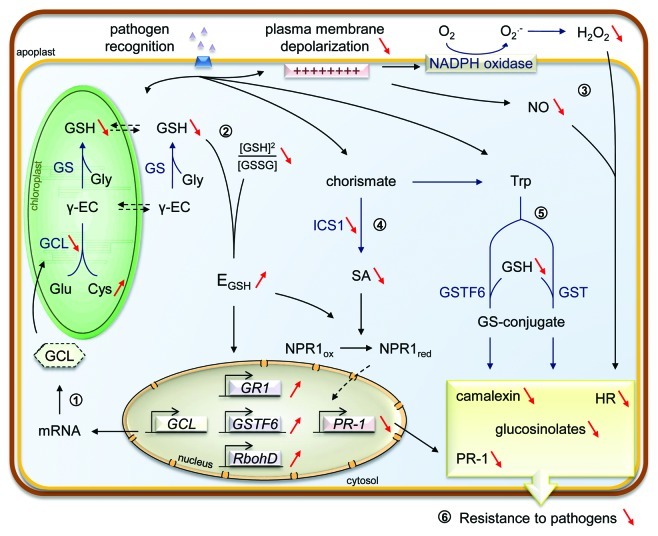
Figure 1. Overview of the glutathione depletion impact on defense signaling processes in pad2-1 mutant under biotic stress. Modification of corresponding molecules or events in pad2-1 mutant compared with wild type are represented with red arrows; γ-EC, γ-glutamylcysteine; Cys, cysteine; EGSH, glutathione redox potential; GCL, glutamate cysteine ligase; Glu, glutamate; Gly, glycine; GR1, glutathione reductase1; GS, glutathione synthase; GS-conjugate, glutathione-conjugated compound; GSH, reduced glutathione; GSSG, oxidized glutathione; GST(F6), glutathione-S-transferase(F6); HR, hypersensitive response; ICS1, isochorismate synthase1; NO, nitric oxide; NPR1ox/red, oxidized/reduced non-expressor of pathogenesis-related genes1; PR-1, pathogenesis-related 1; RbohD, Respiratory burst oxidase homolog D; SA, salicylic acid; Trp, tryptophan.
The Deficiency of Glutathione is Related to a Low Content of GCL Protein
Since pad2-1 displays a normal GCL transcript accumulation compared with wild type, we studied GCL at protein level to determine the cause of the low content of glutathione. Structural prediction of GCL revealed that the pad2-1 mutation (S298N) does not affect the ternary structure of GCL and probably not its enzymatic activity contrary to cad2-1 and rax1-1 mutations localized in substrate binding sites. However, we showed that pad2-1 contains only 48% of wild-type GCL amount.16 Based on these results, one hypothesis suggests that pad2-1 mutation could affect GCL protein folding or degradation leading to glutathione deficiency and correlated with cysteine accumulation (Fig. 1.1).
Pad2-1 Senses a Permanent Oxidative Stress
From many years, glutathione has been shown to be a key molecule of the cellular redox homeostasis through its oxidized/reduced ratio as well as its total concentration. Using a cytosolic targeted redox-sensitive fluorescent probe GRX1-roGFP2, we observed in vivo that glutathione redox potential (EGSH) is less reducing in pad2-1 than in wild type under normal conditions (Fig. 1.2). This result was confirmed with the predominant oxidized form of GCL in pad2-1 although the reduced form is observed in wild type.16 Thus, based on many studies about regulation of gene expression by the redox state, we assume that this altered redox environment could influence gene expression under biotic stress. In that way, marker genes of oxidative stress such as GR1 (Glutathione Reductase1), GSTF6 (Glutathione-S-Transferase F6) and RbohD (Respiratory Burst Oxidase Homolog D) are upregulated in pad2-1 mutant compared with wild type during P. brassicae infection.16
Glutathione Deficiency in pad2-1 Affects Defense-related Signaling Events, which Confers a Susceptibility to Pathogens
In response to biotic stress, pad2–1 displays altered early signaling events such as plasma membrane depolarization and production of H2O2 and nitric oxide (NO; Figure 1.3).16 Our hypothesis is that the less reducing EGSH could affect the corresponding gene expression and/or enzyme activity or upstream signaling events. From these results and based on previous studies, their impairment might explain the weak HR establishment observed in pad2-1 during P. brassicae interaction.16 In parallel, the SA-dependent pathway is blocked in pad2-1 in response to P. brassicae (Fig. 1.4).10 Based on our results, we suggest that the abolished expression of IsoChorismate Synthase 1 (ICS1), encoding a main enzyme of SA biosynthesis, is the origin of the low SA accumulation during P. brassicae infection.16 Consequently, the expression of defense gene PR-1 is not upregulated in pad2-1. Moreover we propose that the low content of glutathione directly affects the accumulation of the antimicrobial compounds camalexin and glucosinolates in pad2-1 since glutathione has been reported to be a sulfur source in their biosynthesis (Fig. 1.5).17,18 Finally, these low defense responses confer a susceptibility of pad2-1 to pathogens (Fig. 1.6). In conclusion, we provided strong evidence that glutathione regulates early signaling events, stress-related gene expression and plant defenses by studying pad2-1 mutant under biotic stress conditions.
Acknowledgments
C.D.-M. was supported by a grant from the French Ministère de l’Enseignement Supe´rieur et de la Recherche. This work was financially supported by the “Conseil Re´gional de Bourgogne.”
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18831
References
- 1.Noctor G, Mhamdi A, Chaouch S, Han YI, Neukermans J, Marquez-Garcia B, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–84. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 2.Vernoux T, Wilson RC, Seeley KA, Reichheld J-P, Muroy S, Brown S, et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–110. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball L, Accotto G-P, Bechtold U, Creissen G, Funck D, Jimenez A, et al. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell. 2004;16:2448–62. doi: 10.1105/tpc.104.022608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanmugam V, Tsednee M, Yeh K-C. ZINC TOLERANCE INDUCED BY IRON 1 reveals the importance of glutathione in the cross-homeostasis between zinc and iron in Arabidopsis thaliana. Plant J. 2012;69:1006–17. doi: 10.1111/j.1365-313X.2011.04850.x. [DOI] [PubMed] [Google Scholar]
- 5.Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. Plant J. 1998;16:73–8. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 6.Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F. Identification of PAD2 as a gamma-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2007;49:159–72. doi: 10.1111/j.1365-313X.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- 7.Glazebrook J, Ausubel FM. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci U S A. 1994;91:8955–9. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, et al. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics. 1997;146:381–92. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuber TL, Plotnikova JM, Dewdney J, Rogers EE, Wood W, Ausubel FM. Correlation of defense gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J. 1998;16:473–85. doi: 10.1046/j.1365-313x.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 10.Roetschi A, Si-Ammour A, Belbahri L, Mauch F, Mauch-Mani B. Characterization of an Arabidopsis-Phytophthora pathosystem: resistance requires a functional PAD2 gene and is independent of salicylic acid, ethylene and jasmonic acid signalling. Plant J. 2001;28:293–305. doi: 10.1046/j.1365-313X.2001.01148.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 2003;35:193–205. doi: 10.1046/j.1365-313X.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- 12.van Wees SC, Chang HS, Zhu T, Glazebrook J. Characterization of the early response of Arabidopsis to Alternaria brassicicola infection using expression profiling. Plant Physiol. 2003;132:606–17. doi: 10.1104/pp.103.022186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohman S, Staal J, Thomma BP, Wang M, Dixelius C. Characterisation of an Arabidopsis-Leptosphaeria maculans pathosystem: resistance partially requires camalexin biosynthesis and is independent of salicylic acid, ethylene and jasmonic acid signalling. Plant J. 2004;37:9–20. doi: 10.1046/j.1365-313X.2003.01927.x. [DOI] [PubMed] [Google Scholar]
- 14.Schlaeppi K, Bodenhausen N, Buchala A, Mauch F, Reymond P. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J. 2008;55:774–86. doi: 10.1111/j.1365-313X.2008.03545.x. [DOI] [PubMed] [Google Scholar]
- 15.Schlaeppi K, Abou-Mansour E, Buchala A, Mauch F. Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J. 2010;62:840–51. doi: 10.1111/j.1365-313X.2010.04197.x. [DOI] [PubMed] [Google Scholar]
- 16.Dubreuil-Maurizi C, Vitecek J, Marty L, Branciard L, Frettinger P, Wendehenne D, et al. Glutathione deficiency of the Arabidopsis mutant pad2-1 affects oxidative stress-related events, defense gene expression, and the hypersensitive response. Plant Physiol. 2011;157:2000–12. doi: 10.1104/pp.111.182667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su T, Xu J, Li Y, Lei L, Zhao L, Yang H, et al. Glutathione-indole-3-acetonitrile is required for camalexin biosynthesis in Arabidopsis thaliana. Plant Cell. 2011;23:364–80. doi: 10.1105/tpc.110.079145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geu-Flores F, Nielsen MT, Nafisi M, Møldrup ME, Olsen CE, Motawia MS, et al. Glucosinolate engineering identifies a gamma-glutamyl peptidase. Nat Chem Biol. 2009;5:575–7. doi: 10.1038/nchembio.185. [DOI] [PubMed] [Google Scholar]