Abstract
Although hydrogen peroxide (H2O2) and nitric oxide (NO) can act as an upstream signaling molecule to modulate the dynamic microtubule cytoskeleton during the defense responses to Verticillium dahliae (VD) toxins in Arabidopsis, it is not known the relationship between these two signaling molecules. Here, we show that VD-toxin-induced NO accumulation was dependent on prior H2O2 production, NO is downstream of H2O2 in the signaling process, and that H2O2 acted synergistically with NO to modulate the dynamic microtubule cytoskeleton responses to VD-toxins in Arabidopsis.
Keywords: Verticillium dahliae toxins, Arabidopsis, H2O2 and NO signals, the dynamic microtubule cytoskeleton
Reactive oxygen species including hydrogen peroxide (H2O2) and nitric oxide (NO) are well established as signaling molecules, mediating a wide range of cellular responses. H2O2 signals have been shown to induce large transcriptional changes and cellular reprogramming that can either protect the plant cell or induce programmed cell death.1-4 Moreover, NO has emerged as an important signaling molecule that mediates many developmental and physiological processes.5-8 It has been demonstrated that NO cooperates with H2O2 to activate the hypersensitive reaction in plants.9-12 However, the interaction of NO and H2O2 is still far from being clearly elucidated.13-17 We have recently demonstrated that NO and H2O2 can act as an upstream signaling molecule to modulate the dynamic microtubule cytoskeleton during the defense responses to Verticillium dahliae (VD) toxins in Arabidopsis.18,19 Here, we provide evidence that NO serves as a signaling intermediate downstream of H2O2 to modulates the dynamic microtubule cytoskeleton during the responses to VD-toxins in Arabidopsis.
The Interaction of NO and H2O2 in VD-Toxins-Induced Responses in Arabidopsis
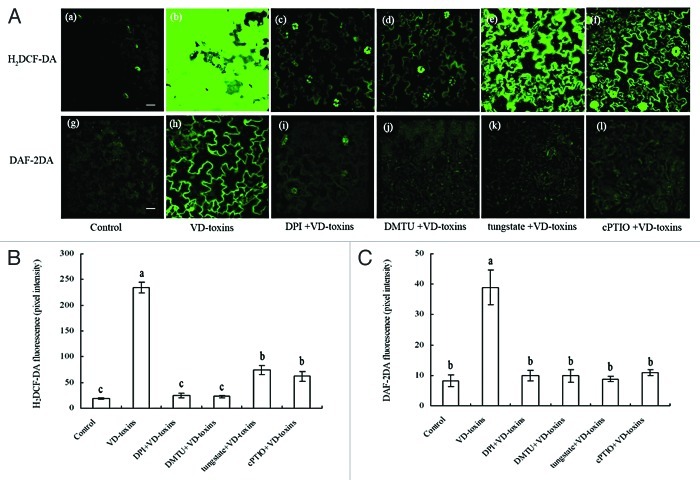
The levels of NO and H2O2 in wild-type Arabidopsis leaves were monitored by cell permeable fluorophores, DAF-2DA and H2DCF-DA, respectively. The fluorescent intensity in leaves significantly increased after treatment with VD-toxins (Fig. 1A, b and h, B and C).
Figure 1. Effect of an inhibitor of NADPH oxidase (DPI), a H2O2 scavenger (DMTU), a NO scavenger (cPTIO) and an inhibitor of nitrate reductase (sodium tungstate) on VD-toxin-induced H2O2 and NO production in the leaves of wild-type Arabidopsis. (A) H2O2 and NO were detected by fluorescence resulting from H2DCF-DA and DAF-2DA. Pictures were taken 60 min post-treatment. Bars = 20 µm. (B) The H2DCF-DA fluorescence intensities in the leaves of wild-type Arabidopsis. (C) The DAF-2DA fluorescence intensities in the leaves of wild-type Arabidopsis. Confocal data are displayed as estimated mean pixel intensities and associated 95% confidence intervals. Error bars indicate standard deviations. Values of each group with the same letters were not significantly different (p < 0.05).
To investigate the interaction between the NO and H2O2 production, wild-type seedlings were co-treated with VD-toxins plus DPI (a potent inhibitor of NADPH oxidase), DMTU (a H2O2 scavenger), cPTIO (a NO scavenger) and sodium tungstate (a potent inhibitor of nitrate reductase).
The VD-toxin-induced H2O2 production was almost completely prevented by supplements of DPI and DMTU, but only partially restricted by supplements of cPTIO or sodium tungstate (Fig. 1A and B). In contrast, the VD-toxin-induced NO production almost completely blocked by supplement of DPI or DMTU, cPTIO or sodium tungstate (Fig. 1A and C). This result showed that NO and H2O2 were signaling molecules in VD-toxin-induced responses in Arabidopsis, and that H2O2 was located upstream of NO in this pathway. Thus, VD-toxin-induced NO accumulation was dependent on H2O2 production in Arabidopsis.
NO and H2O2 Modulates VD-Toxins-Induced Dynamic Microtubule Cytoskeleton
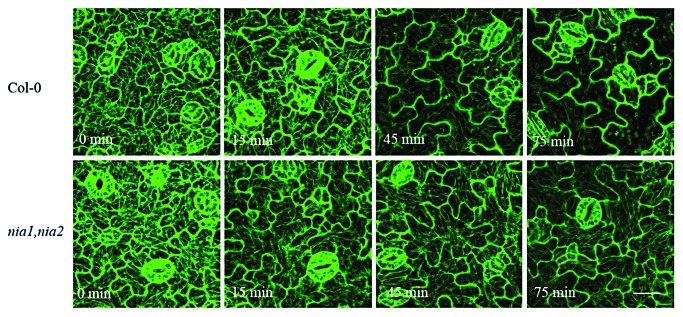
Previous experiments indicated that NO is produced mostly by the nitrate reductase (NR) pathway in response to VD-toxins in Arabidopsis leaves.20 The wild-type and nia1, nia2 NR-deficient mutants of Arabidopsis were used to visualize microtubules in living leaf cells. The results showed that VD-toxins induced a time-dependent microtubule depolymerization, and that microtubule depolymerization was more severe in WT than in nia1, nia2 NR-deficient mutants, especially at the later stages (Fig. 2). The data indicate that NO accumulation was involved in modulating VD-toxins-induced the dynamic microtubule cytoskeleton.
Figure 2. Sequential images of cortical microtubule alterations induced by VD-toxins (150 µg mL–1) in the leaf pavement cells of the wild-type (Col-0) and nia1, nia2 mutants expressing GFP–tubulin of Arabidopsis. Bars = 20 µm.
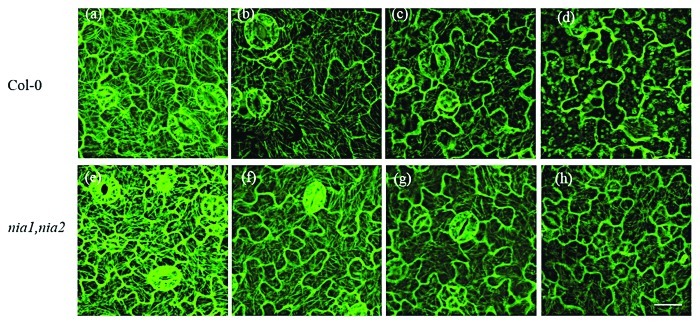
To further examine the role of H2O2 on NO modulation of VD-toxins-induced the dynamic microtubule cytoskeleton, we used different concentrations of exogenous H2O2 to treat the wild-type and nia1, nia2 NR-deficient mutant seedlings. The depolymerization of cortical microtubules increased with increasing concentrations of exogenous H2O2; moreover, microtubule depolymerization was more severe in WT than in nia1, nia2 mutants (Fig. 3). The results suggest that H2O2 modulated the dynamic microtubule cytoskeleton through the activity of NR. It is possible that VD-toxin-induced NO accumulation was dependent on prior H2O2 production, and that H2O2 acted synergistically with NO to modulate the dynamic microtubule cytoskeleton responses to VD-toxins in Arabidopsis.
Figure 3. Disruption of the microtubule cytoskeleton induced by exogenous H2O2 in the leaf pavement cells of wild-type (a–d) and nia1, nia2 mutants (e–h) Arabidopsis. (a) and (e) Control, leaves were untreated; (b) and (f) leaves were treated with 1 mM H2O2; (c) and (g) leaves were treated with 5 mM H2O2; and (d) and (h) leaves were treated with 10 mM H2O2. Pictures were taken 90 min post-treatment. Bar = 20 µm.
Additionally, time course experiments with fluorescent probes showed that there was temporal separation of increases in H2O2 and NO, and NO production occurred after that of H2O2.18,19 Taken together, these data suggest that NO serves as a signaling intermediate downstream of H2O2 to modulates the dynamic microtubule cytoskeleton during the responses to VD-toxins in Arabidopsis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30870203 and 31170249) to Y.-Z.L.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18768
References
- 1.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–93. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 2.Overmyer K, Brosche´ M, Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–42. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 3.Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol. 2005;168:17–20. doi: 10.1083/jcb.200409170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, et al. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006;141:436–45. doi: 10.1104/pp.106.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamattina L, Garci´a-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–36. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 6.Mur LAJ, Carver TLW, Prats E. NO way to live; the various roles of nitric oxide in plant-pathogen interactions. J Exp Bot. 2006;57:489–505. doi: 10.1093/jxb/erj052. [DOI] [PubMed] [Google Scholar]
- 7.Hong JK, Yun BW, Kang JG, Raja MU, Kwon E, Sorhagen K, et al. Nitric oxide function and signalling in plant disease resistance. J Exp Bot. 2008;59:147–54. doi: 10.1093/jxb/erm244. [DOI] [PubMed] [Google Scholar]
- 8.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, et al. Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot. 2008;59:165–76. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 9.Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–9. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polverari A, Molesini B, Pezzotti M, Buonaurio R, Marte M, Delledonne M. Nitric oxide-mediated transcriptional changes in Arabidopsis thaliana. Mol Plant Microbe Interact. 2003;16:1094–105. doi: 10.1094/MPMI.2003.16.12.1094. [DOI] [PubMed] [Google Scholar]
- 11.Leitner M, Vandelle E, Gaupels F, Bellin D, Delledonne M. NO signals in the haze: nitric oxide signalling in plant defence. Curr Opin Plant Biol. 2009;12:451–8. doi: 10.1016/j.pbi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Yoshioka H, Asai S, Yoshioka M, Kobayashi M. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Mol Cells. 2009;28:321–9. doi: 10.1007/s10059-009-0156-2. [DOI] [PubMed] [Google Scholar]
- 13.Dong L, Zhang X, Jiang J, An GY, Zhang LR, Song CP. NO may function in the downstream of H2O2 in ABA-induced stomatal closure in Vicia faba L. Mol Biol. 2005;31:62–70. [PubMed] [Google Scholar]
- 14.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–22. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, et al. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007;175:36–50. doi: 10.1111/j.1469-8137.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Tsuichihara N, Etoh T, Iwai S. Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant Cell Environ. 2007;30:1320–5. doi: 10.1111/j.1365-3040.2007.01711.x. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava N, Gonugunta VK, Puli MR, Raghavendra AS. Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta. 2009;229:757–65. doi: 10.1007/s00425-008-0855-5. [DOI] [PubMed] [Google Scholar]
- 18.Shi FM, Yao LL, Pei BL, Zhou Q, Li XL, Li Y, et al. Cortical microtubule as a sensor and target of nitric oxide signal during the defence responses to Verticillium dahliae toxins in Arabidopsis. Plant Cell Environ. 2009;32:428–38. doi: 10.1111/j.1365-3040.2009.01939.x. [DOI] [PubMed] [Google Scholar]
- 19.Yao LL, Zhou Q, Pei BL, Li YZ. Hydrogen peroxide modulates the dynamic microtubule cytoskeleton during the defence responses to . Verticillium dahliae toxins in Arabidopsis. Plant Cell Environ. 2011;34:1586–98. doi: 10.1111/j.1365-3040.2011.02356.x. [DOI] [PubMed] [Google Scholar]
- 20.Shi FM, Li YZ. Verticillium dahliae toxins-induced nitric oxide production in Arabidopsis is major dependent on nitrate reductase. BMB Rep. 2008;41:79–85. doi: 10.5483/BMBRep.2008.41.1.079. [DOI] [PubMed] [Google Scholar]