Abstract
Plant roots forage the soil for water and nutrients and overcome the soil’s physical compactness. Roots are endowed with a mechanism that allows them to penetrate and grow in dense media such as soil. However, the molecular mechanisms underlying this process are still poorly understood. The nature of the media in which roots grow adds to the difficulty to in situ analyze the mechanisms underlying root penetration. Inhibition of ethylene perception by application of 1-methyl cyclopropene (1-MCP) to tomato seedlings nearly abolished the root penetration in Soilrite. The reversal of this process by auxin indicated operation of an auxin-ethylene signaling pathway in the regulation of root penetration. The tomato pct1–2 mutant that exhibits an enhanced polar transport of auxin required higher doses of 1-MCP to inhibit root penetration, indicating a pivotal role of auxin transport in this process. In this update we provide a brief review of our current understanding of molecular processes underlying root penetration in higher plants.
Keywords: 1-MCP, Auxin transport, BSO, ethylene, mechanical impedance, pct1-2, PIN1, root penetration, tomato
The differences in growth habits of the two major plant organs, the root and the shoot, result from their fundamentally different roles in acquisition of resources for the plant.1 The shoot grows in air practically unhindered by any physical obstruction, except for competition with neighboring plants, primarily to fix carbon via photosynthesis. The root grows in the soil to obtain mineral nutrients and water, and also to provide firm anchorage to the plant.2 The soil in which roots grow and proliferate is a dense medium. Consequently, roots require an additional capability to grow in dense environment to overcome the physical constrains imposed by the soil texture. The dense soil physically obstructs root growth and also reduces the availability of water and oxygen.3 In crop plants the physical constrain imposed by the soil is overcome by tilling prior to sowing. However, in nature seeds germinate and roots grow overcoming soil physical constrains. Roots show a high degree of plasticity in their growth patterns, which are uniquely related to the physical and chemical properties of the soil. To grow in a subterranean environment, roots are endowed with several mechanisms that regulate and optimize their growth. One of the most investigated mechanisms is the gravitropism of roots,4 causing the roots to grow either toward the direction of gravitational vector or at a skewed angle referred to as plagiotropism.
Essentially it is believed that in roots the gravitational signal is sensed through sedimentation of specialized plastids, statoliths, in the columella cells located in the root cap.5,6 The physical signal of statolith sedimentation is converted to physiological information by a mechanism still to be fully deciphered, and transmitted to the root elongation zone located at a distance from the root cap. Several constituents of this physiological response have been identified including changes in pH, reactive oxygen species, Ca+2 induced signaling, and modulation of auxin transport.7-9 In addition to gravity, several other factors regulate the growth of roots in soils, allowing roots to sense availability of moisture and nutrients in the soil and to determine an optimal growth strategy.19,20 Similar to directing the growth of a root toward the gravitational vector, the perception of touch stimuli also plays an important role in the orientation of root growth.21,22 Roots seem able of sensing the touch stimulus and determining the physical impedance exerted by the soil on root growth.10 On encountering of an obstacle such as a hardpan layer of soil, pebbles, or another root, roots either reorient their growth to circumnavigate the obstacle (avoidance) or develop sufficient mechanical force to penetrate through the obstacle (overcoming).23 In either case, the sensing of touch is an important factor that regulates root growth.24
Investigations of root biology of different crop species have highlighted that even within the same species, roots of different cultivars differ in their capacity to penetrate into deeper soil layers to access water and nutrients.11-13 For example, in rice the cultivars that were able to penetrate hardpans had better access to water and were able to avoid drought in rainfed cultivation,14 indicating that the mechanical impedance experienced by roots is one of the major limitations to root growth in soil.12,15 Consistent with this, it has been found that the capability of roots to penetrate in stronger soil layers is genetically controlled.16 Despite the recognition that the response of roots to mechanical impedance has a genetical basis, however, the physiological process triggered in roots to overcome the mechanical impedance is poorly understood.17,18
Compared with other regulatory factors, very limited information is available about the mechanism employed by roots to sense the mechanical impedance, and the signal transduction associated with it. Since obstacles in the soil are first encountered by cells of the root cap, these cells are the primary candidates for sensing the touch and/or mechanical impedance stimuli.25,26 The surgical removal of the root tip results in loss of sensing, and it is regained only after regeneration of a new root tip. The cells of the root cap are in a dynamic state of flux as the root cap continually sheds these cells and replaces them with new ones, to make a smooth passage through the soil. These cells are produced by the root meristem and differentiate to amyloplasts-filled cells, then finally become peripheral and eventually sloughed off.27,28 This transit of cells through different developmental stages has led to the hypothesis that the relative positioning of these cells may determine their differential sensory activities. Evidence indicates that while deep seated columella cells sense gravity, peripheral cells sense touch as evident by touch-induced Ca+2 increase in these cells.29,30 However, these two sensing functions may overlap between the two type of cells.31 Notwithstanding these studies, the details of the signaling systems overcoming mechanical impedance or touch-induced reorientation in root growth remain elusive.
Given the potential mechanistic overlap between graviperception and mechanoperception, Trewavas and Knight32 proposed that the gravisensing represents a modified touch sensing system, as both responses represent some kind of mechanical sensing. It is currently believed that the touch receptor is a mechanosensitive channel, and several lines of evidence point to the existence of stretch-activated channels in the plant plasma membrane.33 On perception of a touch signal, roots respond with an elevation of the cytosolic Ca2+, which in turn triggers transient changes in pH, extracellular ROS, and genome-wide alterations in gene expression patterns in root tissues.34-36 It is believed that the elevation in extracellular pH regulates the activity of several enzymes that affect the cell wall rigidity.37,38 Likewise, ROS production in the apoplast may promote an oxidative cross-linking of cell wall components and thus strengthen the cell wall.39,40 It is expected that these cell wall modifications at the root tip may endow roots with the required rigidity to overcome the physical constrains presented by the compactness of soil.
Though these studies highlight the regulatory role of mechanoperception in root growth, the information about inter- and intracellular signaling in roots, and the change in root morphology upon encountering of an obstacle is scarce. One major limitation is the difficulty to mimic the soil conditions when studying these responses. In a pioneering study, Okada and Shimura41 screened for Arabidopsis root growth mutants by growing seedlings on hard agar (1.5%) on plates inclined at a 45° angle. While the wild type roots showed a wavy growth pattern due to an obstacle-avoidance response, mutant roots either lacked the wavy growth pattern or showed an abnormal pattern. In many cases, the identification of genes of these mutants showed defects in auxin transport, thus highlighting the role of auxin transport and signaling in overcoming mechanical impedance during root growth.42,43
Okamoto et al.44 examined the growth and morphology of roots under mechanical impedance by growing Arabidopsis seedlings on the horizontal surface of an agar plate covered with a dialysis membrane. The roots of these seedlings failed to penetrate the substrate, and showed a typical ethylene-induced morphology, such as a reduction in root growth associated with a decrease in cell elongation and increase in the root diameter. Since the ethylene-overproducing mutant eto1–1 showed a response similar to that in wild type, and the ethylene production was similar in both horizontal or vertical growth conditions, it appears that the mechanical impedance likely enhanced the ethylene response in roots rather than ethylene biosynthesis. Consistent with this view the ethylene signaling mutants, ein2–1 and ctr1–1 failed to show changes in root morphology when subjected to mechanical impedance. In conformity with earlier studies of Okada and Shimura,42 Okamoto et al.44 showed that the mechanical impedance also altered the auxin response in Arabidopsis roots as visualized by a higher expression of DR5::GUS reporter on the lower side of the root tip.
Taken together, these studies are consistent with other reports where it has been shown that an interaction between auxin and ethylene signaling modulates the root elongation in Arabidopsis. Ethylene regulates several facets of auxin action, such as the positive regulation of both acropetal and basipetal auxin transport in the root,45-47 and regulates root growth and development by controlling the auxin biosynthesis and transport in the root tip.45,48 Such synergistic interaction between auxin and ethylene has been observed for several specific responses, such as the regulation of root gravitropism,49 root growth,50 lateral root development51,52 and differentiation and elongation of root hairs.53 The roots of mutants such as tir1, which has a defect in auxin perception, and pin2 and aux1, with defects in auxin efflux and auxin influx transporters, respectively, are resistant to growth inhibition in presence of ethylene.54
Despite the availability of an overwhelming evidence regarding the synergistic interaction between auxin and ethylene in regulating root development and growth, currently little is known about the relative roles of these hormones in regulating the penetration of roots into the soil.55 Current evidence supports that ethylene appears to have a major role in regulating the penetration of roots in the soil. A role of ethylene in root penetration was demonstrated for tomato, where roots of tomato seedlings treated with inhibitors of ethylene action were unable to penetrate into 2% agar but could penetrate 0.5% agar.56 Consistent with this, the root of the ethylene-perception mutant of tomato, Never-ripe (Nr), shows a decreased soil penetration in presence of higher mechanical impedance.57 Roots of the maize mutant Zmacs6, defective in ACC synthase activity, a rate-limiting enzyme in the ethylene biosynthesis pathway, also show a reduced growth in soil, signifying the importance of ethylene in overcoming physical resistance.58
In recent years, the availability of specific inhibitors that block hormonal perception or signaling have greatly aided in deciphering signaling pathways and identification of molecules regulating these pathways. Since excessive production of ethylene during fruit storage causes spoilage of fruits such as banana, tomato and apple, several specific inhibitors that block ethylene perception have been developed.59 Among these inhibitors 1-methylcyclopropane (1-MCP) is the most effective and likely blocks ethylene action by binding to ethylene receptors.60,61
The roots of tomato seedlings grown in presence of 1-MCP failed to penetrate in Soilrite. Since 1-MCP also blocked root penetration in monocots such as rice and wheat, and dicots, such as tobacco and lettuce, this indicated a role of ethylene in regulating root penetration in a wide range of plants.62 The inhibitory effect of 1-MCP on root penetration was reversed by simultaneous application of ethylene, supporting the role of ethylene signaling in regulating root penetration. The loss of root penetration was also associated with a decline in the expression of the auxin-response reporter DR5::GUS in the root tips, suggesting an interaction between ethylene and auxin signaling in the regulation of this response. The application of 1-MCP also reduced the polar auxin transport in tomato roots, suggesting that the auxin-ethylene interaction may be partly mediated by modulation of polar auxin transport. Consistent with this view, the pct1-2 mutant that has a nearly 3-fold higher auxin transport than wild type,63,64 displayed a close to normal root penetration in the Soilrite in presence of 1-MCP. However, the application of a polar auxin transport inhibitor, TIBA, which interferes with the vesicle trafficking of auxin transporters,65 along with 1-MCP partially inhibited the root penetration of the pct1-2 mutant.
Though 1-MCP treatment reduced auxin transport in pct1-2 roots, the PAT remained considerably higher compared with untreated wild type roots. We reasoned that increasing the dosage of 1-MCP would progressively block PAT in pct1-2 roots and would inhibit root penetration even in the pct1-2 mutant. Consistent with our notion, increasing the concentration 1-MCP above 4 μL L−1 inhibited root penetration in pct1-2 to the extent that almost 50% of the roots failed to penetrate in Soilrite (Fig. 1A). In comparison, penetration of wild type roots in Soilrite is almost completely abolished at 2 μL L-1.62 In conformity with the observations made with wild type, increasing the dosage of 1-MCP affected also the growth of hypocotyls and roots of the pct1-2 mutant by stimulating root growth and inhibiting hypocotyl elongation (Fig. 1B).
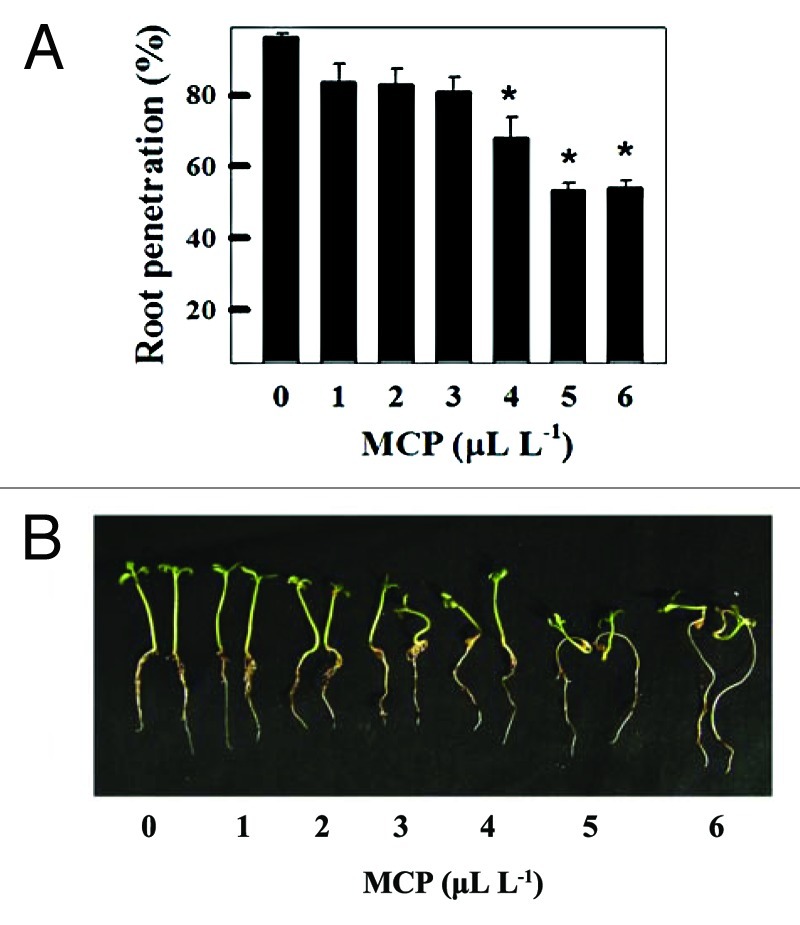
Figure 1. 1-MCP dose response of pct1-2 seedlings. pct1-2 seedlings were grown on Soilrite in presence and absence of 1-MCP as indicated, under continuous white light for 7d. Root penetration (A) and phenotypes (B) of pct1-2 seedlings grown in the presence of different concentrations of 1-MCP. Note that pct1-2 seedlings display partial inhibition of root penetration, a decrease of hypocotyls length, and increase of root elongation at 1-MCP concentrations ≥ 4 μL L−1 . The asterisk (*) indicates a statistically significant difference in response to treatment (p < 0.005 with n = 15–20 per group).
Physiological studies have indicated that the PAT system requires the activity of specific auxin influx and efflux carriers located on the plasma membrane of transporting cells.66,67,68 In Arabidopsis and other plants, PIN proteins have been shown to be intimately linked with polar auxin transport.69,70 In Arabidopsis, ethylene regulates the transcription of several auxin transporters including PIN1, PIN2 and AUX1,71,72 thus enhancing the auxin transport in roots. Recently we demonstrated that the pct1-2 mutant shows enhanced expression of PIN1 in the root, which explains its increased polar transport.73,74 To further explore the link between the enhanced PAT in pct1-2 and its resistance to 1-MCP, we treated pct1-2 seedlings with L-buthionine-(S,R)-sulfoximine (BSO), an inhibitor of glutathione biosynthesis that downregulates the expression of several PIN proteins in Arabidopsis such as PIN1, PIN2 and PIN3.75,76 Similar to the TIBA application,62 application of BSO together with 1-MCP caused a reduction of DR5 activity both in wild type and pct1-2 roots (Fig. 2A). In parallel with the decline in DR5 activity, the root penetration was also inhibited, although much more effectively in wild type than in pct1-2 (Fig. 2B) These results potentially reveal that the decrease in the auxin level and root penetration by 1-MCP is associated with reduced PIN function.
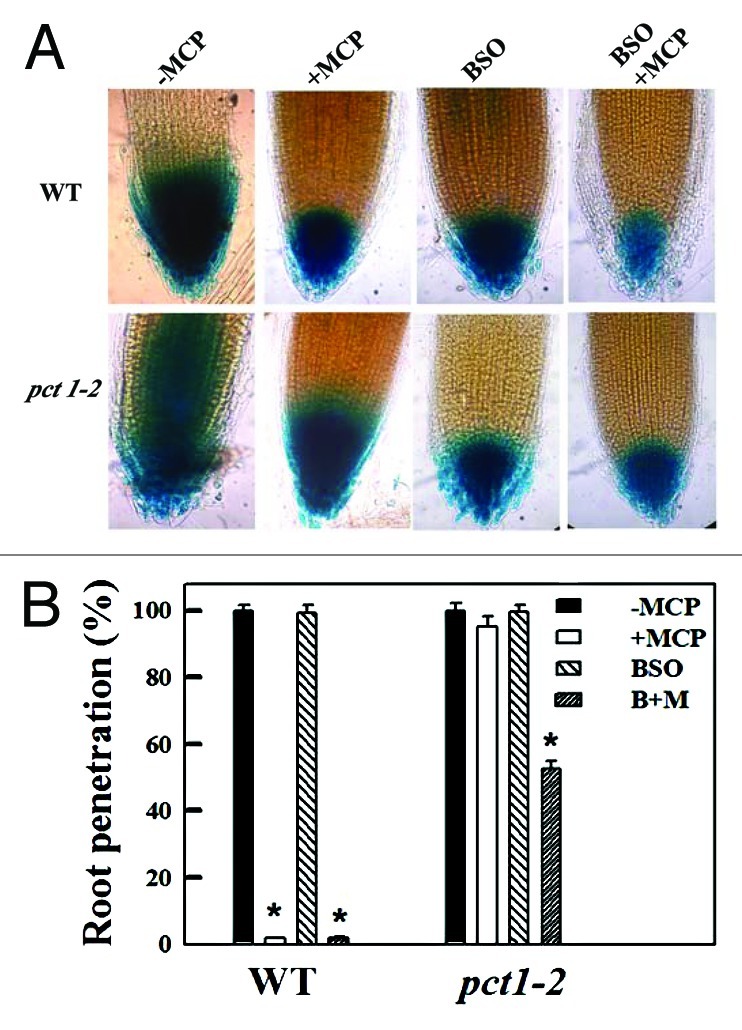
Figure 2. Quantification of the effect of L-buthionine-(S,R)-sulfoximine (BSO) on root penetration in pct1-2 seedlings. Note that simultaneous application of BSO along with 2 μL L−1 1-MCP inhibits DR5::GUS expression in the root tips (A) and root penetration (B) of pct1-2 seedlings. Seedlings were grown on Soilrite moistened with 0.5 mM BSO in the presence or absence of 1-MCP. The control seedlings were grown on Soilrite moistened with water. The asterisk (*) indicates a statistically significant difference in response to treatment (p < 0.005 with n = 15–20 per group).
Our results indicate an intimate interaction between ethylene and the auxin-signaling pathway in regulating root penetration. Our results are consistent with reports showing that in tomato the ethylene-perception Nr mutant shows reduced IAA transport in roots and the ethylene overproducing epi mutant shows increased IAA transport in roots.47,77 Those reports also suggested that ethylene has contrasting roles in auxin transport in roots and hypocotyls, with a positive regulation of PAT in roots and a negative modulation in stem tissue. Similarly, we also observed some contrasting responses in tomato roots and hypocotyls upon treatment with 1-MCP in terms of growth patterns and expression of ethylene and auxin related genes. The fact that the application of 1-MCP reduced both the acropetal and basipetal auxin transport in wild type tomato roots supports that ethylene acts in root penetration and penetration-induced growth changes at least in part by increasing polar auxin transport. In summary, our findings demonstrate an obligatory requirement for ethylene action for executing the root penetration in tomato. We conclude that ethylene regulates root penetration, at least in part, by crosstalk with auxin. In the future, a better knowledge of the root penetration response under impedance should help to develop strategies to breed crops for improved root penetration and foraging function to acquire water and nutrients for plants.
Acknowledgments
This work was supported by International Atomic Energy Agency, Vienna, Austria and Department of Biotechnology, New Delhi, India grant (R.S.), Department of Biotechnology, New Delhi, India grant (Y.S.), University Grants Commission, New Delhi fellowship to P.S., University of Hyderabad fellowship to N.S, and the USDA National Research Initiative Competitive Grants Program (grant 2006-03434) to M.G.I.
Glossary
Abbreviations:
- PAT
polar auxin transport
- BSO
L-butathione-(S,R)-sulfoximine
- TIBA
tri-iodobenzoic acid
- pct
polycotyledon
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18936
References
- 1.Knight T. On the direction of the radicle and germen during the vegetation of seeds. Phil Trans R Soc Lond Ser B. 1806;99:108–20. [Google Scholar]
- 2.Gregory PJ. Plant roots. Growth, activity and interaction with soils. Oxford: Blackwell. 2006. [Google Scholar]
- 3.Taylor HM, Gardner HR. Penetration of cotton seedling tap roots as influenced by bulk density, moisture content and strength of soil. Soil Sci. 1963;96:153–6. doi: 10.1097/00010694-196309000-00001. [DOI] [Google Scholar]
- 4.Morita MT. Directional gravity sensing in gravitropism. Annu Rev Plant Biol. 2010;61:705–20. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- 5.Leitz G, Kang BH, Schoenwaelder ME, Staehelin LA. Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity-sensing Arabidopsis columella cells. Plant Cell. 2009;21:843–60. doi: 10.1105/tpc.108.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita MT, Tasaka M. Gravity sensing and signaling. Curr Opin Plant Biol. 2004;7:712–8. doi: 10.1016/j.pbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell. 2001;13:907–21. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muday GK, Rahman A. Auxin transport and the integration of gravitropic growth. In: Gilroy S, Masson PH, eds. Plant Tropisms. Hoboken NJ: Blackwell Publishing, 2008: 47–78. [Google Scholar]
- 9.Blancaflor EB, Masson PH. Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol. 2003;133:1677–90. doi: 10.1104/pp.103.032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monshausen GB, Swanson SJ, Gilroy S. Touch Sensing and Thigmotropism. In: Gilroy S, Masson PH, eds.Plant Tropisms. Hoboken, NJ: Blackwell Publishing, 2008:91–122. [Google Scholar]
- 11.Materechera SA, Dexter AR, Alston AM. Penetration of very strong soils by seedling roots of different plant-species. Plant Soil. 1991;135:31–41. doi: 10.1007/BF00014776. [DOI] [Google Scholar]
- 12.Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA. Root responses to soil physical conditions; growth dynamics from field to cell. J Exp Bot. 2006;57:437–47. doi: 10.1093/jxb/erj003. [DOI] [PubMed] [Google Scholar]
- 13.Rose TJ, Rengel Z, Ma Q, Bowden JW. Crop species differ in root plasticity response to localised P supply. J Plant Nutr Soil Sci. 2009;172:360–8. doi: 10.1002/jpln.200800031. [DOI] [Google Scholar]
- 14.Clark LJ, Aphale´ SL, Barraclough PB. Screening the ability of rice roots to overcome the mechanical impedance of wax layers: importance of test conditions and measurement criteria. Plant Soil. 2000;219:187–96. doi: 10.1023/A:1004753900945. [DOI] [Google Scholar]
- 15.Bengough AG, Mullins CE. Mechanical impedance to root growth—a review of experimental techniques and root growth responses. J Soil Sci. 1990;41:341–58. doi: 10.1111/j.1365-2389.1990.tb00070.x. [DOI] [Google Scholar]
- 16.Clark LJ, Price AH, Steele KA, Whalley WR. Evidence from near-isogenic lines that root penetration increases with root diameter and bending stiffness in rice. Funct Plant Biol. 2008;35:1163–71. doi: 10.1071/FP08132. [DOI] [PubMed] [Google Scholar]
- 17.Gewin V. Food: An underground revolution. Nature. 2010;466:552–3. doi: 10.1038/466552a. [DOI] [PubMed] [Google Scholar]
- 18.Stirzaker RJ, Passioura JB, Wilms Y. Soil structure and plant growth: impact of bulk density and biopores. Plant Soil. 1996;185:151–62. doi: 10.1007/BF02257571. [DOI] [Google Scholar]
- 19.Gregory PJ. Roots, rhizosphere and soil: the route to a better understanding of soil science? Eur J Soil Sci. 2006;57:2–12. doi: 10.1111/j.1365-2389.2005.00778.x. [DOI] [Google Scholar]
- 20.O’Brien EE, Brown JS, Moll JD. Roots in space: a spatially explicit model for below-ground competition in plants. Proc Biol Sci. 2007;274:929–34. doi: 10.1098/rspb.2006.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migliaccio F, Piconese S. Spiralizations and tropisms in Arabidopsis roots. Trends Plant Sci. 2001;6:561–5. doi: 10.1016/S1360-1385(01)02152-5. [DOI] [PubMed] [Google Scholar]
- 22.Massa GD, Gilroy S. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. Plant J. 2003;33:435–45. doi: 10.1046/j.1365-313X.2003.01637.x. [DOI] [PubMed] [Google Scholar]
- 23.Simmons C, Söll D, Migliaccio F. Circumnutation and gravitropism cause root waving in Arabidopsis thaliana. J Exp Bot. 1995;46:143–50. doi: 10.1093/jxb/46.1.143. [DOI] [Google Scholar]
- 24.Evans M. Touch sensitivity in plants: be aware or beware. Trends Plant Sci. 2003;8:312–4. doi: 10.1016/S1360-1385(03)00133-X. [DOI] [PubMed] [Google Scholar]
- 25.Arnaud C, Bonnot C, Desnos T, Nussaume L. The root cap at the forefront. C R Biol. 2010;333:335–43. doi: 10.1016/j.crvi.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Iijima M, Higuchi T, Barlow PW. Contribution of root cap mucilage and presence of an intact root cap in maize (Zea mays) to the reduction of soil mechanical impedance. Ann Bot. 2004;94:473–7. doi: 10.1093/aob/mch166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawes MC, Bengough G, Cassab G, Ponce G. Root caps and rhizosphere. J Plant Growth Regul. 2003;21:352–67. doi: 10.1007/s00344-002-0035-y. [DOI] [Google Scholar]
- 28.Hawes MC, Gunawardena U, Miyasaka S, Zhao X. The role of root border cells in plant defense. Trends Plant Sci. 2000;5:128–33. doi: 10.1016/S1360-1385(00)01556-9. [DOI] [PubMed] [Google Scholar]
- 29.Blancaflor EB, Fasano JM, Gilroy S. Laser ablation of root cap cells: implications for models of graviperception. Adv Space Res. 1999;24:731–8. doi: 10.1016/S0273-1177(99)00406-8. [DOI] [PubMed] [Google Scholar]
- 30.Legue´ V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasano JM, Massa GD, Gilroy S. Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul. 2002;21:71–88. doi: 10.1007/s003440010049. [DOI] [PubMed] [Google Scholar]
- 32.Trewavas A, Knight M. Mechanical signalling, calcium and plant form. Plant Mol Biol. 1994;26:1329–41. doi: 10.1007/BF00016478. [DOI] [PubMed] [Google Scholar]
- 33.Monshausen GB, Gilroy S. Feeling green: mechanosensing in plants. Trends Cell Biol. 2009;19:228–35. doi: 10.1016/j.tcb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–4. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell. 2009;21:2341–56. doi: 10.1105/tpc.109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimbrough JM, Salinas-Mondragon R, Boss WF, Brown CS, Sederoff HW. The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex. Plant Physiol. 2004;136:2790–805. doi: 10.1104/pp.104.044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monshausen GB, Sievers A. Weak mechanical stimulation causes hyperpolarisation in root cells of Lepidium. Bot Acta. 1998;111:303–6. [Google Scholar]
- 38.Almagro L, Go´mez Ros LV, Belchi-Navarro S, Bru R, Ros Barcelo´ A, Pedreño MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60:377–90. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 39.Kerr EM, Fry SC. Extracellular cross-linking of xylan and xyloglucan in maize cell-suspension cultures: the role of oxidative phenolic coupling. Planta. 2004;219:73–83. doi: 10.1007/s00425-004-1210-0. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci U S A. 2007;104:3639–44. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–6. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- 42.Okada K, Shimura Y. Modulation of root growth by physical stimuli. In: EM Meyerowitz, CR Somerville, eds, Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1994:665–84. [Google Scholar]
- 43.Mochizuki S, Harada A, Inada S, Sugimoto-Shirasu K, Stacey N, Wada T, et al. The Arabidopsis WAVY GROWTH 2 protein modulates root bending in response to environmental stimuli. Plant Cell. 2005;17:37–47. doi: 10.1105/tpc.104.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto T, Tsurumi S, Shibasaki K, Obana Y, Takaji H, Oono Y, et al. Genetic dissection of hormonal responses in the roots of Arabidopsis grown under continuous mechanical impedance. Plant Physiol. 2008;146:1651–62. doi: 10.1104/pp.107.115519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, et al. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–96. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008;55:175–87. doi: 10.1111/j.1365-313X.2008.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Negi S, Sukumar P, Liu X, Cohen JD, Muday GK. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J. 2010;61:3–15. doi: 10.1111/j.1365-313X.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 48.Růzicka K, Ljung K, Vanneste S, Podhorsk´ R, Beeckman T, Friml J, et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buer CS, Sukumar P, Muday GK. Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol. 2006;140:1384–96. doi: 10.1104/pp.105.075671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol. 2002;130:1908–17. doi: 10.1104/pp.010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008;55:335–47. doi: 10.1111/j.1365-313X.2008.03528.x. [DOI] [PubMed] [Google Scholar]
- 52.Ivanchenko MG, Napsucialy-Mendivil S, Dubrovsky JG. Auxin-induced inhibition of lateral root initiation contributes to root system shaping in Arabidopsis thaliana. Plant J. 2010;64:740–52. doi: 10.1111/j.1365-313X.2010.04365.x. [DOI] [PubMed] [Google Scholar]
- 53.Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998;16:553–60. doi: 10.1046/j.1365-313x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 54.Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–85. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark LJ, Whalley WR, Barraclough PB. How do roots penetrate strong soil? Plant Soil. 2003;255:93–104. doi: 10.1023/A:1026140122848. [DOI] [Google Scholar]
- 56.Zacarias L, Reid MS. Inhibition of ethylene action prevents root penetration through compressed media in tomato (Lycopersicon esculentum) seedlings. Physiol Plant. 1992;86:301–7. doi: 10.1034/j.1399-3054.1992.860217.x. [DOI] [Google Scholar]
- 57.Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ. Root formation in ethylene-insensitive plants. Plant Physiol. 1999;121:53–60. doi: 10.1104/pp.121.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallie DR, Geisler-Lee J, Chen J, Jolley B. Tissue-specific expression of the ethylene biosynthetic machinery regulates root growth in maize. Plant Mol Biol. 2009;69:195–211. doi: 10.1007/s11103-008-9418-1. [DOI] [PubMed] [Google Scholar]
- 59.Marti´nez-Romero D, Baile´n G, Serrano M, Guille´n F, Valverde JM, Zapata P, et al. Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: a review. Crit Rev Food Sci Nutr. 2007;47:543–60. doi: 10.1080/10408390600846390. [DOI] [PubMed] [Google Scholar]
- 60.Sisler EC. The discovery and development of compounds counteracting ethylene at the receptor level. Biotechnol Adv. 2006;24:357–67. doi: 10.1016/j.biotechadv.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Blankenship S, Dole JM. 1-Methylcyclopropene: A review. Postharvest Biol Technol. 2003;28:1–25. doi: 10.1016/S0925-5214(02)00246-6. [DOI] [Google Scholar]
- 62.Santisree P, Nongmaithem S, Vasuki H, Sreelakshmi Y, Ivanchenko MG, Sharma R. Tomato root penetration in soil requires a coaction between ethylene and auxin signaling. Plant Physiol. 2011;156:1424–38. doi: 10.1104/pp.111.177014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Hammadi ASA, Sreelakshmi Y, Negi S, Siddiqi I, Sharma R. The polycotyledon mutant of tomato shows enhanced polar auxin transport. Plant Physiol. 2003;133:113–25. doi: 10.1104/pp.103.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madishetty K, Bauer P, Sharada MS, Al-Hammadi ASA, Sharma R. Genetic characterization of the polycotyledon locus in tomato. Theor Appl Genet. 2006;113:673–83. doi: 10.1007/s00122-006-0332-0. [DOI] [PubMed] [Google Scholar]
- 65.Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, et al. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci U S A. 2008;105:4489–94. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muday GK, Murphy AS. An emerging model of auxin transport regulation. Plant Cell. 2002;14:293–9. doi: 10.1105/tpc.140230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baluska F, Samaj J, Menzel D. Polar transport of auxin: carrier-mediated flux across the plasma membrane or neurotransmitter-like secretion? Trends Cell Biol. 2003;13:282–5. doi: 10.1016/S0962-8924(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 68.Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–16. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 70.Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol. 2008;24:447–73. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- 71.Paponov IA, Teale WD, Trebar M, Blilou I, Palme K. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci. 2005;10:170–7. doi: 10.1016/j.tplants.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Petr´sek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertov´ D, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–8. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 73.Kharshiing EV, Kumar GP, Ditengou FA, Li X, Palme K, Sharma R. The polycotyledon (pct1-2) mutant of tomato shows enhanced accumulation of PIN1 auxin transport facilitator protein. Plant Biol (Stuttg) 2010;12:224–8. doi: 10.1111/j.1438-8677.2009.00267.x. [DOI] [PubMed] [Google Scholar]
- 74.Kharshiing EV, Kumar GP, Sharma R. PIN it on auxin: the role of PIN1 and PAT in tomato development. Plant Signal Behav. 2010;5:1379–83. doi: 10.4161/psb.5.11.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, et al. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell. 2010;22:376–91. doi: 10.1105/tpc.109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koprivova A, Mugford ST, Kopriva S. Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Rep. 2010;29:1157–67. doi: 10.1007/s00299-010-0902-0. [DOI] [PubMed] [Google Scholar]
- 77.Lewis DR, Negi S, Sukumar P, Muday GK. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development. 2011;138:3485–95. doi: 10.1242/dev.065102. [DOI] [PubMed] [Google Scholar]


