Abstract
Plants emit volatile organic compounds (VOCs) as a means to warn other plants of impending danger. Nearby plants exposed to the induced VOCs prepare their own defense weapons in response. Accumulated data supports this assertion, yet much of the evidence has been obtained in laboratories under artificial conditions where, for example, a single VOC might be applied at a concentration that plants do not actually experience in nature. Experiments conducted outdoors suggest that communication occurs only within a limited distance from the damaged plants. Thus, the question remains as to whether VOCs work as a single component or a specific blend, and at which concentrations VOCs elicit insect and pathogen defenses in undamaged plants. We discuss these issues based on available literature and our recent work, and propose future directions in this field.
Keywords: cis-jasmone, ethylene, green leaf volatiles, isoprene, methyl jasmonate, methyl salicylate, plant-plant communications, pyrethrins, terpenoids, volatile organic compounds, within-plant communications
Introduction
Plants are exposed to various stress factors such as disease, injury, herbivory, extreme heat/cold, etc. Hence, they must adjust their physiological state either in response to, or in preparation for, such threats to their well-being and survival.1-5 To achieve this adjustment, plants have developed a communication system to transmit information based on volatile organic compounds (VOCs).
Plants emit VOCs under other circumstances besides the threat of danger. Notably, flowers use VOCs to attract pollinators and ensure reproduction.6,7 Induced VOCs provide more than just a scent. In a damaged plant, VOCs are also used as nonvolatile signals to transmit SOS messages within the plant itself. The airborne signals are diffused to reach undamaged plants nearby, giving them the chance to strengthen their own defense system. The receivers are not limited to conspecies. Natural enemies can also catch the SOS signals and locate the place of battle.8-13
By changing the volatile components and their blend ratios, plants can create specific messages for communication. Earlier studies mainly investigated the effects of individual VOCs on plant defense systems because a single compound is easier to test than a blend of compounds. However, there is increasing evidence that VOCs work as blends in plant-plant communication. Thus, we look at the current status of VOCs in studies on within-plant and plant-plant communications to address the question, “Plant communication: mediated by individual or blended VOCs?”
Plant-Plant Communication
The trigger for development in this field was the discovery that undamaged poplar and sugar maple trees accumulated phenolics and tannins when situated close to damaged trees.14 However, in this original report, no active principle was identified. Methyl jasmonate (MeJA) emitted by sagebrush (Artemisia tridentata) was the first compound shown to render intact plants resistant to herbivores by increasing the proteinase inhibitor production.15 Later on, other VOCs emitted by damaged plants were found to influence the receiver plants, regardless of whether or not the receivers were conspecies.16-21
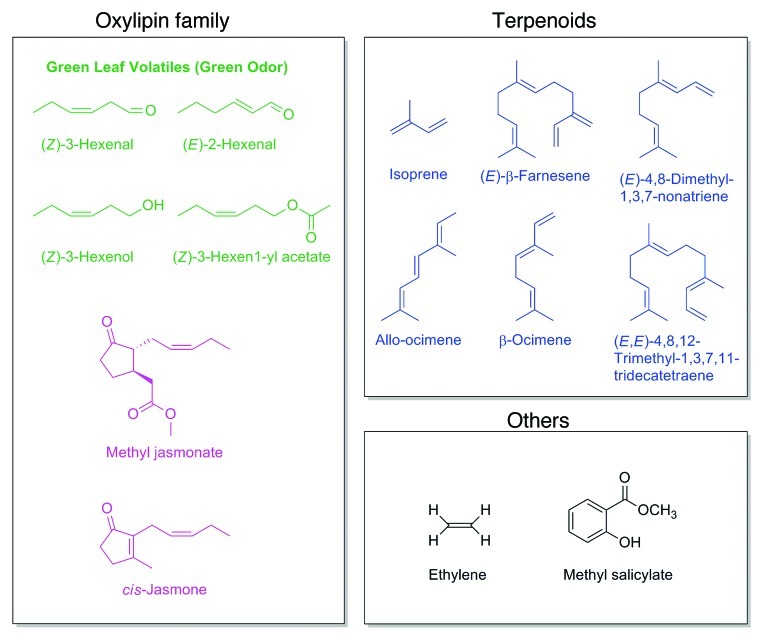
Gas chromatography–mass spectroscopy (GC-MS) has been employed to identify a variety of induced plant VOCs (Fig. 1). One major group is the terpenoids, with subgroups named after the carbon numbers. Although countless terpenoid species have been discovered, only a limited number are involved in plant communications. An equally major group is the green leaf volatiles (GLVs) generated from lipids (Fig. 1). They are produced from C18 fatty acids, particularly linolenic acid, by the action of hydroperoxide lyases and the subsequent shift of the olefinic bond, reduction of the carbonyl group and esterification.21 Furthermore, the small olefins ethylene and isoprene as well as the oxylipin metabolites MeJA and cis-jasmone add to such “ecology tuning” volatiles.
Figure 1. VOCs used for different messages in plant-plant communications. Classified by oxylipin family, terpenoids and others.
In Phaseolus lunatus, (E)-β-ocimene, (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) and (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene (TMTT) (Fig. 1) are emitted in response to spider mite infestation and these VOCs differentially enhance the expression of LOX, FPS and some PR genes in intact leaves.22 On the other hand, in Zea mays, GLVs induce the accumulation of jasmonic acid (JA) with prolonged storage time as well as the release of a greater amount of VOCs.23 Also in this plant species, (Z)-3-hexenol induces the production of (Z)-3-hexenyl acetate and methyl salicylate (MeSA) (Fig. 1) along with the expression of LOX, PAL and maize proteinase inhibitor genes for defense against herbivores.24 In Arabidopsis, GLVs and allo-ocimene enhance resistance to pathogens such as Botrytis cinerea, suggesting that VOCs elicit resistance not only to herbivores, but also to pathogens.25,26
The phytohormones MeSA and ethylene are also volatiles involved in defense. MeSA is emitted from the local infected region to induce systemic acquired resistance (SAR) in the emitter itself and receivers.27 Meanwhile, ethylene, which is known to enhance maturation of various fruits, particularly underlies plant disease response.28,29 Ethylene has also been found to amplify the defense response induced by (Z)-3-hexen-1-ol and amplify the emission of sesquiterpenes in Zea mays.30
cis-Jasmone (Fig. 1) is a metabolite closely related to JA. Arabidopsis was treated with cis-jasmone and the induced gene expression was investigated by microarray to show that this molecule’s mode of action was distinct from that of JA in gene induction.31 Application of cis-jasmone induces defense responses such as synthesis of secondary metabolites and attraction of natural enemies. Furthermore, this signal molecule directly reduces the development of pest infestation, disease and weeds.32,33
Importance of Dose and Ratio
Previous literature as a whole demonstrates that induced VOCs have the ability to enhance plant defense systems. Yet, many of the studies treated their target plants with a single molecule, or native VOC blends, but not with synthetic VOC mixtures of known composition. Also lacking is the concentration-response data to prove that VOCs enhance plant defense at concentrations that plants are actually exposed to in nature. This has recently come to the attention of scientists.34-36 For example, the efficacy of cucumbers to attract melon flies varies among cultivars, and that of grapes to attract the herbivorous moth Paralobesia viteana depends on the VOC blend ratios.37,38 Also, changing herbivore species influences the blend ratio in grapes.39
In our study, we selected the Pyrethrum daisy (Tanacetum cinerariifolium; earlier species name: Chrysanthemum cinerariaefolium) to examine the effects of wound-induced VOCs on the biosynthesis of pyrethrins that are insecticidal metabolites of this plant species.40 The pyrethrin amount in intact young seedlings was increased by placing intact seedlings in the vicinity of wounded seedlings. GS-MS detected significantly enhanced emissions of (Z)-3-hexenal, (E)-2-hexenal, (Z)-3-hexen-1-ol, (Z)-3-hexen-1-yl acetate and (E)-β-farnesene from the wounded T. cinerariifolium seedlings. The blend ratio of the VOCs varied dynamically with time after wounding. The five VOC concentrations were quantified and mixed together at a ratio similar to that observed 35–60 min after wounding to examine the effects of the VOC mixture on pyrethrin biosynthesis. One intriguing observation was that the synthetic VOC was effective only at the concentration at which it was observed in the glassware used to quantify the concentration; both a 10-fold increase and a decrease to 1/10 the concentration resulted in a marked reduction in gene expression of 1-deoxy-D-xylulose 5-phosphate synthase (DXS), chrysanthemyl diphosphate synthase (CPPase) and allene oxide synthase (AOS), which are involved in biosynthesis.
Another interesting discovery in Pyrethrum was that the wound-induced VOCs were effective on pyrethrin biosynthesis only when all five components were mixed. Eliminating just one component from the five-VOC mixture resulted in reduced gene expression of 13-lipoxygenase as well as DXS, CPP and AOS, demonstrating that both the concentration and blend ratio play an important role in establishing plant-plant communications. In sagebrush, VOC-mediated plant-plant communications were observed only when the receiver plants were placed at a certain distance from the emitter plants.12,41 These examples together suggest that plant-plant communication works within a narrow concentration range.
Pyrethrum also relies on within-plant communications to control pyrethrin biosynthesis. Mechanical wounding in older leaves led to increased pyrethrin I in younger intact leaves in the same seedlings, but this effect was prevented by wrapping the receiver leaves, suggesting the contribution of wound-induced VOCs to the increase of pyrethrin I in the intact receiver leaves.42 Unlike the case of plant-plant communications, the concentration of induced VOCs faced by intact leaves is rather high, and so even a single VOC may work as a warning message within wounded plants. Including T. cinerariifolium, more studies are needed to show that the concentration of each VOC reaches the effective concentration at intact leaves within damaged plants.
Future Perspectives
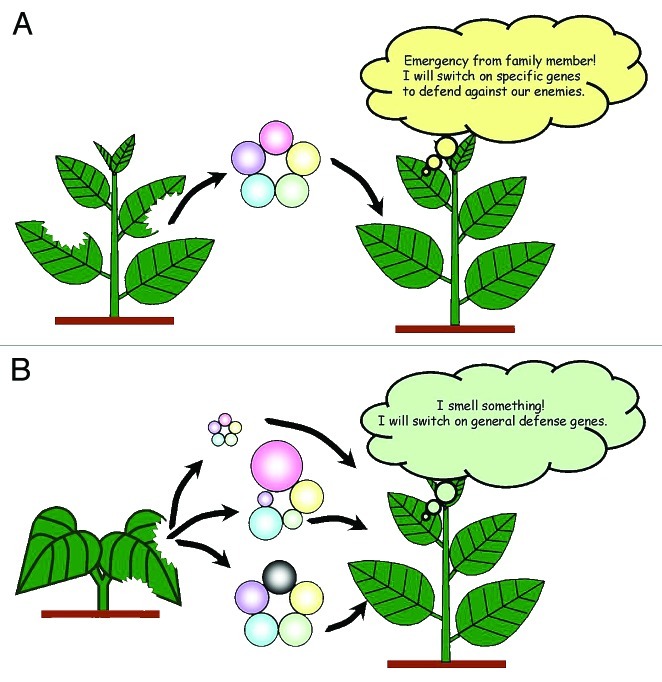
Given the accumulated evidence, we can conclude that both individual and blended VOCs are important in plant communications, but which is most important depends on the concentration. Since the individual VOCs are not species-specific, the blend ratio determines the specificity of VOC-mediated plant-plant communications in conspecies (Fig. 2A), reducing the risk of eavesdropping by other species. This has also led to insect-plant coevolution. However, relying on specific VOCs can lead to an inability to respond to herbivores that target a broad range of plant species. To prepare a defense against generalists, plants eavesdrop on the herbivore-induced VOCs from other species (Fig. 2B).43,44
Figure 2. VOCs emitted by injured plants have a specific ratio and concentration of components. Upon receiving a VOC message from their family, plants respond by inducing a particular defense mechanism. For example, the plant might prepare specific secondary metabolites for defense against herbivores (A). The danger signals emitted by the family provide warning that a species-specific enemy (specialist) is nearby. In contrast, plants that receive a VOC message from other families might elicit a general defense response to prevent damage by herbivores (generalists) attacking various plant species (B). By sharing common VOC information across the plant kingdom, plants are able to prevent attack from a broad range of herbivores.
In this review, we did not discuss the “priming” effect of VOCs.45 After exposure to VOC signals, even if there is no apparent change in metabolites or relevant gene expression, the receiver plants are able to respond more vigorously to herbivore attack compared with naïve plants. At present, little is known about the concentration-response relationship and the individual or blended VOC question for such priming effects, which remains to be investigated. The recent infection with cucumber mosaic viruses has been shown to modulate the volatile blends to attract vector insect vectors.46,47 It has also been shown that the experience of pathogen attack is inherited by the next generation through epigenetics.48 Thus, it is important in the future to consider these topics to enhance our understanding of VOC-mediated plant communication.
Acknowledgments
K.M. was supported in part by Grants-in-Aid for Scientific Research (S) (No. 19101009) and Core-to-Core Program (No. 20004) from the Japan Society for the Promotion of Science. Also the corresponding author was supported by a Strategic Project to Support the Formation of Research Bases at Private Universities: Matching Fund Subsidy (S1101035) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Glossary
Abbreviations:
- AOS
allene oxide synthase
- CPPase
chrysanthemyl diphosphate synthase
- DXS
1-deoxy-D-xylulose 5-phosphate synthase
- GLV
green leaf volatile
- JA
jasmonic acid
- MeJA
methyl jasmonate
- MeSA
methyl salicylate
- VOC
volatile organic compound
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18765
References
- 1.Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol. 2009;5:283–91. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- 2.Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci U S A. 2007;104:5467–72. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science. 2006;311:812–5. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 4.Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, et al. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- 5.Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci. 2006;25:417–40. doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 6.Simpson BB, Neff JL. Floral rewards: alternatives to pollen and nectar. Ann Mo Bot Gard. 1981;68:301–22. doi: 10.2307/2398800. [DOI] [Google Scholar]
- 7.Koptur S. Extrafloral nectary-mediated interactions between insects and plants. In: Bernays EA, ed. Insect-Plant Interactions: CRC Press, Boca Raton, 1992; 81-129. [Google Scholar]
- 8.González-Teuber M, Heil M. Nectar chemistry is tailored for both attraction of mutualists and protection from exploiters. Plant Signal Behav. 2009;4:809–13. doi: 10.4161/psb.4.9.9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–3. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 10.Arimura G, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 2009;50:911–23. doi: 10.1093/pcp/pcp030. [DOI] [PubMed] [Google Scholar]
- 11.Unsicker SB, Kunert G, Gershenzon J. Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol. 2009;12:479–85. doi: 10.1016/j.pbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Heil M, Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol Evol. 2010;25:137–44. doi: 10.1016/j.tree.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Hare JD. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol. 2011;56:161–80. doi: 10.1146/annurev-ento-120709-144753. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin IT, Schultz JC. Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science. 1983;221:277–9. doi: 10.1126/science.221.4607.277. [DOI] [PubMed] [Google Scholar]
- 15.Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990;87:7713–6. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heil M. Indirect defence via tritrophic interactions. New Phytol. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 17.Arimura G, Garms S, Maffei M, Bossi S, Schulze B, Leitner M, et al. Herbivore-induced terpenoid emission in Medicago truncatula: concerted action of jasmonate, ethylene and calcium signaling. Planta. 2008;227:453–64. doi: 10.1007/s00425-007-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tscharntke T, Thiessen S, Dolch R, Boland W. Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa. Biochem Syst Ecol. 2001;29:1025–47. doi: 10.1016/S0305-1978(01)00048-5. [DOI] [Google Scholar]
- 19.Preston CA, Laue G, Baldwin IT. Plant-plant signaling: application of trans- or cis-methyl jasmonate equivalent to sagebrush releases does not elicit direct defenses in native tobacco. J Chem Ecol. 2004;30:2193–214. doi: 10.1023/B:JOEC.0000048783.64264.2a. [DOI] [PubMed] [Google Scholar]
- 20.Tamogami S, Rakwal R, Agrawal GK. Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem Biophys Res Commun. 2008;376:723–7. doi: 10.1016/j.bbrc.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 21.Hatanaka A. The fresh green odor emitted by plants. Food Rev Int. 1996;12:303–50. doi: 10.1080/87559129609541083. [DOI] [Google Scholar]
- 22.Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–5. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 23.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A. 2004;101:1781–5. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farag MA, Fokar M, Abd H, Zhang H, Allen RD, Pare´ PW. (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta. 2005;220:900–9. doi: 10.1007/s00425-004-1404-5. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005;46:1093–102. doi: 10.1093/pcp/pci122. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Analysis of defensive responses activated by volatile allo-ocimene treatment in Arabidopsis thaliana. Phytochemistry. 2006;67:1520–9. doi: 10.1016/j.phytochem.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–6. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- 28.Dong X. SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol. 1998;1:316–23. doi: 10.1016/1369-5266(88)80053-0. [DOI] [PubMed] [Google Scholar]
- 29.Gutterson N, Reuber TL. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol. 2004;7:465–71. doi: 10.1016/j.pbi.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Ruther J, Kleier S. Plant-plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. J Chem Ecol. 2005;31:2217–22. doi: 10.1007/s10886-005-6413-8. [DOI] [PubMed] [Google Scholar]
- 31.Bruce TJ, Matthes MC, Chamberlain K, Woodcock CM, Mohib A, Webster B, et al. cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc Natl Acad Sci U S A. 2008;105:4553–8. doi: 10.1073/pnas.0710305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moraes MC, Birkett MA, Gordon-Weeks R, Smart LE, Martin JL, Pye BJ, et al. cis-Jasmone induces accumulation of defence compounds in wheat, Triticum aestivum. Phytochemistry. 2008;69:9–17. doi: 10.1016/j.phytochem.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Matthes MC, Bruce TJ, Ton J, Verrier PJ, Pickett JA, Napier JA. The transcriptome of cis-jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defence. Planta. 2010;232:1163–80. doi: 10.1007/s00425-010-1244-4. [DOI] [PubMed] [Google Scholar]
- 34.Fontana A, Held M, Fantaye CA, Turlings TC, Degenhardt J, Gershenzon J. Attractiveness of constitutive and herbivore-induced sesquiterpene blends of maize to the parasitic wasp Cotesia marginiventris (Cresson) J Chem Ecol. 2011;37:582–91. doi: 10.1007/s10886-011-9967-7. [DOI] [PubMed] [Google Scholar]
- 35.Danner H, Boeckler GA, Irmisch S, Yuan JS, Chen F, Gershenzon J, et al. Four terpene synthases produce major compounds of the gypsy moth feeding-induced volatile blend of Populus trichocarpa. Phytochemistry. 2011;72:897–908. doi: 10.1016/j.phytochem.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Bruce TJ, Pickett JA. Perception of plant volatile blends by herbivorous insects--finding the right mix. Phytochemistry. 2011;72:1605–11. doi: 10.1016/j.phytochem.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Siderhurst MS, Jang EB. Cucumber volatile blend attractive to female melon fly, Bactrocera cucurbitae (Coquillett) J Chem Ecol. 2010;36:699–708. doi: 10.1007/s10886-010-9804-4. [DOI] [PubMed] [Google Scholar]
- 38.Cha DH, Linn CE, Jr., Teal PE, Zhang A, Roelofs WL, Loeb GM. Eavesdropping on plant volatiles by a specialist moth: significance of ratio and concentration. PLoS One. 2011;6:e17033. doi: 10.1371/journal.pone.0017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierre PS, Jansen JJ, Hordijk CA, van Dam NM, Cortesero AM, Dugravot S. Differences in volatile profiles of turnip plants subjected to single and dual herbivory above- and belowground. J Chem Ecol. 2011;37:368–77. doi: 10.1007/s10886-011-9934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kikuta Y, Ueda H, Nakayama K, Katsuda Y, Ozawa R, Takabayashi J, et al. Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. Plant Cell Physiol. 2011;52:588–96. doi: 10.1093/pcp/pcr017. [DOI] [PubMed] [Google Scholar]
- 41.Karban R, Shiojiri K, Huntzinger M, McCall AC. Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology. 2006;87:922–30. doi: 10.1890/0012-9658(2006)87[922:DRISVA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Ueda H, Matsuda K. VOC-mediated within-plant communications and nonvolatile systemic signals upregulate pyrethrin biosynthesis in wounded seedling of Chrysanthemum cinerariaefolium. J Plant Interact. 2011;6:89–91. doi: 10.1080/17429145.2011.555566. [DOI] [Google Scholar]
- 43.Song YY, Zeng RS, Xu JF, Li J, Shen X, Yihdego WG. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS One. 2010;5:e13324. doi: 10.1371/journal.pone.0013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arimura G, Shiojiri K, Karban R. Acquired immunity to herbivory and allelopathy caused by airborne plant emissions. Phytochemistry. 2010;71:1642–9. doi: 10.1016/j.phytochem.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol. 2006;9:274–80. doi: 10.1016/j.pbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Mauck KE, De Moraes CM, Mescher MC. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc Natl Acad Sci U S A. 2010;107:3600–5. doi: 10.1073/pnas.0907191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauck KE, De Moraes CM, Mescher MC. Effects of Cucumber mosaic virus infection on vector and non-vector herbivores of squash. Commun Integr Biol. 2010;3:579–82. doi: 10.4161/cib.3.6.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaskiewicz M, Conrath U, Peterhänsel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011;12:50–5. doi: 10.1038/embor.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]