A paper by Korol et al. (1) in this issue demonstrates significant premating reproductive divergence between Drosophila melanogaster populations adapted to distinct, but closely adjacent, habitats in “Evolution Canyon” on Mt. Carmel, Israel. The authors suggest that reproductive isolation has evolved in situ as a result of adaptive divergence in response to the contrasting environments of north- and south-facing slopes in Evolution Canyon despite the fact that the populations are within easy “cruising range” (2) of each other. The authors suggest that the divergence of Drosophila occupying distinct habitats in Evolution Canyon represents an early stage in ecological speciation in which divergent natural selection drives the accumulation of genetic differences among populations, resulting in reproductive isolation.
The paper by Korol et al. (1) addresses one of the most persistent questions in evolutionary biology: How do new species arise? As with so many apparently simple questions, there is no simple answer, only complex answers to a number of interrelated questions. How do sexually reproducing organisms become reproductively isolated? How do environment and ecological interactions influence the formation of new species? Are the processes of local adaptation and the evolution of reproductive isolation the same (i.e., both resulting from the accumulation of small, adaptive genetic changes) or are the genetic changes leading to reproductive isolation fundamentally different (i.e., large and rapid genetic changes such as chromosomal rearrangements, genetic revolutions, transilience, or founder events)? Is the disruption of gene flow necessary? What are the relative roles of chance events (e.g., genetic drift) and selection in speciation?
The short answer to all of the above questions is that reproductive divergence can evolve in a number of ways. Both drift and selection can be important depending on the number, degree of interaction, and magnitude of effect of genes involved in reproductive isolation; on the relationship between genes controlling reproductive compatibility and phenotypic characters that may be under ecological selection; and on the historical effective population size of the diverging populations. Reproductive isolation may evolve as a consequence of genetic drift in populations of small effective size (see, e.g., refs. 3–5 for recent treatments), or as a byproduct of adaptive divergence or genetic drift in large populations in allopatry [the classic allopatric divergence model of Mayr (6)]. Reproductive isolation may also evolve in sympatry because of disruptive or divergent selection. For example, natural selection on characters important in both ecological function and mate recognition [such as bill size in Darwin's finches (7) or body size and shape in sticklebacks (8–10)] can lead to premating reproductive isolation without geographic isolation. Rapid chromosomal changes (as in the origin of polyploid plants) can result in instantaneous reproductive isolation, as can changes in genes of large effect that result in rapid evolution of phenotypic characters important in reproduction (e.g., ref. 11). Host shifts in phytophagous insects are well-known to result in essentially instantaneous reproductive isolation (e.g., refs. 12–14). The challenge is to distinguish among alternative hypotheses for diversification in natural populations and to determine the relative roles of selection and drift in speciation.
Natural selection has always been considered a key component of adaptive divergence and speciation (2, 15–17), but the importance of selection has been eclipsed in recent decades by a strong focus on the geography of speciation and on the purely genetic mechanisms by which reproductive isolation evolves (see refs. 18–20 for reviews). Even though selection was seen as critically important in sympatric speciation, sympatric divergence was thought to be rare, and the role of ecology and the environment in diversification received little emphasis. Currently, there is resurgent interest in the role of ecology in speciation. Several recent studies (9, 21–28) have emphasized the importance of ecology and selection in speciation, regardless of the geographic context in which populations diverge, and the term “ecological speciation” has become an important part of the modern lexicon. In a review of 40 years of laboratory experiments on speciation in Drosophila, Rice and Hostert (29) concluded that founder events, drift, and isolation played little role in speciation (but see ref. 20 for critique). On the other hand, diversifying selection was found to contribute substantially to the evolution of reproductive isolation, even when populations were not isolated [see also Barton and Charlesworth (30) who reached a similar conclusion]. This finding bolstered earlier theoretical studies that showed how premating reproductive isolation could evolve as a consequence of ecological selection on characters involved directly in mate recognition or as a consequence of pleiotropy or genetic hitchhiking of genes controlling ecological and reproductive characters (31–33).
The paper by Korol et al. in this issue (1) is important in that it demonstrates significant premating reproductive isolation among populations of D. melanogaster experiencing divergent selection between habitats, whereas there is no reproductive isolation among populations occupying similar habitats. This result is consistent with the evolution of reproductive divergence as a consequence of ecological selection (ecological speciation). However, a key question remains unanswered, namely, did reproductive isolation evolve in situ in response to selection or is the presence of reproductive isolation among populations a result of secondary contact between divergent populations whose ecologies differ and whose ranges abut in Evolution Canyon? This question is important because it bears on the mechanisms by which reproductive isolation has evolved. If the populations are historically divergent, then a degree of reproductive isolation may have evolved because of drift or as a byproduct of adaptive divergence in allopatry before their meeting in Evolution Canyon. If premating reproductive isolation has evolved in situ as a result of local adaptation, then traits under ecological selection must be either directly involved in mate choice, or genetically correlated (via pleiotropy or linkage) with phenotypic characters important in mate choice.
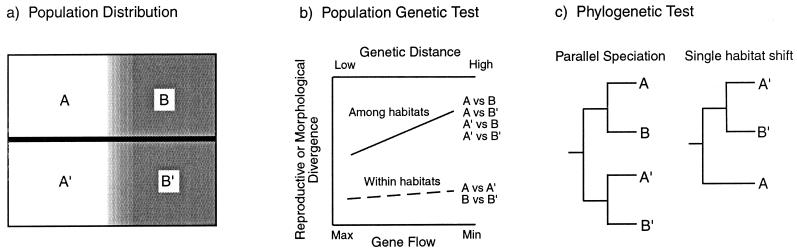
History, and the relative roles of drift and selection, are general issues for empirical studies of speciation in natural populations. One approach to examining the relative roles of drift and selection in population divergence and speciation uses the correlation between reproductive (or morphological) divergence and genetic divergence in neutral molecular characters (Fig. 1). The null model is that reproductive divergence evolves simply as a byproduct of the accumulation of genetic differences among populations because of mutation and drift. Under the null model, populations should show similar levels of reproductive isolation for a given level of genetic distance regardless of their ecological milieu. Several studies have assessed the degree of reproductive and/or morphological divergence in relation to genetic distance within and among populations occupying different habitats and found support for ecological speciation (e.g., 10, 19, 21, 22, 26, 28, 34). In addition, powerful evidence for the role of natural selection in population divergence and speciation comes from examples of “parallel speciation” (ref. 24; Fig. 1c) in which reproductive and/or morphological divergence evolves repeatedly in response to similar selective regimes in evolutionarily independent sets of populations (9, 10, 19, 21, 28).
Figure 1.
Tests for the effect of selection in divergence among populations (modified from refs. 24 and 25). (a) Four regions are defined. Shading indicates different habitats, and the bold horizontal line indicates a partial or complete barrier to gene flow (e.g., geographic distance or a physical barrier). Populations A and A′ occupy one habitat, and populations B and B′ occupy a different habitat. (b) By comparing morphological divergence in fitness-related traits or reproductive divergence among populations, relative roles of drift and selection in divergence can be evaluated. If selection is driving population divergence, then, for a given level of genetic divergence, greater reproductive isolation (or morphological divergence) is expected among populations from different habitats than among populations occupying similar habitats. If the degree of reproductive divergence is similar within and among habitats, then factors acting independent of the environment (e.g., drift) are indicated. (c) Historical relationships among populations provide an additional test of the hypothesis that selection is important in speciation. Populations A and B (also A′ and B′) are sister groups that occupy different habitats whereas populations A and A′ occupy similar habitats but are not sister groups. In parallel speciation (24), similar adaptive divergence of populations occurs independently two or more times. In the single habitat shift scenario, A is the ancestral habitat, and a single shift to habitat B occurs. If reproductive divergence is greater between A and B (and A′ and B′) than between A and A′ (and B and B′), then selection is implicated in divergence.
Whether or not reproductive divergence among Drosophila populations in Evolution Canyon has occurred as a result of selection and local adaptation remains an open question. Additional data on the historical evolutionary relationships of populations in and around Evolution Canyon is required. There have been ca. 80 publications resulting from studies in Evolution Canyon, nearly all showing adaptive divergence to the microscale climate differences in the canyon. Several of these studies examined genetic variation within the canyon, often finding low genetic distances between north- and south-facing slopes, but none examined the regional patterns of variation to determine whether Evolution Canyon may be a zone of secondary contact and introgression. A broader perspective on geographic genetic variation would contribute significantly to understanding the processes producing the patterns observed in Evolution Canyon. Interestingly, there are other canyons in the vicinity of Evolution Canyon that have similar (although perhaps less stark) habitat transitions (E. Nevo, personal communication), which may provide the opportunity to examine parallel divergence in a series of natural replicates. The contrasting microscale environmental differences in Evolution Canyon offer the opportunity for detailed examination of the natural history and genetics of speciation, and we look forward to future studies from this fascinating natural laboratory.
Footnotes
See companion article on page 12637.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240463297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240463297
References
- 1.Korol A, Rashkovetsky E, Iliadi K, Michalak P, Ronin Y, Nevo E. Proc Natl Acad Sci USA. 2000;97:12637–12642. doi: 10.1073/pnas.220041397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayr E. Animal Species and Evolution. Cambridge, MA: Belknap; 1963. [Google Scholar]
- 3.Gavrilets S, Hastings A. Am Nat. 1996;147:466–491. [Google Scholar]
- 4.Gavrilets S. Trends Ecol Evol. 1997;12:307–312. doi: 10.1016/S0169-5347(97)01098-7. [DOI] [PubMed] [Google Scholar]
- 5.Gavrilets S, Boake C R B. Am Nat. 1998;152:706–716. doi: 10.1086/286201. [DOI] [PubMed] [Google Scholar]
- 6.Mayr E. Systematics and the Origin of Species. New York: Columbia Univ. Press; 1942. [Google Scholar]
- 7.Ratcliffe L M, Grant P R. Anim Behav. 1983;31:1139–1153. [Google Scholar]
- 8.Rundle H D, Schluter D. Evolution. 1998;52:200–208. doi: 10.1111/j.1558-5646.1998.tb05153.x. [DOI] [PubMed] [Google Scholar]
- 9.Schluter D. Philos Trans R Soc London B. 1996;351:807–814. [Google Scholar]
- 10.Nagel L, Schluter D. Evolution. 1998;52:209–218. doi: 10.1111/j.1558-5646.1998.tb05154.x. [DOI] [PubMed] [Google Scholar]
- 11.Bradshaw H D, Wilbert S M, Otto K G, Schemske D W. Nature (London) 1995;376:762–765. [Google Scholar]
- 12.Bush G L. Evolution. 1969;23:237–251. doi: 10.1111/j.1558-5646.1969.tb03508.x. [DOI] [PubMed] [Google Scholar]
- 13.Bush G L. Trends Ecol Evol. 1994;9:285–288. doi: 10.1016/0169-5347(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 14.Bush G L, Smith J J. In: Vertical Food Web Interactions: Evolutionary Patterns and Driving Forces. Dettner K, Bauer G, Völkl W, editors. Vol. 130. Heidelberg: Springer; 1997. pp. 3–19. [Google Scholar]
- 15.Dobzhansky T. Genetics and the Origin of Species. New York: Columbia Univ. Press; 1951. [Google Scholar]
- 16.Fisher R A. The Genetical Theory of Natural Selection. Oxford: Oxford Univ. Press; 1930. [Google Scholar]
- 17.Wright S. Ann Eugen. 1951;1:323–334. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- 18.Coyne J A, Orr H A. Philos Trans R Soc London B. 1998;353:287–305. doi: 10.1098/rstb.1998.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schluter D. In: Endless Forms: Species and Speciation. Howard D J, Berlocher S H, editors. New York: Oxford Univ. Press; 1998. pp. 114–129. [Google Scholar]
- 20.Templeton A R. Evolution. 1996;50:909–915. doi: 10.1111/j.1558-5646.1996.tb03899.x. [DOI] [PubMed] [Google Scholar]
- 21.Schneider C J, Smith T B, Larison B, Moritz C. Proc Natl Acad Sci USA. 1999;96:13869–13873. doi: 10.1073/pnas.96.24.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith T B, Wayne R K, Girman D J, Bruford M W. Science. 1997;276:1855–1857. [Google Scholar]
- 23.Vamosi S M, Hatfield T, Schluter D. J Fish Biol. 2000;57:109–121. [Google Scholar]
- 24.Schluter D, Nagel L M. Am Nat. 1995;146:292–301. [Google Scholar]
- 25.Orr M R, Smith T B. Trends Ecol Evol. 1998;13:502–506. doi: 10.1016/s0169-5347(98)01511-0. [DOI] [PubMed] [Google Scholar]
- 26.Lu G Q, Bernatchez L. Evolution. 1999;53:1491–1505. doi: 10.1111/j.1558-5646.1999.tb05413.x. [DOI] [PubMed] [Google Scholar]
- 27.Benkman C W. Am Nat. 1999;153:S75–S91. doi: 10.1086/303213. [DOI] [PubMed] [Google Scholar]
- 28.Rundle H D, Nagel L, Boughman J W, Schluter D. Science. 2000;287:306–308. doi: 10.1126/science.287.5451.306. [DOI] [PubMed] [Google Scholar]
- 29.Rice W, Hostert E. Evolution. 1993;47:1637–1653. doi: 10.1111/j.1558-5646.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 30.Barton N, Charlesworth B. Annu Rev Ecol Syst. 1984;15:133–164. [Google Scholar]
- 31.Rice W R. Evol Ecol. 1987;1:301–314. [Google Scholar]
- 32.Rice W R. Evolution. 1984;38:1251–1260. doi: 10.1111/j.1558-5646.1984.tb05647.x. [DOI] [PubMed] [Google Scholar]
- 33.Slatkin M. Evolution. 1982;36:263–270. doi: 10.1111/j.1558-5646.1982.tb05040.x. [DOI] [PubMed] [Google Scholar]
- 34.Schluter D. Am Nat. 1996;148:S40–S64. [Google Scholar]