Abstract
A common adaptation in angiosperms is the deposition of hydrophilic mucilage into the apoplast of seed coat epidermal cells during the course of their differentiation. Upon imbibition, seed mucilage, composed mainly of carbohydrates (i.e. pectins, hemicelluloses and glycans) expands rapidly, encapsulating the seed and aiding in seed dispersal and germination. The FEI1/FEI2 receptor-like kinases and the SOS5 extracellular GPI-anchored protein were previously shown to act on a pathway regulating cellulose biosynthesis during Arabidopsis root elongation. In the highlighted study, we demonstrated that FEI2 and SOS5 regulate the production of the cellulosic rays deposited across the inner adherent-layer of seed mucilage. Mutations in either fei2 or sos5 disrupted the formation of rays, which was associated with an increase in the soluble, outer layer of pectin mucilage and accompanied by a reduction in the inner adherent-layer. Mutations in CELLULOSE SYNTHASE 5 also led to reduced rays and mal-partitioning of the pectic component of seed mucilage, further establishing a structural role for cellulose in seed mucilage. Here, we show that FEI2 expressed from a CaMV 35S promoter complemented both root and seed mucilage defects of the fei1 fei2 double mutant. In contrast, expression of FEI1 from a 35S promoter complemented the root, but not the seed phenotype of the fei1 fei2 double mutant, suggesting that unlike in the root, FEI2 plays a unique and non-redundant role in the regulation of cellulose synthesis in seed mucilage. Altogether, these data suggest a novel role for cellulose in anchoring the pectic component of seed mucilage to the seed surface and indicate that the FEI2 protein has a function distinct from that of FEI1, despite the high sequence similarity of these RLKs.
Keywords: AGP, cellulose, mucilage, RLK, seed coat
During differentiation, the maternally derived seed coat epidermal cells (also called mucilage secretory cells; MSC) from Arabidopsis thaliana secrete mucilage composed primarily of pectins into the apoplast. Upon hydration of the mature seed, the mucilage expands, rupturing the tangential cell wall and encapsulating the seed.1,2 Once released, the seed coat mucilage is organized in two distinct domains: an inner, dense layer, tightly attached to the seed surface and an outer, water soluble, diffuse layer. Both layers are composed primarily of the pectin rhamnogalacturonan I (RG-I), with the inner layer also containing other pectins and minor amounts of hemicellulose and β-glucans.1,3-6 Genetic and biochemical analyses of seed coat mucilage secretory cells has led to new insights regarding the complex mechanism involved in pectin biosynthesis and in muro modification, and has demonstrated the utility of seed mucilage as a system for further study of cell wall polymer biosynthesis and modification.7-9 The precise physiological role of seed mucilage is yet to be determined, but has been proposed to play a role in seed dispersal and germination.5,9-11
A number of previous studies have provided data supporting a role for cellulose in seed mucilage: (1) Calcofluor and Congo red stains suggested that β-glucans are present in a set of rays deposited across the inner layer of seed mucilage3,4,12; (2) These β-glucan rays were resistant to pectolytic enzymes, unless these were combined with cellulases,3 (3) Fluorescently labeled cellulose binding modules identified cellulose in seed mucilage with distinct binding patterns for crystalline and amorphous cellulose.6,13,14
Cellulose microfibrils are synthesized at the plasma membrane by protein complexes comprised primarily of CELLULOSE SYNTHASE A (CESA), which is encoded by a family of ten genes in Arabidopsis.15 In primary cell wall biosynthesis, CESA1 and 3 are unique, non-redundant components of the cellulose synthase complex, while CESA2, 5, 6 and 9 act as a third component in a partially redundant manner.16,17 CESA4, 7 and 8 play a role in cellulose biosynthesis in secondary cell walls, and CESA10 has not as yet been assigned any function.18-20
The leucine rich repeat receptor like kinases (RLKs) FEI1 and FEI2 act redundantly in the regulation of cellulose biosynthesis during root elongation.21 The fei1 fei2 double mutant displays root growth arrest, root tip swelling and a reduction in the biosynthesis of crystalline cellulose in the presence of elevated sucrose or salt.21 SALT OVERLY SENSITIVE 5, which encodes a GPI-anchored fasciclin-like arabinogalactan protein,21,22 was shown by genetic interaction to act on the same pathway as the FEIs in the regulation of cellulose biosynthesis.21 Interestingly, the root phenotype of both fei1 fei2 and sos5 is suppressed by inhibitors of ethylene biosynthesis, but not by disruption of the ethylene signaling pathway, suggesting a role for ACC as a signaling molecule in this pathway.21,22
The FEI-SOS Pathway Plays a Non-Conditional Role in Seed Coat Mucilage
Upon imbibition of wild type Arabidopsis seeds, the transparent mucilage rapidly expands, resulting in the gaps observed between the seeds and leading to an increase in the overall volume occupied by the hydrated seeds. In both fei1 fei2 and sos5 mutant seeds, these gaps are greatly reduced compared to wild-type, leading to the reduced overall volume occupied by the seeds (Fig. 1). Staining of the seed mucilage for acidic pectins using the cationic dye ruthenium red confirmed that the adherent inner layer of seed mucilage, which remains attached to the seed surface following mild shaking, is reduced in both fei1 fei2 and sos5 mutant seeds. These data were supported by immunolabeling with the CCRC-M36 antibody, which recognizes the unbranched backbone of RGI in seed mucilage and by monosaccharide analysis of soluble vs. adherent seed mucilage extracts. Interestingly, the reduced inner layer of mucilage in the mutants, was associated with a corresponding increase in the outer-soluble layer of seed mucilage, as observed by both ruthenium red stain and monosaccharide analysis. The role of FEI1 and FEI2 in the regulation of cellulose biosynthesis during root elongation suggested they may affect cellulose biosynthesis also in seed mucilage. We therefore used both calcofluor and pontamine fast scarlet S4B stains in order to visualize crystalline cellulose in seed mucilage. In wild-type, both calcofluor and pontamine stains revealed a set of rays radiating the seed, deposited from the tip of the columella and across the inner layer of seed mucilage. This set of rays was almost completely absent or substantially compromised in either fei1 fei2 or sos5 seed mucilage, respectively. Together these data suggest that the depleted cellulose rays in fei1 fei2 and sos5 leads to mal-partitioning of the pectic component between the inner and outer domains of seed mucilage.
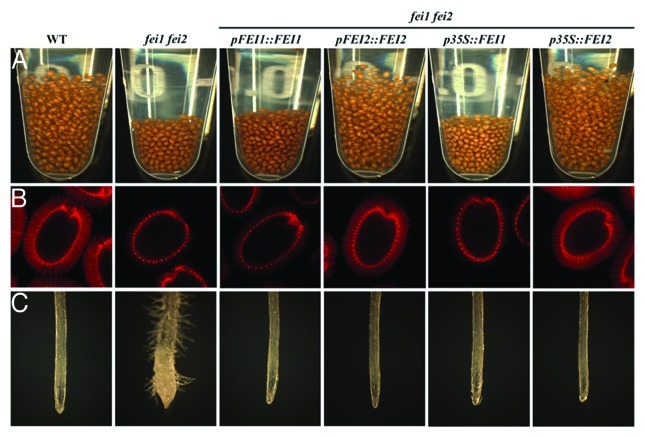
Figure 1. FEI2 plays a non-redundant role in the regulation of cellulose synthesis in seed mucilage and cannot be replaced by ectopic expression of FEI1. Phenotypes of the wild-type (WT) or fei1 fei2 mutants transformed with FEI1 or FEI2 expressed from their native promoter of a CaMV 35S promoter as indicated above each column. (A) Seed volumes of 10 mg of seeds suspended in water. (B) Pontamine staining for cellulose following gentle shaking in water. Pontamine stain labels the columella, tangential cell wall remnants and rays deposited across the inner layer of mucilage in the wild-type. (C) Root phenotype of seedlings grown on MS medium plus 0% sucrose for 4 days and then transferred to medium supplemented with 4.5% sucrose for another 5 days.
CESA5 Plays a Role in Cellulose Biosynthesis in Seed Mucilage
In order to further investigate the mechanism involved in crystalline cellulose deposition into the rays of Arabidopsis seed mucilage, we examined the structure of seed mucilage in cesA2, cesA5, cesA6 and cesA9 mutants. Calcofluor and pontamine staining revealed that only the disruption of cesA5 compromised the rays across the inner layer of seed mucilage, resulting in a phenotype similar to that observed in fei1 fei2 and sos5. Consistent with this, Sullivan et al., 2011 and Mendu et al., 2011 recently observed similar phenotypes for other mutant alleles of cesA5, as well as additional, partially redundant roles for CESA2, CESA5 and CESA9 in cellulose deposition during seed development.23,24 Together, these studies demonstrate that cellulose is an essential component of seed mucilage and that it acts to anchor the pectic component of seed mucilage to the seed surface, resulting in an inner layer of seed mucilage that remains attached to the seed most likely in order to maintain a hydrated environment during the first phase of seed germination.
FEI2, But Not FEI1, Plays a Unique and Non-Redundant Role in Seed Mucilage
The fei1 fei2 double mutant displays a swollen root phenotype as a result of reduced cellulose biosynthesis, but neither single mutant displays any detectable root phenotype.21 In contrast, fei2 single mutants, but not fei1 mutants, display a mucilage defect similar to the double fei1 fei2 mutant phenotype, suggesting that FEI2 plays a non-redundant role in seed mucilage. Consistent with this, expression of FEI2 from its endogenous promoter complemented both the root and the seed mucilage defect of the double fei1 fei2 mutant. Expression of FEI1 driven by its endogenous promoter complemented only the root phenotype of the double mutant. Thus, only FEI2 is involved in the regulation of cellulose biosynthesis into the cellulosic rays deposited across the inner layer of seed mucilage.
The question arises is whether the unique and non-redundant function of FEI2 in seed mucilage is a result of a distinct expression pattern of the gene as compared to FEI1, or is due to differences in the function of the two proteins, which are highly similar in sequence (82% amino acid identity). To address this question, we expressed FEI1 and FEI2 from the CaMV 35S promoter in fei1 fei2 double mutant background and assessed the ability of each to complement the root and seed phenotypes. As each transgene is expressed from the same constitutive promoter, any difference in the ability to complement these phenotypes must be due to differences in the function of the encoded proteins. Consistent with the results obtained by the expression from its native promoter, expression of FEI2 from the CaMV 35S promoter complemented both the root and seed phenotypes in the double fei1 fei2 mutant background (Fig. 1). Likewise, expression of FEI1 from the CaMV 35S promoter complemented the root phenotype of the double fei1 fei2 mutant (in all ten different lines examined). However, the CaMV 35S::FEI1 transgene failed to complement the seed mucilage phenotype of the fei1 fei2 double mutant (Fig. 1). These data indicate that despite the high sequence similarity shared by these LRR-RLKs, the FEI2 protein has a distinct function in the regulation of cellulose biosynthesis in seed mucilage. This suggests that there are likely distinct signaling inputs and/or outputs acting through FEI2 to regulate cellulose biosynthesis during the production of seed coat mucilage. The intracellular domain of both FEI1 and FEI2, encompassing the C-terminal kinase domain, share more than 90% identity in sequence, while the extracellular domain, containing the leucine-rich repeats is more variable (62% identity in sequence). This suggests that perhaps the extracellular domain is more likely to harbor a unique function in seed mucilage related cellulose deposition, though further studies examining the structure-function relationship of FEI1 and FEI2 will be required to clearly define the domains imparting the unique function to FEI2.
Mucilage secretory cells have been shown to be a powerful model system for the study of pectin metabolism and in muro modification.7,8 We have established cellulose as an essential component of seed mucilage and identified CESA5, FEI2 and SOS5 as regulators of cellulose deposition into the rays radiating the seed across the inner layer of seed mucilage. Other recent studies have defined additional, partially redundant functions for CESA2, CESA5 and CESA9 in cellulose deposition into the radial cell walls of mucilage secretory cells.23-25 These studies set the stage for the use of mucilage secretory cells as a novel tool for further research aimed to uncover new players regulating cellulose biosynthesis.
Acknowledgements
We thank Gyeong Mee Yoon for fruitful discussions. This project was supported by National Science Foundation grant IOS 0624377 (to J.J.K.) and by a Natural Sciences and Engineering Research Council Discovery Grant (to T.L.W.). S.H-S. is a recipient of a Vaadia-BARD postdoctoral fellowship award No. FI-411-2008 from BARD, The United States—Israel Binational Agricultural Research and Development Fund.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18819
References
- 1.Western TL, Skinner DJ, Haughn GW. Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol. 2000;122:345–56. doi: 10.1104/pp.122.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haughn G, Chaudhury A. Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci. 2005;10:472–7. doi: 10.1016/j.tplants.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Macquet A, Ralet M-C, Kronenberger J, Marion-Poll A, North HM. In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol. 2007;48:984–99. doi: 10.1093/pcp/pcm068. [DOI] [PubMed] [Google Scholar]
- 4.Willats WGT, McCartney L, Knox JP. In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta. 2001;213:37–44. doi: 10.1007/s004250000481. [DOI] [PubMed] [Google Scholar]
- 5.Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW. MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell. 2001;13:2777–91. doi: 10.1105/tpc.010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young RE, McFarlane HE, Hahn MG, Western TL, Haughn GW, Samuels AL. Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell. 2008;20:1623–38. doi: 10.1105/tpc.108.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Western TL. Changing spaces: the Arabidopsis mucilage secretory cells as a novel system to dissect cell wall production in differentiating cells. Can J Bot. 2006;84:622–30. doi: 10.1139/b06-008. [DOI] [Google Scholar]
- 8.Arsovski AA, Haughn GW, Western TL. Seed coat mucilage cells of Arabidopsis thaliana as a model for plant cell wall research. Plant Signal Behav. 2010;5:796–801. doi: 10.4161/psb.5.7.11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Western TL. The sticky tale of seed coat mucilages: production, genetics, and role in seed germination and dispersal. Seed Sci Res. 2012;22:1–25. doi: 10.1017/S0960258511000249. [DOI] [Google Scholar]
- 10.Gutterman Y, Shem-Tov S. Structure and function of the mucilaginous seed coats of Plantago coronopus inhabiting the Negev Desert of Israel. J Plant Sci. 1996;44:125–33. [Google Scholar]
- 11.Yang X, Zhang W, Dong M, Boubriak I, Huang Z. The achene mucilage hydrated in desert dew assists seed cells in maintaining DNA integrity: adaptive strategy of desert plant Artemisia sphaerocephala. PLoS One. 2011;6:e24346. doi: 10.1371/journal.pone.0024346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Windsor JB, Symonds VV, Mendenhall J, Lloyd AM. Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J. 2000;22:483–93. doi: 10.1046/j.1365-313x.2000.00756.x. [DOI] [PubMed] [Google Scholar]
- 13.Blake AW, McCartney L, Flint JE, Bolam DN, Boraston AB, Gilbert HJ, et al. Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. J Biol Chem. 2006;281:29321–9. doi: 10.1074/jbc.M605903200. [DOI] [PubMed] [Google Scholar]
- 14.Dagel DJ, Liu YS, Zhong L, Luo Y, Himmel ME, Xu Q, et al. In situ imaging of single carbohydrate-binding modules on cellulose microfibrils. J Phys Chem B. 2011;115:635–41. doi: 10.1021/jp109798p. [DOI] [PubMed] [Google Scholar]
- 15.Somerville C. Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol. 2006;22:53–78. doi: 10.1146/annurev.cellbio.22.022206.160206. [DOI] [PubMed] [Google Scholar]
- 16.Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, et al. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2007;104:15572–7. doi: 10.1073/pnas.0706569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, et al. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci U S A. 2007;104:15566–71. doi: 10.1073/pnas.0706592104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor DR, Ingvarsson PK. Common features of segregation distortion in plants and animals. Genetica. 2003;117:27–35. doi: 10.1023/A:1022308414864. [DOI] [PubMed] [Google Scholar]
- 19.Turner SR, Somerville CR. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell. 1997;9:689–701. doi: 10.1105/tpc.9.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc Natl Acad Sci U S A. 2003;100:1450–5. doi: 10.1073/pnas.0337628100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu SL, Rahman A, Baskin TI, Kieber JJ. Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell. 2008;20:3065–79. doi: 10.1105/tpc.108.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi H, Kim Y, Guo Y, Stevenson B, Zhu J-K. The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell. 2003;15:19–32. doi: 10.1105/tpc.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan S, Ralet MC, Berger A, Diatloff E, Bischoff V, Gonneau M, et al. CESA5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiol. 2011;156:1725–39. doi: 10.1104/pp.111.179077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendu V, Griffiths JS, Persson S, Stork J, Downie AB, Voiniciuc C, et al. Subfunctionalization of cellulose synthases in seed coat epidermal cells mediates secondary radial wall synthesis and mucilage attachment. Plant Physiol. 2011;157:441–53. doi: 10.1104/pp.111.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stork J, Harris D, Griffiths J, Williams B, Beisson F, Li-Beisson Y, et al. CELLULOSE SYNTHASE9 serves a nonredundant role in secondary cell wall synthesis in Arabidopsis epidermal testa cells. Plant Physiol. 2010;153:580–9. doi: 10.1104/pp.110.154062. [DOI] [PMC free article] [PubMed] [Google Scholar]