Abstract
Control of final organ size is a fundamental and core process of development of all multicellular organisms, but the mechanisms that set the final size of determinate organs are largely unknown. In a recent study, we demonstrated that the Mediator complex subunit 25 (MED25/PFT1), which is involved in the transcriptional regulation of gene expression, controls the final size of determinate organs by restricting both cell proliferation and cell expansion. med25 mutants formed large organs with larger and slightly more cells, whereas plants overexpressing MED25 produced small organs due to a reduction in both cell number and cell size. Here, we show that a loss-of-function mutation in the Mediator complex subunit 8 (MED8) causes small flowers as a result of reduced cell expansion. Analysis of the med8 med25–2 double mutant reveals that MED8 acts independently of MED25 to regulate cell expansion and organ growth. Taken together, our findings show that MED8 and MED25 play an important role in regulating organ size. Further identification of upstream and downstream components of MED8 and MED25 will help understand how the Mediator complex is involved in organ size control in plants.
Keywords: cell expansion, MED25, MED8, organ size control
The Mediator complex was first identified in yeast as an activity required for the transcriptional activation and later as a multiprotein complex that functions to transmit various signals from activators, repressors, as well as general transcription factors, to the RNA polymerase II complex to initiate transcription.1-3 The Mediator complex subunits are conserved in all eukaryotes from yeast to humans. In Arabidopsis, 21 conserved and six putative plant-specific Mediator subunits have recently been identified.4 Several Mediator subunits were described to regulate flowering time, embryo development, stress responses and microRNA biogenesis in Arabidopsis.5-9 The Mediator complex subunit 25 (MED25/PFT1) was first described as a positive regulator of shade avoidance and flowering time and later as a regulator of jasmonate-dependent defense and abiotic stress responses.4,6,7,10 In a recent study, we discovered a new role of MED25 in plant organ size control through a genetic screen for mutations that enhance the floral organ size of the da1-1 mutant; DA1 is a negative regulator of cell proliferation in Arabidopsis.11,12 Loss-of-function mutants in MED25 formed large organs, whereas plants overexpressing MED25 produced small organs.12 med25 mutants predominantly increased cell expansion but also increased cell proliferation slightly. MED25 functions to restrict cell expansion and organ size independently of MED25-mediated phytochrome signaling and the jasmonate pathway.12 We showed that cell enlargement in med25 petals might, in part, result from increased expression of particular expansin genes.12 In addition, our genetic analyses revealed that the med25-2 mutation synergistically enhanced the cell number phenotype of da1-1,12 indicating that da1-1 is required for the dramatic effects of the med25 mutations on cell proliferation and also suggesting that MED25 acts redundantly with DA1 to limit cell proliferation. Thus, MED25 may function as a hub that provides a link between cell proliferation and cell expansion pathways within the transcriptional machinery.
Previous studies showed that Arabidopsis med25/pft1 and med8 mutants appear to similarly affect both flowering time and pathogen resistance.7 We therefore asked whether MED8 is involved in organ size regulation in Arabidopsis. To address this question, we obtained the med8 mutant.7 Surprisingly, in contrast to med25 mutants, med8 exhibited smaller flowers than wild type (Fig. 1A and B). Transformation of the med8 mutant with a wild-type MED8 cDNA driven by its own promoter restored a wild-type phenotype (Fig. 1F). To investigate the cellular basis of the decrease in flower size, we measured the number and size of adaxial epidermal cells in petals. The size of epidermal cells in the maximal width region of med8 petals was significantly decreased compared with wild type (Figs. 1C, D and 2B), while the number of epidermal cells in med8 petals was similar to that in wild-type petals (Fig. 1E), indicating that the med8 mutation restricts cell expansion. To determine whether MED8 and MED25 function antagonistically in a common pathway to regulate cell expansion, we generated a med8 med25-2 double mutant and analyzed its cell and organ size phenotypes together with those of the med8 and med25-2 single mutants. Genetic interactions between med8 and med25-2 were essentially additive for petal size and epidermal cell area compared with their parental lines (Fig. 2), suggesting that MED8 acts to regulate cell expansion and organ growth separately from MED25. It is possible that MED8 and MED25 may transmit distinct signals from different classes of activators to the RNA polymerase II complex to initiate transcripts of their respective downstream target genes involved in organ size control. Therefore, further identification of their activators and downstream targets in organ-size control pathways will help understand how the Mediator complex controls organ size in plants.
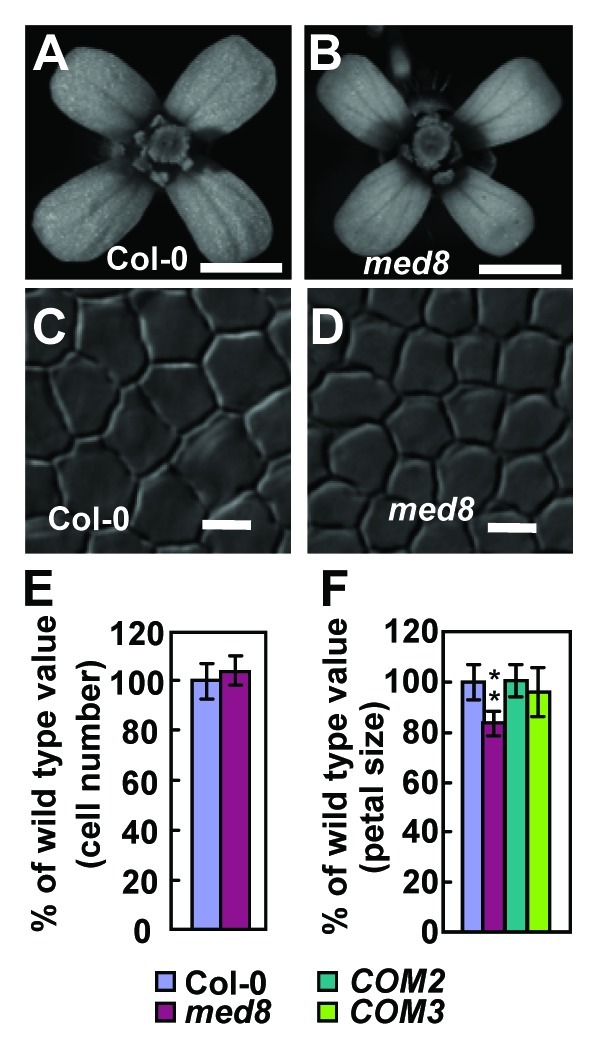
Figure 1.med8 mutant forms small flowers. (A and B) Flowers of Col-0 and med8. (C and D) Adaxial epidermal cells in Col-0 and med8 petals. (E) The number of adaxial epidermal cells in Col-0 and med8 petals. Each value represents measurements from more than 10 petals. (F) Petal area of Col-0, med8, COM2 and COM3. COM is med8 transformed with MED8 cDNA sequence driven by the 2362 bp MED8 promoter. Petals from opened flowers (stage 14) were used to measure petal area. Each value for petal area represents measurements from more than 30 petals. Values (E and F) are given as mean ± standard deviation (s.d.) relative to the respective wild-type values. **, p < 0.01 compared with the wild type (Student’s t-test). Scale bar, (A and B), 1mm; (C and D), 10μm.
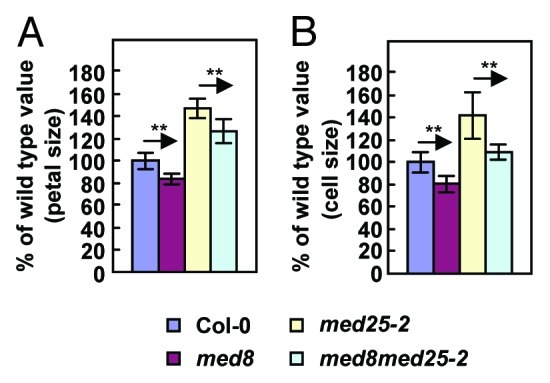
Figure 2.MED8 acts independently of MED25 to regulate cell and organ size. (A) Petal area of Col-0, med8, med25–2 and med8 med25–2 double mutant. Petals from opened flowers (stage 14) were used to measure petal area. Each value for petal area represents measurements from more than 30 petals. (B) The size of adaxial epidermal cells in the maximal width region of Col-0, med8, med25–2 and med8 med25–2 petals. More than 50 cells in the maximal width region of petals were measured. Each value represents measurements from more than 10 petals. Values (A and B) are given as mean ± s.d. relative to the respective wild-type values. **Difference indicated by the arrow is statistically significant at p < 0.01 (Student’s t-test).
Acknowledgments
We thank ABRC for the med8 mutant. This work was supported by National Basic Research Program of China (2009CB941503) and National Natural Science Foundation of China (30870215; 30921003; 91017014).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18803
References
- 1.Kelleher RJ, 3rd, Flanagan PM, Kornberg RD. A novel mediator between activator proteins and the RNA polymerase II transcription apparatus. Cell. 1990;61:1209–15. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Björklund S, Li Y, Sayre MH, Kornberg RD. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 3.Koleske AJ, Young RA. An RNA polymerase II holoenzyme responsive to activators. Nature. 1994;368:466–9. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 4.Bäckström S, Elfving N, Nilsson R, Wingsle G, Björklund S. Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol Cell. 2007;26:717–29. doi: 10.1016/j.molcel.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Autran D, Jonak C, Belcram K, Beemster GT, Kronenberger J, Grandjean O, et al. Cell numbers and leaf development in Arabidopsis: a functional analysis of the STRUWWELPETER gene. EMBO J. 2002;21:6036–49. doi: 10.1093/emboj/cdf614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerd´n PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–5. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- 7.Kidd BN, Edgar CI, Kumar KK, Aitken EA, Schenk PM, Manners JM, et al. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell. 2009;21:2237–52. doi: 10.1105/tpc.109.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillmor CS, Park MY, Smith MR, Pepitone R, Kerstetter RA, Poethig RS. The MED12-MED13 module of Mediator regulates the timing of embryo patterning in Arabidopsis. Development. 2010;137:113–22. doi: 10.1242/dev.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YJ, Zheng B, Yu Y, Won SY, Mo B, Chen X. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J. 2011;30:814–22. doi: 10.1038/emboj.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elfving N, Davoine C, Benlloch R, Blomberg J, Brännström K, Müller D, et al. The Arabidopsis thaliana Med25 mediator subunit integrates environmental cues to control plant development. Proc Natl Acad Sci U S A. 2011;108:8245–50. doi: 10.1073/pnas.1002981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Zheng L, Corke F, Smith C, Bevan MW. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev. 2008;22:1331–6. doi: 10.1101/gad.463608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu R, Li Y. Control of final organ size by Mediator complex subunit 25 in Arabidopsis thaliana. Development. 2011;138:4545–54. doi: 10.1242/dev.071423. [DOI] [PubMed] [Google Scholar]


