Abstract
Nitric oxide (NO) is a highly inducible molecule and overaccumulated during stress responses, such as drought, cold and pathogen infection. Several key developmental processes within a plant life cycle have been reported to be signaled by this gaseous molecule, and among them seed germination, de-etiolation, gravitropic response or root growth are well-characterized. The importance of NO as a plant growth and stress regulator is emerging considerably, despite the current knowledge about its signaling pathway is still limited. Therefore, the identification and characterization at the molecular level of NO targets is essential to get a deeper insight into this pathway. Here we characterize the effect of NO on root development in Arabidopsis and found that NO application reduces cell lengths in differentiation zone. Additionally, the contribution of the gibberellin (GA) signaling pathway to the NO root-related phenotypes, mainly through DELLA repressors, is also depicted.
Keywords: crosstalk, DELLA repressor, gibberellins, nitrossative stress, plant growth regulator, reactive nitrogen species
In our previous report,1 we demonstrated that NO causes root apical meristem defects and growth inhibition while reducing PIN1-dependent acropetal auxin transport. Here we characterize the involvement of NO in GA-regulated root growth and describe the identification of the GA DELLA repressors as molecular targets of this gaseous molecule.
Nitric Oxide Role during Root Growth and Development
Root growth is a complex process involving and integrating various exogenous and endogenous signals. A physiological role for nitric oxide (NO) in the regulation of root growth has previously been described. NO is able to diminish primary root (PR) growth and to promote lateral root (LR) development in tomato.2 Furthermore, demonstrations of NO requirements for the molecular events involved in auxin-induced adventitious root3 and LR development2 have arisen. The inhibition of root growth by NO has already been used as a phenotype for screen NO-hypersensitive mutants.4 This screening resulted in the isolation of the NO overproducer mutant nox1 and the identification of chlorophyll a/b binding protein (CAB) underexpressed 1 (CUE1) as the mutated gene.
It has been previously suggested that NO is interpreted differentially in a dose-dependent manner: exogenous application of high levels of NO inhibits plant growth, but however application of low levels of NO promotes it.4 A detailed analysis of plant gravitropic response, which involve altered endogenous NO levels, seems to confirm this hypothesis: low concentration of NO in the upper side could promote root elongation, whereas the high concentration of NO in the lower side would suppress elongation, thus effecting gravitropic bending.5 But, however, the NO-related structural modifications involved in these precise cellular responses are not well understood yet.
To this end, we got insight into NO function during early plant growth (i.e., root organogenesis and elongation) using a phenotypic, cellular, genetic and molecular analysis in Arabidopsis. In our previous report,1 we demonstrated that NO inhibits PR growth of wild-type Arabidopsis seedlings. Cell division and cell elongation are the main final contributors to this process. While cell division mainly occurs at the root apical meristem (RAM), cell elongation takes place at the elongation-differentiation zone (EDZ). Detailed analysis revealed that the number and size of root meristem cells are significantly different after the addition of NO donors and scavengers, and in mutant background with enhanced NO levels (cue1), supporting a pivotal role of NO in the modulation of RAM activity. Auxin is a key hormone involved in root organogenesis and expression pattern of auxin reporter DR5:GUS/GFP is altered in cue1 and after NO application. Additionally, we found differences in the accumulation of the PIN1 transporter in the presence of NO donors and in cue1, confirming the involvement of NO in acropetal (rootward) auxin transport.
Nitric Oxide, Root Elongation and Cell Sizes
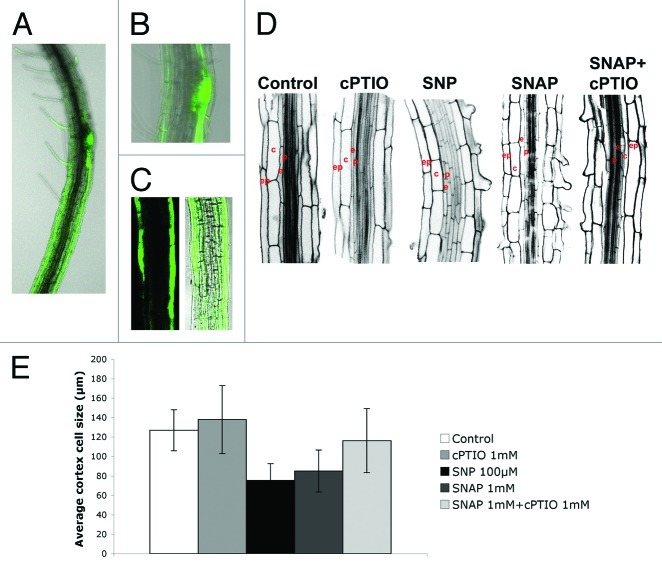
The inhibition of PR growth after NO treatment might be also related to differences in cell lengths in the differentiation zone. To test this fact, the specific site of NO action may be important to exert the different physiological functions during root growth and development. Defining the precise sites of NO production within the plant root is a preliminary task to understand NO-dependent function in different plant organs (revised in ref. 6). Sites of NO production in plant tissues have been routinely identified by using the fluorescence indicator 4,5-diaminofluorescein diacetate (DAF-2DA).2,7 DAF-2DA is a permeable compound hydrolyzed inside the cells and able to emit fluorescence when is nitrosylated by endogenous NO. Labeling of seven-day-old wild-type roots with DAF-2DA revealed localization of NO production mainly in epidermis cell files in the maturation zone (Fig. 1A–C).
Figure 1. High levels of NO inhibit cell elongation in the EDZ. (A–C) Detection of endogenous NO accumulation in the EDZ using DAF-2DA. Plants were grown for 7 d on agar plates and then subjected to DAF-2DA incubation. (D and E) Confocal images and cell sizes in cortex layer of root hair zone. (D) Note that cell elongation in cells of the EDZ is reduced in the presence of high levels of NO. (E) Average cell size in cortex layer for root hair cortical cells of wild-type seedlings grown for 8 d on MS agar plates untreated, or wild-type seedlings supplemented with 1 mM cPTIO, 100 µM SNP, 1 mM SNAP and 1 mM SNAP plus 1 mM cPTIO. Measurements were taken 5 d after the treatment of 3-d-old seedlings. Ep, epidermis; c, cortex; e, endodermis; p, pericycle.
In order to elucidate whether high levels of NO could also be affecting the rate of cell elongation in the EDZ, Arabidopsis thaliana wild-type seeds (ecotype Columbia-0, Col-0) were germinated on plates containing NO released by NO donors S-N-acetyl-DL-penicillamine (SNAP) and sodium nitroprusside (SNP). After 8 d of root growth, average size of cortical cells in the EDZ was measured, where cell differentiation is observed in the formation of root hairs on specific epidermal cell files. Remarkably, we found significant differences in cell lengths between those seedlings treated with SNP (75.36 µm ± 17.13) and SNAP (85,06 µm ± 21,56), and those non-treated seedlings (127 µm ± 21.10), seedlings treated with the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) (137.96 mm ± 34.97), or seedlings treated with SNAP plus cPTIO (116,25 mm ± 32,95) (Fig. 1D and E). As shown in Figure 1D and 1E, NO scavenged by cPTIO partially blocked the action of the NO specific releasing compound SNAP, clearly establishing that NO is a major contributing element in the inhibition of cell elongation in the EDZ. Therefore, our results show that overaccumulation of NO promotes a reduction of cell elongation in EDZ, supporting the hypothesis that NO causes PR growth inhibition by affecting both root apical meristem activity and cell elongation in the EDZ.
Cells in the basal meristem respond differently from the cells in the main elongation zone. In many cases the responses of the two sets of cells to a variety of environmental signals (i.e., gravi- and thigmostimulation, electrotropic stimulation, water stress and responses to auxin) are opposite (revised in ref. 8). For example, gravistimulation inhibits the elongation of cells in the main elongation zone but promotes the elongation of cells along the upper side of the basal meristem (revised in refs. 9 and 10). Our results confirm that NO application mimics this biphasic effect associated to the response of roots to a variety of environmental signals. We have compelling evidence that overaccumulation of NO promotes the elongation of cells, mainly in the basal meristem, and therefore decreases the pool of dividing cells,1 but however inhibits the elongation of cells in the EDZ (Fig. 1).
Cytoskeletal proteins seem to be good candidates for these NO-related structural modifications. Interestingly, a recent study reported that the actin cytoskeleton acts as a downstream effector of NO signal transduction in root cells and that the extent of such modification is cell-type and developmental stage-specific.11 Based on that report, cells of the basal meristem reacted most strongly to the presence of exogenous NO, suggesting specific physiological properties of cells along the basal meristem in relation to NO action.
Gibberellins and Nitric Oxide Involvement in Cell Elongation Processes
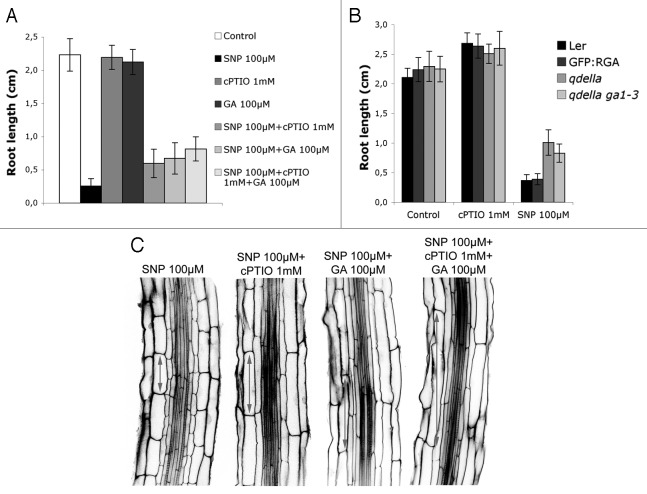
Gibberellins (GAs) are well-characterized positive regulators of cell elongation in plants.12,13 We hypothesized whether combined addition of GAs and NO released by NO-donors could restore root growth and cell elongation in the EDZ. To this end, wild-type seeds were germinated on plates during 3 d and treated with NO released by the NO donor SNP (100 µM) and GA (100 µM) for 5 d. At that time (i.e., 8-d-old seedlings), PR length was measured and average size of cortical cells in the EDZ was also quantified. Our results show that combined addition of GAs and NO partially restores PR growth (Fig. 2A) and cell elongation in the EDZ (Fig. 2C) and interestingly, GAs seem to potentiate the effect of NO depletion after cPTIO treatment (Fig. 2A and C). Clearly, the extension of cells is recovered either after scavenge of NO, GA application or the combination of both.
Figure 2. Action of GAs partially restores the inhibition of cell elongation due to high levels of NO. (A) Addition of GAs to seedlings treated in the presence (100µM SNP) or absence (1mM cPTIO) of NO. Wild-type seedlings grown for 8 d on MS agar plates untreated (control), or supplemented with 100 µM SNP, 1 mM cPTIO, 100 µM GAs, 100 µM SNP plus 1 mM cPTIO, 100 µM SNP plus 100 µM GAs and 100 µM SNP plus 1 mM cPTIO and plus 100 µM GAs. (B) Effect of the impairment of DELLA proteins (qdella mutant) and qdella in a GA deficient background (qdella;ga1–3) (kindly provided by M.A. Blazquez and S. Prat). Measurements were taken 5 d after the treatment of 3-d-old seedlings. (C) Confocal images of root hair zone from wild-type seedlings grown for 8 d on MS agar plates supplemented with 100 µM SNP, 100 µM SNP plus 1 mM cPTIO, 100 µM SNP plus 100 µM GAs and 100 µM SNP plus 1 mM cPTIO and plus 100 µM GAs. Note that cell elongation in cells of the EDZ is reduced in the presence of NO.
With these premises, we reasoned that inhibition of cell elongation in the EDZ by high levels of NO could be mediated through the coordinated action of DELLA repressors. With this purpose, seeds of quadruple della mutant (qdella, impaired in four out of the five DELLA proteins) and qdella in a GA deficient background (qdella;ga1–3) were germinated on plates containing NO released by SNP (100 µM) or the NO scavenger cPTIO (1mM). Our results show that both qdella and qdella;ga1–3 mutants are partially resistant to the effect of NO in the PR growth inhibition (Fig. 2B). These data support the hypothesis that NO inhibits cell elongation in the EDZ by promoting DELLA activity.
GA application restores the inhibition of root growth caused by NO mainly through the promotion of root growth by increasing cell expansion in the elongation zone. In agreement with these results, it has been previously described that NO is able to repress hypocotyl growth in dark grown lettuce and Arabidopsis plants.14 During plant etiolated growth, the so called skotomorphogenesis, cell elongation of the hypocotyl is the only driving force, providing an excellent system to the study of this process. Recently, NO capability to reduce hypocotyl elongation during dark growth has been demonstrated in Arabidopsis15 where NO promotes photomorphogenic development acting as a negative regulator of PHYTOCHROME INTERACTING FACTOR (PIF) genes and as a positive regulator of DELLA proteins.
Conclusions and Perspectives
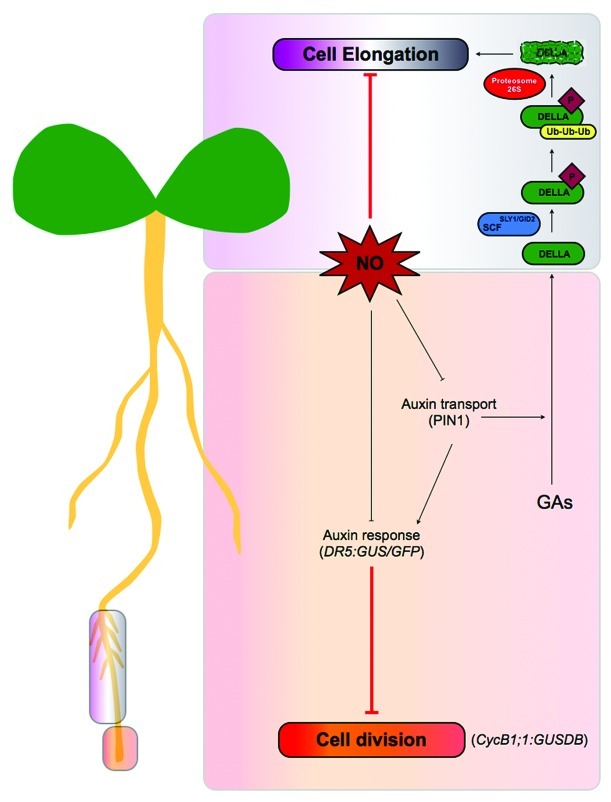
According to our previous results1 and the presented data, we suggest that high levels of NO inhibit PR growth by both affecting root apical meristem activity and cell elongation in the EDZ. Fu and Harberd, (2003)16 provided evidence about the auxin promotion of Arabidopsis root growth through the modulation of GA response. These authors showed that attenuation of auxin transport or signaling delayed the GA-induced degradation of the DELLA protein RGA from root cell nuclei. Consequently, since auxin transporter PIN1 is decreased after NO addition,1 we hypothesize that inhibition of cell elongation in the EDZ by high levels of NO could partially involve the promotion of DELLA activity through PIN1 degradation (Fig. 3). However, to uncover the precise role of NO in these processes, genetic analysis in Arabidopsis should be conducted.
Figure 3. Schematic overview of the physiological role of NO in Arabidopsis primary root growth. NO affects root apical meristem activity and cell elongation during primary root growth. High levels of NO diminish cell division (CycB1;1:GUSDB) and promotes cell elongation in root meristem cells, consistent with an inhibition of auxin response (DR5:GUS/GFP) and auxin polar transport (by disappearance of PIN1 protein).1 Since auxin promotes Arabidopsis root growth through the modulation of GA response16 and auxin transporter PIN1 is decreased after NO addition,1 inhibition of cell elongation in the EDZ by high levels of NO could partially involve the promotion of DELLA activity through PIN1 disappearance.
Acknowledgments
We thank Roberto Solano, Gregorio Nicol´s and Dolores Rodriguez for critical reading of the manuscript and stimulating discussions. We also thank CIC-USAL for excellent technical fluorescence microscopy assistance. This work was financed by grants BIO2008–04698, BIO2011-26940, CSD2007–00057 (TRANSPLANTA) from the Ministerio de Educacio´n y Ciencia (Spain) and SA048A10–2 (to O.L.) from Junta de Castilla y Leo´n. M.F-M. is supported by a FPI fellowship from the Junta de Castilla y Leo´n and L.S. by a Marie Curie European Reintegration Grant (FP7-PEOPLE-ERG-2008).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18895
References
- 1.Fern´ndez-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O. Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci U S A. 2011;108:18506–11. doi: 10.1073/pnas.1108644108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–5. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- 3.Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002;129:954–6. doi: 10.1104/pp.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y, Tang RH, Hao Y, Stevens RD, Cook CW, Ahn SM, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–71. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, Neill SJ, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 2005;137:663–70. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stöhr C, Stremlau S. Formation and possible roles of nitric oxide in plant roots. J Exp Bot. 2006;57:463–70. doi: 10.1093/jxb/erj058. [DOI] [PubMed] [Google Scholar]
- 7.Ille´s P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluska F, Ovecka M. Aluminium toxicity in plants: internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot. 2006;57:4201–13. doi: 10.1093/jxb/erl197. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa H, Evans ML. Specialized zones of development in roots. Plant Physiol. 1995;109:725–7. doi: 10.1104/pp.109.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masson PH. Root gravitropism. Bioessays. 1995;17:119–27. doi: 10.1002/bies.950170207. [DOI] [PubMed] [Google Scholar]
- 10.Blancaflor EB, Masson PH. Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol. 2003;133:1677–90. doi: 10.1104/pp.103.032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasprowicz A, Szuba A, Volkmann D, Baluska F, Wojtaszek P. Nitric oxide modulates dynamic actin cytoskeleton and vesicle trafficking in a cell type-specific manner in root apices. J Exp Bot. 2009;60:1605–17. doi: 10.1093/jxb/erp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowling RJ, Harberd NP. Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J Exp Bot. 1999;50:1351–7. doi: 10.1093/jexbot/50.337.1351. [DOI] [Google Scholar]
- 13.de Lucas M, Davière JM, Rodri´guez-Falco´n M, Pontin M, Iglesias-Pedraz JM, Lorrain S, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–4. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 14.Beligni MV, Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–21. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- 15.Lozano-Juste J, Leo´n J. Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis. Plant Physiol. 2011;156:1410–23. doi: 10.1104/pp.111.177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–3. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]