Abstract
The highly conserved target of rapamycin (TOR) Ser/Thr kinase promotes protein synthesis under favorable growth conditions in all eukaryotes. Downregulation of TOR signaling in the model unicellular green alga Chlamydomonas reinhardtii has recently revealed a link between control of protein synthesis, endoplasmic reticulum (ER) stress and the reversible modification of the BiP chaperone by phosphorylation. Inhibition of protein synthesis by rapamycin or cycloheximide resulted in the phosphorylation of BiP on threonine residues while ER stress induced by tunicamycin or heat shock caused the fast dephosphorylation of the protein. Regulation of BiP function by phosphorylation/dephosphorylation events was proposed in early studies in mammalian cells although no connection to TOR signaling has been established so far. Here I will discuss about the coordinated regulation of BiP modification by TOR and ER stress signals in Chlamydomonas.
Keywords: BiP chaperone, Chlamydomonas, endoplasmic reticulum stress, phosphorylation/dephosphorylation, protein synthesis, TOR kinase
BiP is a member of the HSP70 family of molecular chaperones that resides within the lumen of the ER.1 This chaperone assists the folding and assembly of newly-synthesized proteins as they are translocated into the ER and also binds to misfolded, underglycosylated or unassembled proteins.2 Mammalian BiP can be post-translationally modified by phosphorylation and ADP-ribosylation and both modifications have been associated to oligomeric forms of the protein that likely represent an inactive state of BiP.3 According to this theory, unmodified BiP monomers are more active and bind to and promote folding of substrate proteins. Whether regulation of BiP function by phosphorylation and/or ADP-ribosylation is conserved in other systems is unclear.
We have recently shown that inhibition of TOR signaling by rapamycin in Chlamydomonas led to the phosphorylation of BiP on threonine residues, suggesting a role of TOR in the control of BiP modification.4 Phosphorylation of BiP occurred in a highly conserved region of the peptide-binding domain,4 which plays an important role in the regulation of the chaperone activity of BiP.2 The finding that cycloheximide also induced threonine phosphorylation of Chlamydomonas BiP pointed to inhibition of protein synthesis as one of the origins of BiP phosphorylation. But why would downregulation of protein synthesis lead to BiP phosphorylation? Inhibition of protein synthesis might reduce the load of BiP substrates in the ER and hence the requirement for an elevated chaperone activity in this cellular compartment. Based on the model that modified BiP represents an inactive form of the protein,3 it is therefore possible that BiP becomes phosphorylated under certain conditions to reduce its function.
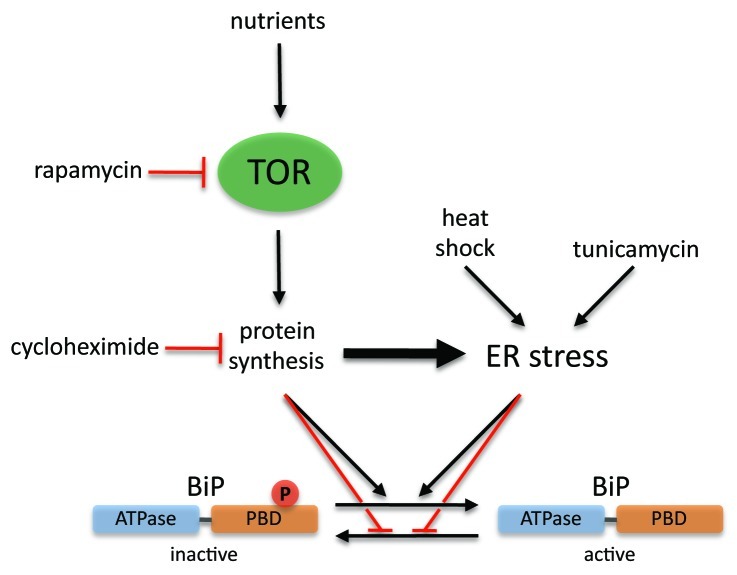
Our results indicate that TOR controls BiP phosphorylation in Chlamydomonas through the regulation of protein synthesis (Fig. 1). Studies mainly performed in yeast and mammalian cells have demonstrated that TOR, in association with other conserved proteins that constitute the so-called TOR complex 1 or TORC1, is a key regulator of translation.5 TOR can also interact with other proteins to form a structurally and functionally distinct complex termed TORC2, which promotes cell survival and mediates organization of the actin cytoskeleton.5 Homologs to the TORC1-specific partner KOG1/raptor have been identified in plants and algae,6-8 suggesting that this signaling complex is present in photosynthetic eukaryotes. Accordingly, association of AtTOR and AtRaptor1 has been demonstrated in Arabidopsis.9 Moreover, experimental evidence indicate that TORC1 is functionally conserved in plants since control of protein synthesis, one of the best-characterized TORC1 functions, is downregulated in plants with reduced TOR activity.10,11 However, the absence of key upstream regulators of TORC1, such as the TSC1/2 complex, in plants and algae strongly suggests that this signaling complex might be differently regulated in these organisms. No obvious homologs exist for the TORC2-specific proteins AVO1/hSIN1 and AVO3/rictor in plant and algal genomes,8,12 raising the question of whether TORC2 is structurally conserved in photosynthetic organisms. Nevertheless, given the elevated conservation of TORC2 components in non-photosynthetic eukaryotes,5 plants and algae might functionally maintain a TORC2 complex, although the proteins that constitute this putative complex must substantially differ from their yeast and mammalian counterparts.
Figure 1. Control of BiP phosphorylation by the TOR pathway and ER stress in Chlamydomonas. Phosphorylation occurs at a conserved region within the peptide-binding domain (PBD) of BiP4 and is associated to an inactive state of the protein that can be efficiently converted into an active, dephosphorylated form in response to increased protein synthesis or specific ER stress. Regulation of BiP function by phosphorylation/dephosphorylation events is therefore coordinated with nutritional and environmental inputs via the TOR pathway and ER stress signaling components.
To our knowledge, the TOR pathway has not been linked to the control of BiP modification in any system different to Chlamydomonas. Modification of BiP by phosphorylation in this microalga can be suppressed under conditions that require the chaperone activity of BiP, such as heat shock or tunicamycin treatment, which inhibits glycosylation of proteins in the ER.4 This finding is in agreement to early studies showing dephosphorylation of mammalian BiP in cells that accumulated non-transported proteins or subjected to glucose starvation.3 Our data suggest that Chlamydomonas BiP exists in two different forms that can be interconverted: a phosphorylated and probable inactive protein, and a dephosphorylated and active form (Fig. 1). Interconversion of these two states must be catalyzed by protein kinase(s) and phosphatase(s), the activity of which must be finely regulated in response to protein synthesis requirements and/or ER stress signals. According to this model, a rapamycin-sensitive TOR pathway may control BiP function in Chlamydomonas either by positively regulating the activity of a BiP phosphatase or inhibiting a BiP kinase (Fig. 1), although none of these proteins have been reported so far. Similar to BiP, the activity of the molecular chaperone HSP90 is also regulated by phosphorylation in mammalian cells. Hyperphosphorylation of HSP90 in response to phosphatase inhibition resulted in reduced association with its substrates and HSP90 phosphorylation appears to play an important role in its chaperoning function.13 Control of HSP90 phosphorylation is better characterized and understood than BiP phosphorylation and several HSP90 kinases have already been identified.13
TOR signaling has been functionally linked to ER stress. Loss of TSC1/2, an upstream negative regulator of mTORC1, causes increased translation due to the upregulation of mTORC1 signaling, which in turn induces ER stress.14 It is thus tempting to speculate that enhanced protein synthesis may also trigger ER stress in Chlamydomonas (Fig. 1). Physical association of the TOR kinase to ER membranes has been reported in mammals.15 However, different cellular locations have been assigned to TOR in lower and higher eukaryotes, including the cytoplasm, the Golgi and ER compartments, the nucleus, the plasma membrane, endosomes, autophagosomes and the vacuolar membrane16 (and references therein). This extremely diverse pattern of TOR cellular distribution is likely due to the large number of processes controlled by this kinase, although some studies point to a prevacuolar compartment and the vacuolar membrane as a main platform for TORC1 signaling.16,17 Interestingly, mTORC2 has been recently shown to associate with ribosomes, likely with the subset of membrane-bound ribosomes at the ER and Golgi apparatus.18 Biochemical fractionation assays revealed that TORC1 associates, at least in part, with ER membranes in Chlamydomonas,19 which may reflect a functional link between TOR signaling and this cellular compartment, in consonance with the demonstrated control of protein synthesis and BiP phosphorylation by TOR. Future work should concentrate on the identification of new components operating in this signaling pathway in Chlamydomonas and plants, where TORC1 (but not TORC2) components are structurally and functionally conserved.7,9,20
Acknowledgments
I thank Anna M. Lindahl for critical comments on the manuscript. This study was supported by the Spanish Ministry of Science and Innovation (BFU2009-07368).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18767
References
- 1.Schroda M. The Chlamydomonas genome reveals its secrets: chaperone genes and the potential roles of their gene products in the chloroplast. Photosynth Res. 2004;82:221–40. doi: 10.1007/s11120-004-2216-y. [DOI] [PubMed] [Google Scholar]
- 2.Otero JH, Liz´k B, Hendershot LM. Life and death of a BiP substrate. Semin Cell Dev Biol. 2010;21:472–8. doi: 10.1016/j.semcdb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendershot LM, Ting J, Lee AS. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol Cell Biol. 1988;8:4250–6. doi: 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di´az-Troya S, Pe´rez-Pe´rez ME, Pe´rez-Marti´n M, Moes S, Jeno P, Florencio FJ, et al. Inhibition of protein synthesis by TOR inactivation revealed a conserved regulatory mechanism of the BiP chaperone in Chlamydomonas. Plant Physiol. 2011;157:730–41. doi: 10.1104/pp.111.179861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GH, Veit B, Hanson MR. The Arabidopsis AtRaptor genes are essential for post-embryonic plant growth. BMC Biol. 2005;3:12. doi: 10.1186/1741-7007-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deprost D, Truong HN, Robaglia C, Meyer C. An Arabidopsis homolog of RAPTOR/KOG1 is essential for early embryo development. Biochem Biophys Res Commun. 2005;326:844–50. doi: 10.1016/j.bbrc.2004.11.117. [DOI] [PubMed] [Google Scholar]
- 8.Pe´rez-Pe´rez ME, Crespo JL. Elucidating TOR signaling in Chlamydomonas reinhardtii In: Fujuhiko Tamanoi MNH, ed. The Enzymes: Academic Press (Elsevier), 2010:245-61. [Google Scholar]
- 9.Mahfouz MM, Kim S, Delauney AJ, Verma DP. Arabidopsis TARGET OF RAPAMYCIN interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell. 2006;18:477–90. doi: 10.1105/tpc.105.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, Nicolaï M, et al. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007;8:864–70. doi: 10.1038/sj.embor.7401043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sormani R, Yao L, Menand B, Ennar N, Lecampion C, Meyer C, et al. Saccharomyces cerevisiae FKBP12 binds Arabidopsis thaliana TOR and its expression in plants leads to rapamycin susceptibility. BMC Plant Biol. 2007;7:26. doi: 10.1186/1471-2229-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau M, Sormani R, Menand B, Veit B, Robaglia C, Meyer C. The TOR complex and signaling pathway in plants. In: Michael N. Hall FT, ed. The Enzymes: Academic Press (Elsevier), 2010:285-301. [Google Scholar]
- 13.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–49. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, Manning BD, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–51. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Zheng XF. Endoplasmic reticulum and Golgi localization sequences for mammalian target of rapamycin. Mol Biol Cell. 2007;18:1073–82. doi: 10.1091/mbc.E06-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturgill TW, Cohen A, Diefenbacher M, Trautwein M, Martin DE, Hall MN. TOR1 and TOR2 have distinct locations in live cells. Eukaryot Cell. 2008;7:1819–30. doi: 10.1128/EC.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 2007;26:663–74. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–68. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Di´az-Troya S, Florencio FJ, Crespo JL. Target of rapamycin and LST8 proteins associate with membranes from the endoplasmic reticulum in the unicellular green alga Chlamydomonas reinhardtii. Eukaryot Cell. 2008;7:212–22. doi: 10.1128/EC.00361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menand B, Desnos T, Nussaume L, Berger F, Bouchez D, Meyer C, et al. Expression and disruption of the Arabidopsis TOR (target of rapamycin) gene. Proc Natl Acad Sci U S A. 2002;99:6422–7. doi: 10.1073/pnas.092141899. [DOI] [PMC free article] [PubMed] [Google Scholar]