Abstract
Biological timekeeping is essential for proper growth and development. Organisms such as the model plant Arabidopsis use the circadian clock to coordinate biological processes with the environment so that changes in conditions are anticipated and processes favorably phased. Despite the identification of numerous clock genes, knowledge of their molecular connectivity and influence on output programs remains limited. We recently showed LUX encodes a sequence-specific DNA-binding protein that directly regulates expression of the morning clock gene PRR9. We also showed that LUX interacts with the evening-phased proteins ELF3 and ELF4 to form a complex called the Evening Complex (EC). The EC binds the PIF4 and PIF5 promoters to control hypocotyl growth as a clock output. Here we provide evidence that LUX also recruits ELF3 to the PRR9 promoter. As with the PIF4 and PIF5 promoters, both LUX and its close homolog NOX are required for recruitment. Hence the entire EC likely functions together as part of the core clock oscillator to optimize plant fitness.
Keywords: Arabidopsis, circadian clock, Evening Complex, feedback loops, transcriptional regulation
Organisms evolved circadian clocks to coordinate endogenous processes with the approximately 24-h fluctuations in the environment. Clocks are reset daily and seasonally by input cues including light and temperature. Oscillations in gene expression occur in nearly 90% of Arabidopsis transcripts1 and output processes are phased throughout the day. This ensures responses like hypocotyl growth and flowering time occur under optimal conditions. Organisms with disrupted clocks exhibit reduced fitness, supporting that clocks provide adaptive advantages across organisms.
While many clock genes have been identified, few have been positioned mechanistically within the clock architecture. The core clock consists of a series of interlocking transcription- and translation-feedback loops.2 The first involves reciprocal regulation between the pseudo-response regulator TOC1 and the homologous MYB factors CCA1 and LHY. A second loop involves repression of CCA1/LHY by the TOC1 homologs PRR9 and PRR7. In each loop, proteins occupy the promoter of the other’s gene to control expression. CCA1 and the TCP transcription factor CHE form an additional loop by mutual repression of gene expression. Computational modeling has predicted another loop incorporating the novel protein GI, which activates TOC1; GI expression is repressed by CCA1, LHY and TOC1. Fine-tuning of clock protein and activity levels occurs post-translationally. Clock proteins also influence output genes. For example, TOC1 occupies the promoter of the ABA-related gene ABAR and modulates dehydration responses.3 While this provides a framework, a comprehensive picture of the clock network remains lacking. Modeling predictions suggest additional components are required,4 and others already isolated remain to be precisely positioned. Direct connections to output pathways are also scarce. In recent studies, we determined LUX is a direct transcriptional repressor of PRR9 and the hypocotyl growth genes PIF4 and PIF5.5,6 The latter regulation requires formation of the Evening Complex (EC) including the LUX homolog NOX and the novel clock proteins ELF3 and ELF4. We present data supporting the EC likely has a similar function in regulating PRR9 since ELF3 recruitment is dependent on LUX and NOX. This establishes new mechanistic connections within the core oscillator.
LUX is a Sequence Specific DNA-Binding Protein that Directly Represses PRR9 Expression
LUX (also known as PHYTOCLOCK1) was identified through genetic screens as a regulator of hypocotyl growth and circadian gene oscillations.7,8 lux mutant seedlings exhibit clock defects including long hypocotyl, early flowering, and arrhythmic circadian gene expression. Since LUX shares with TOC1 similar expression patterns and promoter binding by CCA1 and LHY, it was proposed to fit within the clock architecture in a similar manner.7 However, like TOC1, little was known about its biochemical function. LUX was predicted to encode a MYB-like GARP factor. To determine if it binds DNA in a sequence-specific manner, we took an unbiased in vitro approach using bacterial-expressed LUX and protein binding microarrays (PBMs). We determined the LUX binding site (LBS) to be GATWCG (where W represents A or T). This sequence is found in intergenic regions throughout the genome, including the PRR9 and LUX promoters. LUX binding to the LBS was confirmed in yeast using multimerized perfect- and mutated-LBS sequences. The LUX homolog NOX, which shares 97% identical amino acids in the DNA-binding domain, also binds the LBS in yeast.
To test whether the LBS is bound in vivo, we generated LUX minigene lines (LUX::LUX-GFP in lux-4 mutant background) for chromatin immunoprecipitation (ChIP) assays. Consistent with our PBM and yeast results, this experiment showed amplicons containing or close to the LBS of the PRR9 promoter produced the greatest enrichment. lux-4 seedlings have high levels of PRR9 indicating LUX binding has direct transcriptional effects. In the context of the clock architecture, this supports that LUX activates CCA1 by downregulating its repressor PRR9. As CCA1 represses LUX expression,7 this completes a regulatory loop within the clock. In addition, we showed LUX binds the LBS in its own promoter. This provided the first evidence for direct auto-regulation within the Arabidopsis clock. Finally, to assess the transcriptional potential of LUX, we compared the ability of LUX-translational fusions to complement lux-4 defects. When transformed with LUX fused to either a strong activation (LUX::LUX-VP64) or repression (LUX::LUX-CRES) domain, only the repressive LUX-CRES variant rescued hypocotyl and oscillation defects. Together these studies provide the first in vivo function for LUX as a direct repressor of PRR9 and LUX within the core circadian clock.
The Evening Complex Binds the PRR9 Promoter to Regulate the Core Clock
We recently showed LUX interacts in vivo with the evening proteins ELF3 and ELF4 to form the Evening Complex (EC).6 ELF3 is required to tether the interaction between LUX and ELF4, and this complex regulates diurnal hypocotyl growth through LUX- and NOX-mediated binding to promoters of the key regulatory genes PIF4 and PIF5. Since the EC represses clock output genes, it might also play a key role within the core clock oscillator. Interestingly, ELF3 and ELF4 occupy the same region of the PRR9 promoter as LUX and based on genetic studies, confer similar repressive activities.9,10 While their participation in clock function has been established by genetic studies,10-12 the mechanism of ELF3 and ELF4 action is unknown as they possess no domains of known function.
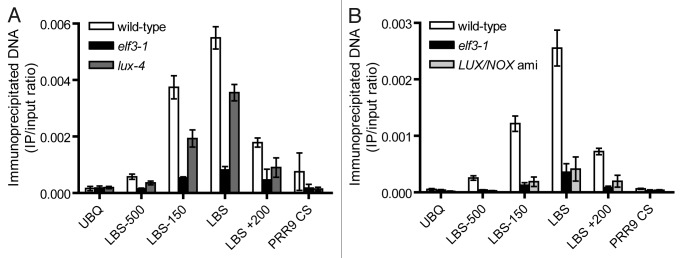
Since ELF3 acts as an adaptor between ELF4 and LUX,6 the recruitment of ELF3 and ELF4 to the PRR9 promoter may require LUX. To test whether ELF3 associates with the PRR9 promoter in a LUX-dependent manner, we performed ChIP experiments. We used PRR9 primers, an antibody against the endogenous ELF3 protein, and methods as reported previously.5,6 Seedlings were entrained in 12-h light/12-h dark cycles for 12 d, and then transferred to constant light for collection at Zeitgeber time 14 (ZT14). As reported previously for YFP-tagged ELF3,9 we showed that endogenous ELF3 selectively binds the PRR9 promoter at the same regions as LUX in wild-type seedlings (Fig. 1A). The greatest enrichment was centered over the LBS, and decreased at more distal regions. No enrichment was observed at the control UBQ promoter. Binding is specific to ELF3 since enrichment is not observed when ChIP is performed using the elf3–1 mutant. To test if ELF3 occupation of the PRR9 promoter is dependent on LUX, we also performed these experiments using lux-4 seedlings. A significant reduction was observed in lux-4 relative to wild-type. However, as ELF3 binding could still be detected, we also tested the LUX/NOX double amiRNA line (LUX/NOX ami).6 In this case, we found ELF3 occupancy was abolished (Fig. 1B). These results support that ELF3, and thus likely ELF4, requires LUX and NOX for binding the PRR9 promoter and provide a mechanism by which both proteins that lack known DNA-binding domains are recruited to the LUX binding sites. Interestingly, similar to LUX, ELF4 expression is directly controlled by CCA1,13 suggesting they may fit within the clock mechanism in a similar manner. Together our results are consistent with a role of ELF3, LUX and NOX (and likely the entire EC including ELF4) in regulating the core clock oscillator as well as output programs (Fig. 2).
Figure 1. ELF3 binds the PRR9 promoter in vivo through recruitment by LUX and NOX. Endogenous ELF3 ChIP on the PRR9 promoter at ZT14 under constant light using: wild-type (CAB2::LUC); elf3–1; lux-4 (CAB2::LUC) (A); and LUX/NOX ami (B). UBQ, UBIQUITIN; LBS, LUX-binding site; CS, coding sequence. Numbering is relative to the LBS positioned -166 base pairs from the transcriptional start site. The data are presented as the average of two technical immunoprecipitations; error bars represent the standard deviation from the mean. Similar results were obtained with a second biological replicate.
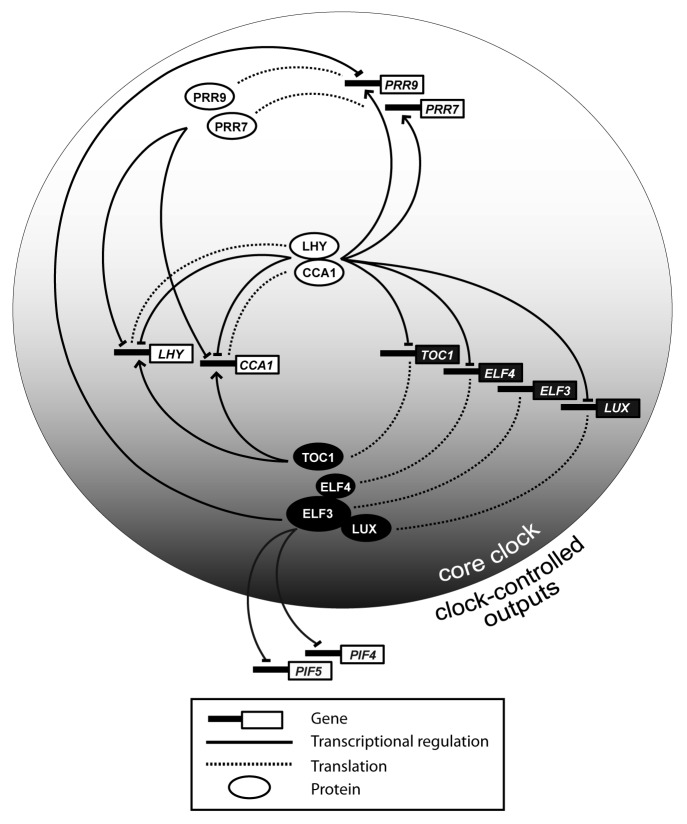
Figure 2. Model for the role of the Evening Complex in the Arabidopsis clock. The Evening Complex (LUX-ELF3-ELF4) is responsible for the downregulation of PRR9 and the output genes PIF4 and PIF5 during late night. Morning-expressed genes and proteins are represented in white; evening-expressed genes and proteins are represented in black. Certain components of the clock network were omitted for simplicity.
Complexities of the Complex
Transcription factor-DNA interactions form the crux of gene regulatory networks. As transcription factors function within complexes, understanding clock function will require the characterization of protein associations. This has recently begun with in vivo interactions observed between several clock proteins.14-19 Our recent discovery of the EC and its binding to the LBS opens up many exciting questions. For instance, where else in the genome does the EC reside? Obvious candidate loci include flowering time genes such as CO which has three LBS motifs within 500 bp upstream of its coding region. Interestingly, GI associates with the CO promoter, and ELF3-GI interaction has been described.16,20 To gain a global perspective of EC distribution, ChIP-Seq (ChIP followed by high-throughput sequencing of immunoprecipiated DNA) will be necessary. High-resolution maps of LUX, ELF3 and ELF4 binding will identify new molecular targets of the EC and determine if members function solely as a unit. ELF3 has been reported to interact with different partners,16,21 so it is possible other transcription factors may recruit ELF3 and ELF4 to DNA. In this case, scanning target promoters for cis-elements will be helpful to identify potential co-factors. ChIP data sets should also be coupled to expression data from EC mutants to determine if there is a direct transcriptional consequence to binding, as well as the polarity of regulation. The latter is of particular interest because of differences in LUX and NOX activity. While we showed LUX and NOX directly bind PRR9 and PIF4/5 to repress expression,6 NOX (also called BOA) has been shown to directly activate CCA1.22 This suggests NOX and LUX may act as bi-functional transcription factors by possessing both activator and repressor activities. Functional analysis may identify inherent activation and repression domains like those in the Arabidopsis transcription factor WUSCHEL.23 Dual transcriptional activities may also be due to dynamic protein associations with trans-acting activators or repressors as suggested for the floral identity proteins SEP3, AP1, AP2, and LFY.24-26 Although these proteins have no known function in the clock, they may share common mechanisms of transcriptional regulation with the members of the EC. This leads to another important question about the EC: do other proteins associate with the EC? Protein partners of each EC member can be identified by yeast two-hybrid assays or immunoprecipitation followed by mass spectrometry.27 The latter will distinguish in vivo context-dependent interactions with proteins such as activators/repressors, co-activators/co-repressors, and chromatin remodelers to reveal the underlying mechanism of action.28 While LUX binds DNA and ELF3 has adaptor functions, the biochemical function of ELF4 is unknown and remains an important question to be addressed. Since LUX, ELF3 and ELF4 homologs are found in other plants, it will be essential to assess whether EC function is conserved. The answers to these questions will undoubtedly yield exciting insights into how the EC participates within the clock transcriptional network to confer plant fitness.
Acknowledgments
We thank Naden Krogan, Elizabeth Hamilton and Josh Gendron for critical reading of the manuscript. This work was supported by grants R01 GM67837 and GM50006 to S.A.K., by National Research Service Award GM083585 fellowship to D.A.N. and by European Molecular Biology Organization ALTF 236–2005 fellowship to A.H.
Disclosure of Potential Conflicts of Interest
There were no potential conflicts of interest to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18766
References
- 1.Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD, et al. Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet. 2008;4:e14. doi: 10.1371/journal.pgen.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClung CR, Gutie´rrez RA. Network news: prime time for systems biology of the plant circadian clock. Curr Opin Genet Dev. 2010;20:588–98. doi: 10.1016/j.gde.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legnaioli T, Cuevas J, Mas P. TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 2009;28:3745–57. doi: 10.1038/emboj.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pokhilko A, Hodge SK, Stratford K, Knox K, Edwards KD, Thomson AW, et al. Data assimilation constrains new connections and components in a complex, eukaryotic circadian clock model. Mol Syst Biol. 2010;6:416. doi: 10.1038/msb.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helfer A, Nusinow DA, Chow BY, Gehrke AR, Bulyk ML, Kay SA. LUX ARRHYTHMO encodes a nighttime repressor of circadian gene expression in the Arabidopsis core clock. Curr Biol. 2011;21:126–33. doi: 10.1016/j.cub.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, et al. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature. 2011;475:398–402. doi: 10.1038/nature10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc Natl Acad Sci U S A. 2005;102:10387–92. doi: 10.1073/pnas.0503029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onai K, Ishiura M. PHYTOCLOCK 1 encoding a novel GARP protein essential for the Arabidopsis circadian clock. Genes Cells. 2005;10:963–72. doi: 10.1111/j.1365-2443.2005.00892.x. [DOI] [PubMed] [Google Scholar]
- 9.Dixon LE, Knox K, Kozma-Bognar L, Southern MM, Pokhilko A, Millar AJ. Temporal repression of core circadian genes is mediated through EARLY FLOWERING 3 in Arabidopsis. Curr Biol. 2011;21:120–5. doi: 10.1016/j.cub.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolmos E, Nowak M, Werner M, Fischer K, Schwarz G, Mathews S, et al. Integrating ELF4 into the circadian system through combined structural and functional studies. HFSP J. 2009;3:350–66. doi: 10.2976/1.3218766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McWatters HG, Kolmos E, Hall A, Doyle MR, Amasino RM, Gyula P, et al. ELF4 is required for oscillatory properties of the circadian clock. Plant Physiol. 2007;144:391–401. doi: 10.1104/pp.107.096206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thines B, Harmon FG. Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proc Natl Acad Sci U S A. 2010;107:3257–62. doi: 10.1073/pnas.0911006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G, Siddiqui H, Teng Y, Lin R, Wan XY, Li J, et al. Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nat Cell Biol. 2011;13:616–22. doi: 10.1038/ncb2219. [DOI] [PubMed] [Google Scholar]
- 14.Para A, Farre´ EM, Imaizumi T, Pruneda-Paz JL, Harmon FG, Kay SA. PRR3 Is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell. 2007;19:3462–73. doi: 10.1105/tpc.107.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim WY, Fujiwara S, Suh SS, Kim J, Kim Y, Han L, et al. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature. 2007;449:356–60. doi: 10.1038/nature06132. [DOI] [PubMed] [Google Scholar]
- 16.Yu JW, Rubio V, Lee NY, Bai S, Lee SY, Kim SS, et al. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol Cell. 2008;32:617–30. doi: 10.1016/j.molcel.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu SX, Knowles SM, Andronis C, Ong MS, Tobin EM. CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis. Plant Physiol. 2009;150:834–43. doi: 10.1104/pp.108.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruneda-Paz JL, Breton G, Para A, Kay SA. A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science. 2009;323:1481–5. doi: 10.1126/science.1167206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Fujiwara S, Somers DE. PRR5 regulates phosphorylation, nuclear import and subnuclear localization of TOC1 in the Arabidopsis circadian clock. EMBO J. 2010;29:1903–15. doi: 10.1038/emboj.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawa M, Nusinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–5. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu XL, Covington MF, Fankhauser C, Chory J, Wagner DR. ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell. 2001;13:1293–304. doi: 10.1105/tpc.13.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai S, Wei X, Pei L, Thompson RL, Liu Y, Heard JE, et al. BROTHER OF LUX ARRHYTHMO is a component of the Arabidopsis circadian clock. Plant Cell. 2011;23:961–72. doi: 10.1105/tpc.111.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell. 2009;21:3493–505. doi: 10.1105/tpc.109.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellmer F, Riechmann JL. Gene networks controlling the initiation of flower development. Trends Genet. 2010;26:519–27. doi: 10.1016/j.tig.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, Wollmann H, et al. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell. 2010;22:2156–70. doi: 10.1105/tpc.110.075606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter CM, Austin RS, Blanvillain-Baufume´ S, Reback MA, Monniaux M, Wu MF, et al. LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Dev Cell. 2011;20:430–43. doi: 10.1016/j.devcel.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Sardiu ME, Washburn MP. Building protein-protein interaction networks with proteomics and informatics tools. J Biol Chem. 2011;286:23645–51. doi: 10.1074/jbc.R110.174052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krogan NT, Long JA. Why so repressed? Turning off transcription during plant growth and development. Curr Opin Plant Biol. 2009;12:628–36. doi: 10.1016/j.pbi.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]