Abstract
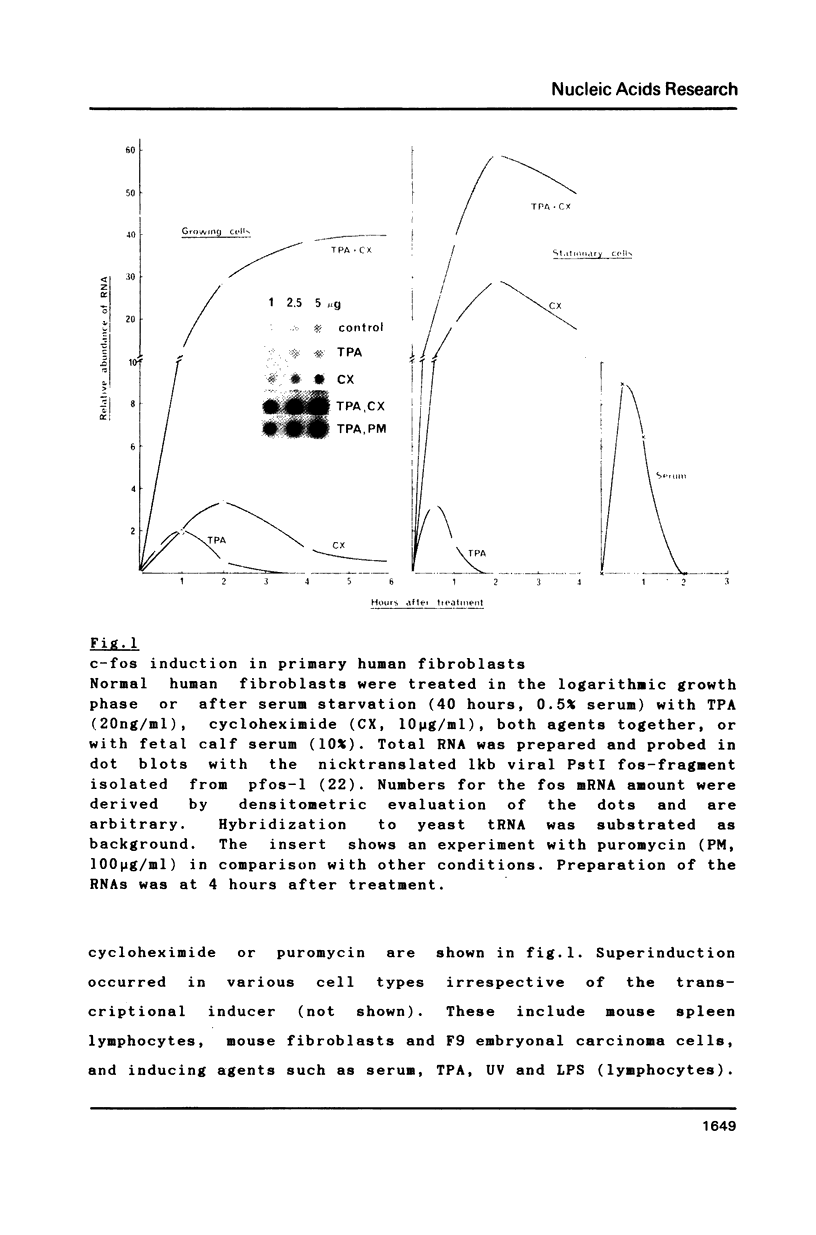
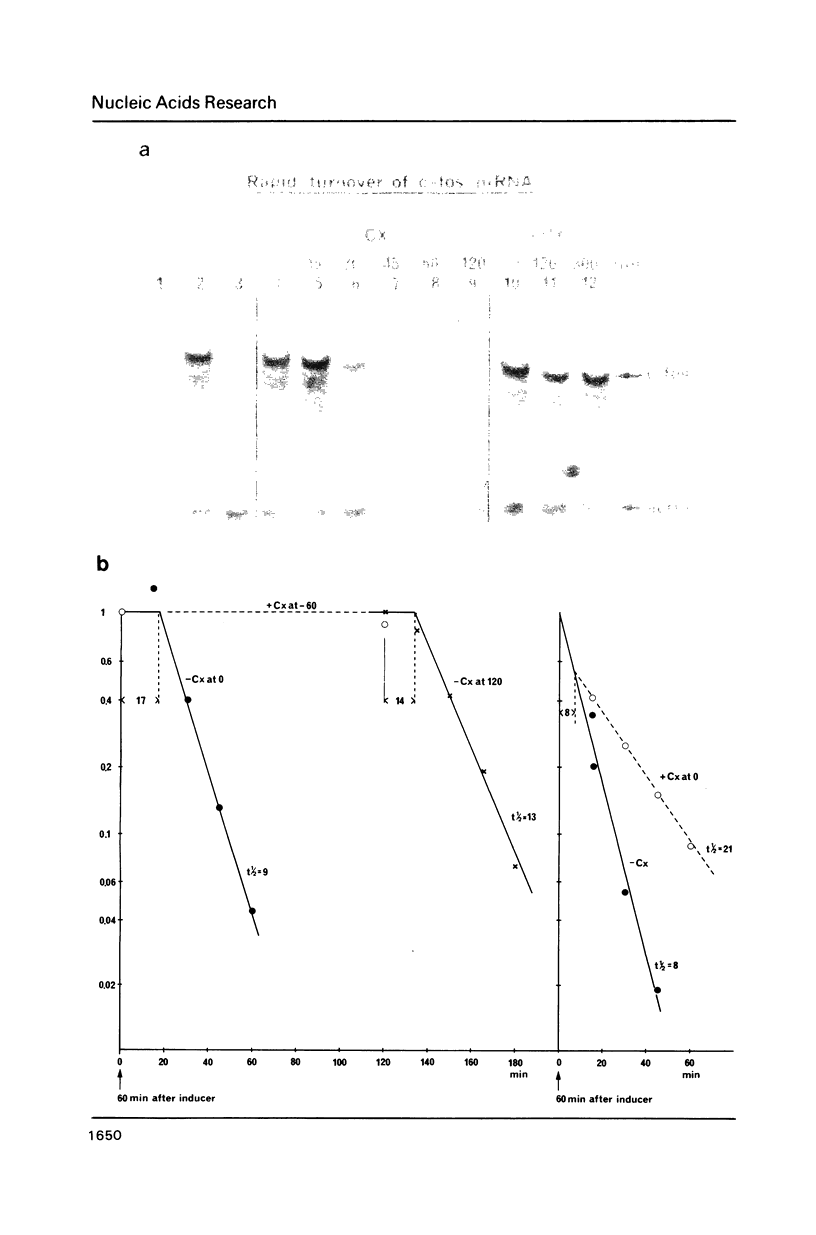
The transient induction of c-fos mRNA and protein suggests that regulation occurs not only by transcriptional activation but also at the level of turnover of the gene product. Here we present evidence for the rapid turnover of c-fos mRNA and some of the requirements for its specific degradation. The half life of induced mature cytoplasmic c-fos mRNA is 9 min in both serum-starved and growing primary human fibroblasts and in NIH 3T3 cells. A structure present at the 3' end of the c-fos mRNA molecule is involved in its low stability since the substitution or the removal of the untranslated 3' portion prolongues the RNA life time. The rapid turnover of fos mRNA requires, in addition, continued protein synthesis. Treatment of cells with cycloheximide stabilizes c-fos mRNA. Washing out cycloheximide reestablishes the rapid turnover. Both changes occur with lag periods of less than 17 minutes.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Pöting A., Mallick U., Rahmsdorf H. J., Schorpp M., Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986 May;6(5):1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Mechti N., Piechaczyk M., Lebleu B., Jeanteur P., Blanchard J. M. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4896–4899. doi: 10.1073/pnas.82.15.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Finkel M. P., Biskis B. O., Jinkins P. B. Virus induction of osteosarcomas in mice. Science. 1966 Feb 11;151(3711):698–701. doi: 10.1126/science.151.3711.698. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Kazmaier M., Brüning E., Ryffel G. U. Post-transcriptional regulation of albumin gene expression in Xenopus liver. EMBO J. 1985 May;4(5):1261–1266. doi: 10.1002/j.1460-2075.1985.tb03770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijlink F., Curran T., Miller A. D., Verma I. M. Removal of a 67-base-pair sequence in the noncoding region of protooncogene fos converts it to a transforming gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4987–4991. doi: 10.1073/pnas.82.15.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Mitchell R. L., Henning-Chubb C., Huberman E., Verma I. M. c-fos expression is neither sufficient nor obligatory for differentiation of monomyelocytes to macrophages. Cell. 1986 May 23;45(4):497–504. doi: 10.1016/0092-8674(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Curran T., Müller D., Guilbert L. Induction of c-fos during myelomonocytic differentiation and macrophage proliferation. Nature. 1985 Apr 11;314(6011):546–548. doi: 10.1038/314546a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Müller D., Guilbert L. Differential expression of c-fos in hematopoietic cells: correlation with differentiation of monomyelocytic cells in vitro. EMBO J. 1984 Aug;3(8):1887–1890. doi: 10.1002/j.1460-2075.1984.tb02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Verma I. M., Adamson E. D. Expression of c-onc genes: c-fos transcripts accumulate to high levels during development of mouse placenta, yolk sac and amnion. EMBO J. 1983;2(5):679–684. doi: 10.1002/j.1460-2075.1983.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Wagner E. F. Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature. 1984 Oct 4;311(5985):438–442. doi: 10.1038/311438a0. [DOI] [PubMed] [Google Scholar]
- Ponta H., Ball R., Steinmetz M., Groner B. Hormonal regulation of cell surface expression of the major histocompatibility antigen H-2Ld in transfected cells. EMBO J. 1985 Dec 16;4(13A):3447–3453. doi: 10.1002/j.1460-2075.1985.tb04103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Harth N., Eades A. M., Litfin M., Steinmetz M., Forni L., Herrlich P. Interferon-gamma, mitomycin C, and cycloheximide as regulatory agents of MHC class II-associated invariant chain expression. J Immunol. 1986 Mar 15;136(6):2293–2299. [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Wagner E. F., Müller R. Analysis of the differentiation-promoting potential of inducible c-fos genes introduced into embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1775–1781. doi: 10.1002/j.1460-2075.1985.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shuin T., Billings P. C., Lillehaug J. R., Patierno S. R., Roy-Burman P., Landolph J. R. Enhanced expression of c-myc and decreased expression of c-fos protooncogenes in chemically and radiation-transformed C3H/10T1/2 Cl 8 mouse embryo cell lines. Cancer Res. 1986 Oct;46(10):5302–5311. [PubMed] [Google Scholar]
- Simcox A. A., Cheney C. M., Hoffman E. P., Shearn A. A deletion of the 3' end of the Drosophila melanogaster hsp70 gene increases stability of mutant mRNA during recovery from heat shock. Mol Cell Biol. 1985 Dec;5(12):3397–3402. doi: 10.1128/mcb.5.12.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]