Abstract
Senescence marker protein 30 (SMP30) is a multifunctional protein involved in cellular Ca2+ homeostasis and the biosynthesis of ascorbate in non-primate mammals. The primary structure of the protein is highly conserved among vertebrates, suggesting the existence of a significant physiological function common to all mammals, including primates. Enzymatic activities of SMP30 include aldonolactone and organophosphate hydrolysis. Protective effects against apoptosis and oxidative stress have been reported. X-ray crystallography revealed that SMP30 is a six-bladed β-propeller with structural similarity to paraoxonase 1, another protein with lactonase and organophosphate hydrolase activities. SMP30 has recently been tied to several physiological conditions including osteoporosis, liver fibrosis, diabetes, and cancer. This review aims to describe the recent advances made toward understanding the connection between molecular structure, enzymatic activity and physiological function of this highly conserved, multifaceted protein.
Keywords: beta-propeller, divalent metal binding, gluconolactonase, oxidative stress, regucalcin, SMP30
Introduction
The designation of senescence marker protein 30 (SMP30) as a marker of senescence or aging implies that its cellular levels would increase with aging, when, in fact, the opposite is true. Analysis of age-associated changes in the expression of soluble liver proteins in rats showed that expression of SMP30 decreases androgen-independently with age. SMP30 comprises 2% of soluble rat liver proteins and its levels decrease by 60–70% in aged rats (1). The molecular weight of SMP30 was initially estimated as 30 kD, and hence the 30 in its name. More accurate measurements and cloning of SMP30 later revealed that the molecular weight is actually 34 kD (2). The gene for SMP30, located on the X-chromosome, consists of 7 exons, and has an open reading frame of 897 bp coding for 299 amino acids (3). While it is primarily found in liver and kidney tissues, low amounts of SMP30 have also been detected in the lungs, ovaries, testes, epidermis, stomach, brain, mammary glands, prostate, epididymis, and seminal vesicles using both immunostaining and mRNA analysis (2–7). Furthermore, the amino acid sequence of SMP30 is highly conserved (70–90% identity) in vertebrates, which is suggestive of an important, even essential, biological function.
Although Fujita et al. (2) were the first to isolate cDNA clones and determine the amino acid sequence of SMP30, this protein was independently “discovered” in the soluble fraction of rat liver homogenates four separate times. Interestingly, the four independent discoveries correspond to four different proposed functionalities: Yamaguchi et al. (8) claimed to have found a novel Ca2+-binding protein involved in Ca2+ homeostasis, Fujita et al. (1) suggested it was an anti-aging molecule protecting cells from apoptosis and oxidative stress, Billecke et al. (9) reported the isolation of an organophosphate (OP) hydrolase, and Lehninger (10) reported the identification of an aldonolactonase thought to be involved in ascorbate biosynthesis. Isolation and sequencing of cDNA clones ultimately revealed that these four distinct functions could all be ascribed to a single gene product (11).
Over a decade before the reports of SMP30 as an aging marker, Yamaguchi et al. (8) named this rat liver protein regucalcin and proposed that it was a novel Ca2+-binding protein involved in the regulation of free cellular Ca2+ concentrations. Unlike other Ca2+-binding proteins, regucalcin lacks a known Ca2+-binding domain, such as an EF-hand motif. The Ca2+-binding ability of regucalcin/SMP30 was called into question when later studies failed to detect binding of 45Ca2+ and indicated that the enzyme’s ability to hydrolyze diisopropyl phosphorofluoridate (DFP) was not dependent on Ca2+(12). However, later kinetics experiments performed in our lab using highly purified human SMP30 overexpressed from E. coli revealed that SMP30 does exhibit lactonase activity in the presence of high concentrations of Ca2+ such as those that would be seen in cells under stress (13). It is believed by some that SMP30 affects Ca2+ homeostasis indirectly through interaction with calmodulin and membrane Ca2+ pumps (14–16). This role of SMP30 in Ca2+ signaling and homeostasis has been reviewed by Yamaguchi (16, 17) and will not be covered in depth here.
The two remaining “discoveries” of SMP30 revealed enzymatic functions of the protein. SMP30 is capable of hydrolyzing DFP and other OPs such as sarin, soman, and tabun in the presence of divalent cations such as Mg2+ and Mn2+ (9, 12). Sarin, soman, and tabun are nerve agents that have been used as chemical weapons. The OP hydrolase activity of SMP30 makes this enzyme an interesting target for the development of bioscavengers. However, since OPs are man-made compounds that were not synthesized until the 1930s, this OP hydrolase activity provides little insight into the physiological function of the protein.
In contrast, the second observed enzymatic function of SMP30 has clear biological significance in non-primate mammals. SMP30 also functions as a lactonase and catalyzes the penultimate step in the ascorbate (vitamin C) biosynthetic pathway. While studying the conversion of D-glucuronic acid to L-ascorbate, Lehninger purified an aldonolactonase from rat liver and demonstrated its ability to catalyze the reversible interconversion of L-gulonate and L-gulono-γ-lactone (10, 18, 19). In the formation of ascorbate, L-gulonate is closed to form L-gulono-γ-lactone which is then converted to L-ascorbate by gulonolactone oxidase (20). Lehninger’s aldonolactonase was determined to be SMP30 in a study showing that SMP30 knockout mice fed a vitamin C deficient diet developed scurvy-like symptoms such as brittle bones, low body weight, and shortened lifespan. In addition to gulono-γ-lactone, SMP30 also displayed lactonase activity with other aldonolactones (21). Most mammals synthesize their own ascorbate via the pathway including SMP30, however, for some species, including primates of the Haplorrhini suborder and guinea pigs, this compound must be obtained through diet. Primates and guinea pigs no longer synthesize ascorbate because they lack a functional copy of gulonolactone oxidase, the final enzyme in the pathway. Yet, the amino acid sequence of SMP30 is remarkably well conserved, even in these species that do not synthesize ascorbate; the protein sequence of the human form is 88% identical and 93% similar to the mouse form. Thus, the primary physiological function of SMP30 in humans remains unclear.
Recent studies describe the relationship of SMP30 with a number of physiological effects. Counter to other aging observations, overexpression of SMP30 appears to cause bone loss and osteoporosis (22, 23). On the other hand, SMP30 deficiency leads to decreased glucose tolerance and abnormal lipid accumulation in the liver (22, 24–28). SMP30 has also been associated with control of cell proliferation and is down-regulated in human prostate and breast cancers (29, 30). As the effect of SMP30 in these conditions appears to be largely independent of vitamin C, they are likely clues to the physiological relevance of this protein in humans and other mammals lacking the capacity to synthesize ascorbate. Although SMP30 has been implicated in bone loss, abnormal lipid metabolism, decreased glucose tolerance and certain cancers, its role in these conditions has not been described on a metabolic or molecular level. The crystal structure of SMP30 was recently solved and described (13). Further structural characterization of this protein and the elucidation of its reaction mechanism should help to identify and further elaborate on each of the physiologic functions of SMP30 in humans.
Highly Conserved in Vertebrates
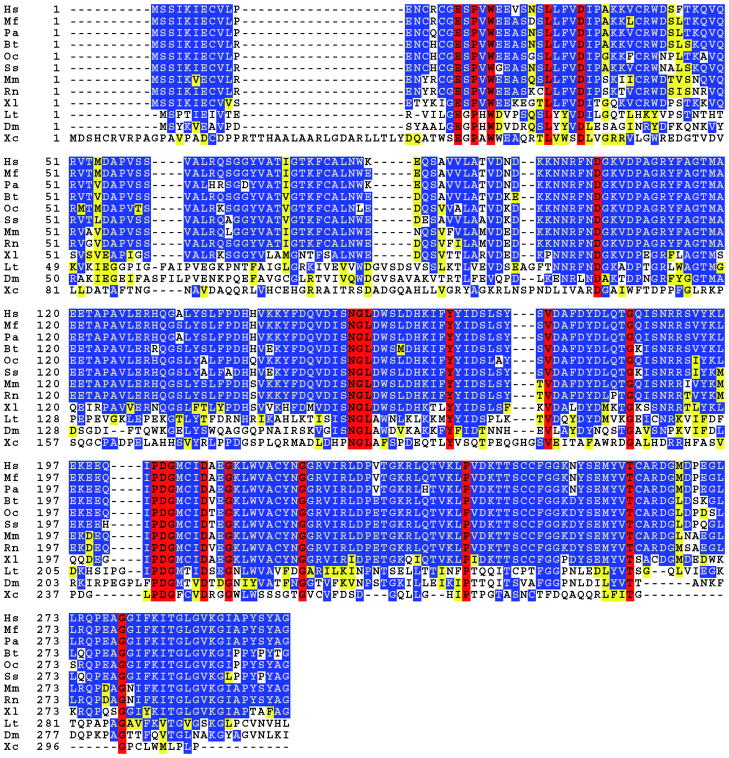
Evolutionary conservation of protein sequence often indicates that a protein has an important biological function. SMP30 homologs have been identified in at least 16 different species ranging from vertebrates to insects, bacteria and fungi (Figure 1). Vertebrate forms of SMP30, which include human, monkey, orangutan, cow, hamster, rabbit, pig, mouse, rat, and frog homologs, are 70–90% identical to one another. Sequence similarity drops to 25–45% in insects, bacteria and fungi. No known homolog exits in C. elegans however a 51-amino acid segment of the SMP30 protein exhibits homology with a yeast RNA polymerase (31–33).
Figure 1.
Amino acid sequence alignment of SMP30 homologs in vertebrates and invertebrates. Red indicates completely conserved, blue indicates identical, and yellow indicates similar residues. Identity between vertebrates ranges from 70–90%, and drops to about 30% between vertebrate and invertebrate forms of SMP30. The amino acid sequence of SMP30 is highly conserved among all mammals, regardless of ability to synthesize ascorbate. Latin and common names as well as gi accession numbers for the above represented species are as follows: Hs, Homo Sapiens, human, 23111021; Mf, Macaca fascicularis, monkey, 115502619; Pa, Pongo abeilii, orangutan, 197101437; Bt, Bos Taurus, cow, 13633941; Oc, Oryctolagus cuniculus, rabbit, 20178120; Ss, Sus scrofa, pig, 122131846; Mm, Mus musculus, mouse, 15215231; Rn, Rattus Norvegicus, rat, 68067383; Xl, Xenopus laevis, frog, 147905135; Lt, Lampyris turkestanicus, firefly LRE, 301068495; Dm, Drosophila melanogaster, fruit fly, 23171287; Xc, Xanthomonas campestris, bacteria, 21233020. This sequence alignment was prepared using the Biology Workbench (81).
Insect homologs of SMP30 include firefly luciferin-regenerating enzyme (LRE), flesh fly and Drosophila anterior fat body protein, and Drosophila SMP30. Sequence identity between the insect homologs and vertebrate SMP30 ranges between 32 and 38% (34). Firefly LRE, a 34kD protein composed of 308 amino acids, catalyzes the recycling of oxyluciferin to luciferin in firefly lanterns. Light is produced when luciferase converts luciferin to oxyluciferin in the presence of ATP and molecular oxygen. Oxyluciferin is thought to be a competitive inhibitor of luciferase. Oxyluciferin is converted back to luciferin in a two-step process; LRE first catalyzes the conversion of oxyluciferin to 2-cyano-6-hydroxybenzothiazole which then combines with D-cysteine to form luciferin (34–36). An SMP30 homolog in Drosophila was found to be up-regulated after exposure to cold temperatures (15 °C). Interestingly, expression of the Drosophila SMP30 homolog increased in senescence, in contrast to the vertebrate homologs, for which expression decrease with age (37). Significant protein sequence homology (34% identity, 50% similarity) to mammalian SMP30 was also seen in anterior fat body protein (AFP) of Sarcophaga peregrina, a 34 kD protein found in the larval fat body. AFP levels in the anterior fat body decrease after pupation in a similar trend with mammalian SMP30 homologs, and like SMP30, it does not appear to have a strong affinity for Ca2+ (38).
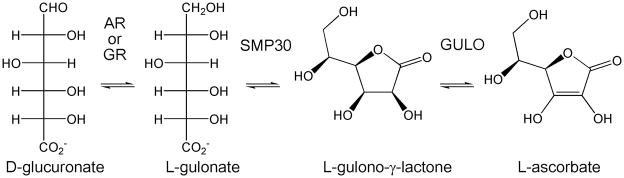
Inferences about the biological significance of SMP30 can be made from sequence analysis of homologs from different species. The role of SMP30 in ascorbate biosynthesis was first discovered in mice (21). Although most invertebrates do not synthesize ascorbate, a large number of vertebrates do (39–41). The trait of ascorbate biosynthesis most likely appeared before the divergence of plant and animal species, and well before the emergence of terrestrial vertebrates (21, 42). Cold-blooded vertebrates, such as amphibians, reptiles, and some fish, synthesize ascorbate in their kidneys, while most warm-blooded mammals synthesize ascorbate in their livers (21, 42–44). Organisms that have lost the ability to synthesize ascorbate include teleost fishes, some, but not all, passeriform birds, some bat species, guinea pigs, and Haplorrihini primates, which includes monkeys, apes, and humans. It was commonly assumed that all primates lack the ability to synthesize ascorbate, however studies have shown that primates of the suborder Strepsirrhini (prosimians) including lemurs, lorises, and galagos synthesize ascorbate (42, 44). Additionally, ascorbate is also synthesized in plants, and fermented in bacteria (21). Thus, of the organisms with identified SMP30 homologs, all produce their own ascorbate except humans, monkeys, orangutans and flies. SMP30 is the penultimate enzyme and gulonate oxidase (GULO) the final enzyme in the ascorbate biosynthetic pathway as shown in Figure 2. The gene for gulonolactone oxidase in Haplorrhini primates has undergone genetic drift, as expected for a protein evolving without functional constraint; however, SMP30 sequences remained highly conserved, indicating the existence of another biologically significant function.
Figure 2.
The final 3 steps of the biosynthesis of L-ascorbate from D-glucuronate. First, D-glucuronate is converted to L-gulonate by either aldose reductase (AR, 15%) or aldehyde reductase (GR, 85%) (67). SMP30 then closes L-gulonate to the 5-membered lactone, L-gulono-γ-lactone. In the final step, gulonolactone oxidase (GULO) converts L-gulono-γ-lactone to L-ascorbate. In mammals lacking the ability to synthesize ascorbate, GULO is non-functional and highly mutated.
SMP30 and Apoptosis
A protective effect of SMP30 against apoptosis has been observed and partially attributed to the ability of SMP30 to regulate cellular Ca2+ concentrations (14, 45). Sustained, elevated cellular Ca2+ levels induce apoptosis. Human Hep G2 cells and pig renal tubular epithelial cells overexpressing SMP30 showed enhanced Ca2+ efflux following a transient spike in cellular free Ca2+ levels induced by ATP (14, 45). When pig kidney cells transfected with SMP30 were treated with a calmodulin inhibitor, the rate of Ca2+ efflux was reduced, indicating that SMP30 indirectly regulates the activity of a calmodulin-dependent membrane Ca2+ pump by interacting with calmodulin (45). Interaction of SMP30 with calmodulin also appears to regulate the activation of survival factor Akt (46). The ability of SMP30 to stimulate membrane Ca2+ pumps was also demonstrated in vivo using isolated rat liver plasma membranes and microsomes (47, 48).
In addition to preventing apoptosis induced by Ca2+ influx, SMP30 also exhibits a protective effect against apoptosis induced by other signaling pathways. Tumor necrosis factor-α (TNF-α) induces apoptosis by binding to membrane receptors that activate multiple intracellular signaling pathways. Primary cultured hepatocytes from SMP30 knockout mice are more susceptible to apoptosis induced by treatment with tumor necrosis factor-α (TNF-α)/actinomycin D than wild type hepatocytes (15). Likewise, rat hepatoma H4-II-E cells overexpressing SMP30 were significantly less affected by treatment with TNF-α than control cells (49). The Fas ligand is another member of the TNF family that induces apoptosis by binding to membrane death receptors. Livers from SMP30 knockout mice display an increased sensitivity to Fas-mediated apoptosis, an observation that supports a protective role of SMP30 against apoptosis (15).
Other important events in the induction of apoptosis include activation of the caspase cascade and protein kinases. Lipopolysaccharide (LPS) activates many proapoptotic genes including caspase-8 and caspase-3 (50). Dibucaine is a Ca2+-dependent protein kinase inhibitor and has been shown to activate caspase-3, -6, -8, and -9. PD98059 is an ERK inhibitor that induces apoptosis by inactivating Bcl-2. Rat hepatoma H4-II-E cells overexpressing SMP30 were shown to be less likely to undergo apoptosis when treated with LPS, PD98059, and Dibucaine (51). Furthermore, rat kidney NRK52E cells overexpressing SMP30 were less likely to undergo apoptosis when treated with TNF-α, LPS, Bay K 8644, and thapsigargin than wild type rat kidney NRK52E cells. Addition of a caspase-3 inhibtor to wild type NRK52E cells prevented apoptosis caused by TNF-α, LPS, and Bay K 8644. Expression of caspase-3 mRNA was increased in wild type NRK52E cells treated with TNF-α; however no such change was observed in NRK52E cells overexpressing SMP30 (52). These results indicate that SMP30 prevents apoptosis by inhibiting the caspase cascade.
SMP30 and Oxidative Stress
A protective effect of SMP30 against oxidative stress has also been observed. Levels of protein carbonyls (a known marker of oxidative stress) were higher in SMP30 knockout mice than in wild type mice of the same age. After an eight week exposure to cigarette smoke SMP30 knockouts developed pulmonary emphysema due to elevated oxidative stress, whereas wild type mice did not (53). Elevated levels of oxidative stress were also observed in the brains of SMP30 knockout mice (54). Furthermore, overexpression of SMP30 in P19 cells (from a multipotent mouse embryonic carcinoma) protects the cells from t-butylhydroperoxide induced cytotoxicity (55).
Discovery of SMP30’s role as a lactonase involved in the biosynthesis of ascorbate provided an explanation for the increased oxidative stress observed in tissues from knockout mice. Ascorbate, more commonly known as vitamin C, is a powerful, water-soluble antioxidant. In 2006 Kondo et al. (21) discovered that SMP30 was the enzyme that catalyzes the penultimate step in the biosynthesis of ascorbate (Figure 2). Therefore, observed antioxidant effects of SMP30 can largely be attributed to its role in ascorbate production. SMP30 knockout mice have recently been used as a model system in studies concerning the role of ascorbate (56–61). In most cases, dietary vitamin C supplementation restores a normal phenotype to these SMP30 knockouts. However, one study indicates that the antioxidant effect of SMP30 may be due to more than ascorbate production. Handa et al. (62) overexpressed SMP30 in human hepatoma cells, Hep G2, and observed a decrease in reactive oxygen species (ROS), lipid peroxidation, superoxide dismutase activity, and GSH levels compared to control Hep G2 cells. They also found that recombinant human SMP30 was not able to directly scavenge radicals in vitro. As noted above, human cells lack gulonolactone oxidase, the final enzyme in the ascorbate biosynthesis pathway, and cannot produce vitamin C. Consequently, ascorbate production does not explain the antioxidant effect of SMP30 in this study which utilized human hepatoma cells. Handa et al. (62) proposed that altered Ca2+ modulation in SMP30 transfectants played a role in the decrease of ROS levels.
Gluconolactonase Activity
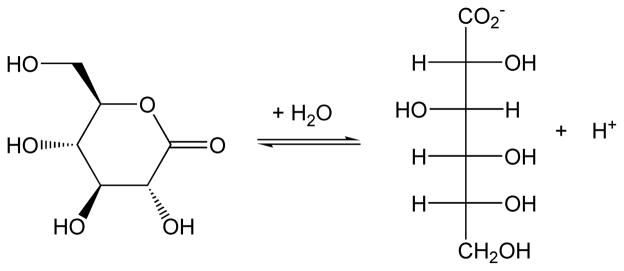
SMP30 has the ability to hydrolyze various aldonolactones. Kondo et al. (21) tested the enzyme’s gluconolactonase ability after noticing sequence similarity to several bacterial gluconolactonases. Using rat SMP30 purified from E. coli, the specific activity towards D- and L-glucono-δ-lactone, D- and L-gulono-γ-lactone, and D- and L-galactono-γ-lactone was determined in the presence of 75 μM Zn2+. In each case, it was determined that D-aldonolactones were the more reactive stereoisomeric substrates for the reaction shown in Figure 3. The specific activity in vitro of rat SMP30 with D-glucono-δ-lactone was 18- to 22-fold higher than with D-gulono- and D-galactono-γ-lactones, respectively (21). Glucono-δ-lactone is a six-membered ring, whereas gulono-γ-lactone and galactono-γ-lactone are five-membered rings. The closing of L-gulonate to L-gulono-γ-lactone is the penultimate step of the ascorbate biosynthetic pathway (Figure 2).
Figure 3.
SMP30 catalyzes the interconversion of D-glucono-δ-lactone and D-gluconic acid in the presence of divalent cations including Mn2+, Mg2+, Ca2+, and Zn2+. Opening of the lactone ring releases a proton. Of all the aldonolactone subtrates tested, SMP30 showed the greatest activity with D-glucono-δ-lactone.
The activity of human SMP30 with D-glucono-δ-lactone and its dependence on various divalent cations has also been examined (13). In the presence of 75 μM Zn2+, the KM, kcat, and kcat/KM values were determined to be 2.7 mM, 341 s−1, and 126 mM−1s−1. The Kd values for Zn2+, Mn2+, Mg2+, and Ca2+ were determined in the presence of D-glucono-δ-lactone. Although SMP30 had the highest gluconolactonase activity in the presence of Zn2+, this is unlikely the metal of physiological relevance because the Kd for Zn2+ is above the free metal ion cellular concentration range. In light of the fact that SMP30 was thought to be a Ca2+-binding protein that played a significant role in Ca2+ regulation, it is interesting to note that SMP30 had the lowest affinity for Ca2+, and the free concentration of Ca2+ in a resting cell is far below the determined Kd value. It was speculated (13) that the lactonase activity of SMP30 may depend on Mn2+ or Mg2+ most of the time, switching to a Ca2+-dependent activity only in times of stress when Ca2+ levels are known to increase.
SMP30 is a Six-Bladed β-propeller
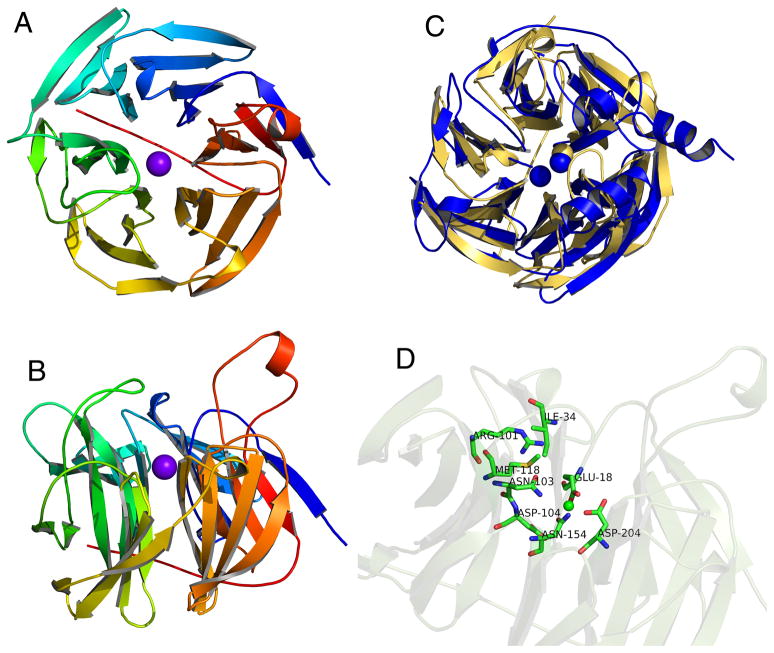
The crystal structure of human SMP30 was recently solved to a resolution of 1.4 Å (13). The structure reveals that SMP30 is a six-bladed β-propeller with 24 β-strands arranged in six β-sheets that form a torus around a central tunnel as shown in Figure 4. A single metal binding site is found inside the solvent-filled central tunnel and crystal structures bound to Zn2+ and Ca+ have been solved. In structural homologs of SMP30, such as a drug resistant protein from S. aureus named Drp35, squid DFPase, paraoxonase 1 (PON1), and a protein from X. campestris, there are four conserved residues that bind a catalytic Ca2+ atom. In SMP30, these residues correspond to E18, N154, D204, and N103; however, based on structures solved with Ca2+ or Zn2+ bound, only three of these residues actually coordinate with the metal ion in SMP30 (13). Although it is in close proximity, the side chain of N103 is rotated relative to its position in the structural homologs and does not appear to bind to the metal ion. The putative active site consists of four residues in the central tunnel just above the metal binding site (R101, M118, I34, D104). A flexible loop made up of six residues is located at the top of the central tunnel, forming a lid-like structure above the active site that could possibly play a role in substrate specificity or as a gatekeeper. The C-terminal tail runs across the opposite side of the central tunnel (13).
Figure 4.
Ca2+-bound crystal structure of human SMP30. A) A top view of SMP30 shows the six-bladed β-propeller fold. The C-terminal tail traverses the central tunnel of the propeller and the bound Ca2+ ion is shown in purple. B) A side view of SMP30 shows several loops that extend above the propeller near the active site which is located just above the bound Ca2+ ion. C) Overlay of the crystal structures of human SMP30 (gold) and PON1 (blue). Although both proteins have a six-bladed β-propeller fold, PON1 binds two Ca2+ ions whereas SMP30 only binds one. Furthermore, PON1 is specific for Ca2+, while SMP30 can bind several different divalent cations. D) Side view of SMP30 with the metal-binding and putative active site residues highlighted. E18, D204, and N154 are bound to the metal ion. N103 and D104 are not close enough to bind the metal. Other putative active site residues, which are located just above the metal binding site include, I34, R101, and M118.
SMP30 as an Organophosphate Hydrolase
OP compounds, first synthesized in Germany in the 1930s, are acetylcholinesterase inhibitors that are found in insecticides and used as nerve agents in chemical warfare (63). Billecke et al. (9) identified an enzyme in rat liver capable of hydrolyzing DFP, soman, sarin, and tabun that Kondo et al. later confirmed to be SMP30 (12). The activity of SMP30 was tested with substrates of human PON1 (another mammalian enzyme capable of hydrolyzing OPs) to determine how closely related the two enzymes were. The enzymes are each six-bladed β-propeller enzymes (Figure 4C). SMP30 was unable to hydrolyze any of the putative physiological PON1 substrates such as phenyl acetate and napthylacetates, and 7-acetoxy-N-methylquinolinium (9). Furthermore, the DFPase hydrolytic activity of SMP30 is dependent on divalent cations such as Mg2+, Mn2+, and Co2+, with the highest activity observed in the presence of Mg2+ (9). Unlike PON1, which requires Ca2+ for activity, SMP30 was unable to hydrolyze DFP with Ca2+ (12). Although the ability of an enzyme to hydrolyze OPs is of potential interest in the engineering of bioscavengers, it tells us little about the enzymes true physiological purpose, since OPs are synthetic substrates that have been around for less than a century.
There are several enzymes that are structural and potential functional homologs of SMP30. SMP30 is one of a select few enzymes that have been shown to hydrolyze both lactones and OPs, including PON1, and bacterial phophotriesterases (64). Some six-bladed β-propellers, such as Drp35 from S. aureus and XC5397 from X. campestris hydrolyze lactones but not OPs, while still others, such as DFPase from the squid L. vulgaris, hydolyze OPs but not lactonases (65). As is characteristic for β-propeller proteins, there is very low sequence identity between any of the above mentioned proteins (13, 64, 65). It is known that for PON1, different residues in the active site catalyze the OP hydrolase and lactonase mechanisms (64, 65). The relationship and involvement of specific active site residues between OP hydrolase and lactonase activity in SMP30 is unknown.
Physiological Effects
High sequence conservation of SMP30 among vertebrates is suggestive of a significant, conserved physiological function; however, no such conserved function is known for this protein. Although SMP30 is a key enzyme in the synthesis of ascorbate in most non-primate mammals, there is no direct metabolic evidence of an essential biological function in primates and other mammals that lack the capacity to synthesize their own ascorbate. Furthermore, in SMP30 knockout mice, most differences in phenotype, such as brittle bones and low body weight, are corrected by a diet supplemented with vitamin C. Recent studies have tied SMP30 to a number of physiological effects of medical relevance that are independent of a link to vitamin C. Connections between SMP30 and osteoporosis, fatty liver diseases, glucose intolerance and cancer will be discussed next.
SMP30 and Osteoporosis
Brittle bones and scurvy-like symptoms were observed in SMP30 knockout mice as a result of vitamin C deficiency (21). Ascorbate is a known cofactor in the hydroxylation of lysine and proline, which are required for collagen formation. Since collagen synthesis is a precursor of bone mineralization, ascorbate is required for bone formation (66). In addition, ascorbate contributes to bone formation by suppressing the activity of bone-resorbing osteoclasts and promoting the formation of bone-forming osteoblasts (67). Ascorbate deficiency is associated with the development of osteoporosis in elderly humans (67). Curiously, rats overexpressing SMP30 also showed bone loss and osteoporosis, with females being more affected than males (22). In SMP30 transgenic rats, SMP30 levels were elevated to 110% and 150% of wild type levels in the femoral-diaphyseal tissues of male and female rats respectively, and to 136% and 205% of wild type levels in the femoral-metaphyseal tissues of male and female rats (23). Bone morphologic changes were observed in femoral-diaphyseal and femoral-metaphyseal tissues alike, with greater changes observed in female transgenic rats. SMP30 transgenic rats had less highly mineralized and calcified tissue than wild type rats. In addition, the polar strength strain index and cortical thickness were significantly decreased in female SMP30 transgenic rats (23). SMP30 was also enhanced in bone marrow cells of transgenic rats. Differentiation of marrow cells into bone resorbing osteoclasts is stimulated by bone-resorbing factors such as the receptor activator of NF-κB ligand, parathyroid hormone, 1α,25-dihydroxyvitamin D3 and prostaglandin E2. When marrow cells from wild type and SMP30 transgenic rats were cultured in the absence of bone-resorbing factors, increased osteoclast formation was seen in cultures from SMP30 transgenic rats (68, 69). These results implicate SMP30 in bone resorption and the development of osteoporosis.
Nonalcoholic Fatty Liver Disease and Liver Fibrosis in SMP30 Knockouts
The designation, nonalcoholic fatty liver disease (NAFLD) applies to a range of fatty liver diseases including simple hepatic steatosis (the abnormal accumulation of lipids in cells), steatohepatitis, and even cirrhosis that are caused by neither alcoholic abuse nor hepatitis infection (27, 70, 71). It was estimated that in 2006, NAFLD affected 16–23% of the US population (70). Hyperlipidemia, insulin resistance, oxidative stress, and especially obesity are believed to contribute to NAFLD development (27, 70, 71). Over 90 percent of people with a body mass index of 39 kg/m2 or greater have NAFLD (70). Furthermore, NAFLD is one of the most common causes of fibrosis, the build-up of scar tissue (71).
Recent observations in humans and knockout mice indicate an association between SMP30 and NAFLD. Among the phenotypic characteristics of SMP30 knockout mice is an accumulation of lipids, phospholipids, and neutral lipids in the liver. Lipid droplets can be seen in SMP30-deficient hepatocytes and around the central vein in the liver, suggesting a role for SMP30 in lipid metabolism (26). Since hyperlipidemia is a putative contributing factor to NAFLD development, abnormal lipid metabolism and lipid accumulation in SMP30 knockout mice indicates that SMP30 deficiency may indirectly lead to NAFLD. Observations in human subjects further support this hypothesis. Park et al. (27) observed decreased SMP30 levels in liver and blood samples from human NAFLD patients. Patients with the most severe degree of NAFLD had the lowest hepatic SMP30 levels and the highest low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) levels. Interestingly, VLDL1 production in the liver is commonly observed during development of insulin resistance, which is another one of the key risk factors for NAFLD occurrence (27). An estimated 70% of people with type 2 diabetes also have NAFLD (72). Decreased hepatic SMP30 levels were also observed in mice with D-galactosamine/lipopolysaccharide-induced acute liver failure (ALF) (73). Interestingly, plasma SMP30 levels were elevated in these mice as well as in human ALF patients (over 3 times those of healthy humans) (73). While these results do not conclusively determine whether decreased hepatic SMP30 expression is a cause or a result of NAFLD and ALF, they indicate that SMP30 is involved in the pathogenesis of this increasingly prevalent disease.
Advanced stages of NAFLD are characterized by liver fibrosis which can lead to cirrhosis. Hepatic stellate cells (HSCs), usually found in a quiescent state, are the main cells implicated in collagen production during liver fibrosis. Activation of HSCs is stimulated by transforming growth factor beta, insulin, and leptin and inhibited by peroxisome proliferator-activated receptor gamma (PPAR-γ) and adiponectin (71, 74). In contrast to the previously mentioned human studies in which decreased levels of SMP30 are associated with more severe NAFLD cases, another study done in SMP30 knockout mice demonstrated that the lack of SMP30 protected the mice from carbon tetrachloride (CCl4)-induced liver fibrosis (74). Fibrosis was restored to levels seen in CCl4-treated wild type mice when SMP30 knockouts were given a vitamin C enriched diet, however the level of fibrosis in wild type mice did not change upon addition of vitamin C. Furthermore, PPAR-γ was up-regulated in SMP30 knockout mice. Park et al. (74) concluded that vitamin C deficiency in SMP30 knockout mice indirectly inhibits HSC activation by up-regulating PPAR-γ. These results appear to be in conflict with those of the previously mentioned studies. However, the protective effects of SMP30 deficiency observed here were the result of vitamin C deficiency, a nutrient that humans must obtain through dietary intake.
SMP30 and Insulin Resistance
Declining glucose tolerance is seen in normal aging. Type 2 diabetes generally occurs later in life (25). Paradoxically, both overexpression of SMP30 and decreasing levels of SMP30 have been implicated in the development of insulin resistance. Overexpression of SMP30 in rat hepatoma H4-II-E cells led to insulin resistance. Genes that were normally up-regulated in response to treatment with insulin or glucose were not affected by glucose or insulin in SMP30 transfectants (75). The mechanism by which SMP30 inhibits expression of insulin signaling-related proteins is still unclear. Furthermore, H4-II-E cells over-expressing SMP30 demonstrated increased lipid production and glucose utilization (75). An in vivo study using SMP30 knockout mice showed that blood glucose levels were significantly higher and insulin levels significantly lower than in wild type mice thirty minutes after glucose administration. In islet cells isolated from the knockout mice, treatment with glucose or KCl caused less insulin secretion than in islet cells from wild type mice, indicating impaired insulin secretion in the absence of SMP30 (25). Interestingly, insulin stimulates SMP30 expression in human hepatoma cells (Hep G2); treatment of Hep G2 cells with 0.1 μM insulin for twelve hours caused increased SMP30 mRNA and protein levels (76). These studies indicate that SMP30 may be involved in the development of glucose intolerance and type 2 diabetes, however, little is known about this putative involvement.
SMP30 and Cancer
SMP30 appears to be involved in the regulation of cell proliferation and has recently been linked to breast and prostate cancers. Following treatment with carbon tetrachloride (CCl4), liver cells from SMP30 knockout mice showed increased mitosis compared to those from wild type mice. In addition, SMP30 expression was up-regulated in response to treatment with a mitogen, lead nitrate. From this data, Ishigami et al. (29) proposed that SMP30 has a suppressive effect on cell proliferation and that it is important in regulating liver regeneration following hepatic injury. Other studies have also shown that SMP30 has a suppressive effect on cell proliferation in rat liver and kidney cells (77, 78). In addition, over-expression of SMP30 alters the expression of tumor-related genes such as c-myc, Ha-ras, c-src, p53, and Rb (retinoblastoma protein). In rat liver cells with over-expressing SMP30, the expression of the oncogenes c-myc, Ha-ras, and c-src was suppressed, while expression of the tumor suppressor genes p53 and Rb were up-regulated compared to wild type cells (79, 80). This suggests a protective role of SMP30 against cancer development. Furthermore, a recent study showed that SMP30 expression was decreased in human breast and prostate cancer tissues and cell lines and that lower SMP30 levels were associated with further progressed tumors (30). Whether decreased SMP30 levels are a cause or effect of cancer progression remains unclear.
Outlook
On a molecular level, little is known about how SMP30 affects cancer development, liver fibrosis, glucose intolerance, and bone loss. More research is required to determine the significance of the associations of SMP30 with these physiological effects. The recently solved crystal structure of SMP30 will allow putative physiological functions to be explored on the molecular level. The combined future exploration of structure, enzyme activity, metabolomics and in vivo studies will undoubtedly reveal which previously assigned SMP30 functions are physiologically relevant and significant.
Figure 5.
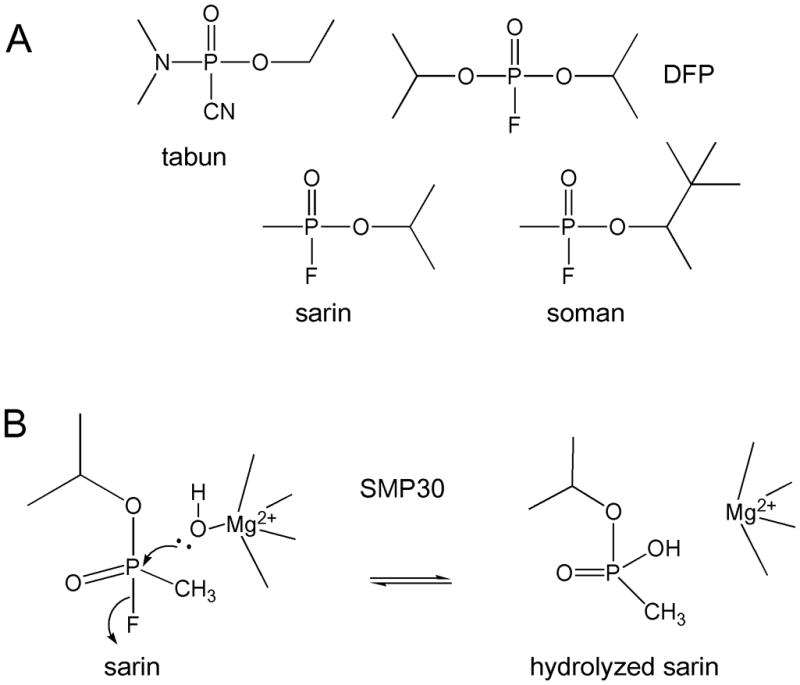
Organophosphates hydrolyzed by SMP30 (9). A) Structures of tabun, DFP, sarin, and soman. B) Hydrolysis of sarin by SMP30 and Mg2+.
Highlights.
SMP30 is highly conserved (70–90%) in vertebrates irrespective of whether the species is capable of synthesizing ascorbate. This indicates an additional important physiological function of SMP30.
SMP30, predominantly found in the liver and kidneys, has also been detected in the lungs, ovaries, testes, bone, epidermis, stomach and brain. Ascorbate biosynthesis occurs only in the liver in capable species. The metabolic and molecular role of SMP30 in other tissues is currently unknown.
Homologs have been identified in insects, bacteria and fungi; however sequence identity is less than 50%.
Cells over-expressing SMP30 have enhanced Ca2+-pumping activity. SMP30 appears to regulate Ca2+-pumping activity of plasma and microsomal membranes indirectly via interactions with calmodulin.
SMP30 protects cells from apoptosis. This protective effect is likely the result of the protein’s role in regulating Ca2+ homeostasis.
SMP30 is involved in the biosynthesis of ascorbate, a powerful antioxidant molecule. A major protective effect of SMP30 against oxidative stress is the result of ascorbate synthesis.
SMP30 is a lactonase capable of hydrolyzing various aldonolactones such as D- and L-gulono-γ-lactone, D- and L-glucono-δ-lactone, and D- and L-galactono-γ-lactone. Greatest activity was seen with D-glucono-δ-lactone in the presence of Zn2+, a six-membered lactone.
The crystal structure of human SMP30 reveals a six-bladed β-propeller. The active site is located in the central tunnel of the propeller, just above the single metal-binding site.
Unlike structural homologs, such as PON1, that bind two Ca2+ atoms, the slightly altered geometry of the SMP30 metal-binding site allows it to bind a single metal, yet with a selectivity for a wider variety of divalent cations (Ca2+, Zn2+, Mg2+ and Mn2+).
SMP30 hydrolyzes DFP and other OPs in the presence of Mg2+ and Mn2+. OPs are not naturally occurring compounds so this activity is a non-physiological, yet fortuitous function of SMP30, and this function sheds little light on the physiological function.
Over-expression of SMP30 induces bone loss and osteoporosis in rats.
SMP30 deficiency causes accumulation of lipids in the liver and is associated with NAFLD and liver fibrosis.
SMP30 expression has been associated with decreasing glucose tolerance and the development of insulin resistance, suggesting a role of SMP30 in the development of type 2 diabetes.
SMP30 suppresses cell proliferation and modulates expression of oncogenes and tumor suppressor genes.
Expression of SMP30 in human breast and prostate cancers is significantly lower than in non-cancerous breast and prostate cells.
Little is known about how SMP30 affects bone development, hepatic lipid accumulation and fibrosis, insulin resistance, and cancer development on the molecular level. Future studies are necessary to determine the significance of these associations.
Acknowledgments
The review was partly supported by Award Number R01HL084366 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
References
- 1.Fujita T, Uchida K, Maruyama N. Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim Biophys Acta. 1992;1116:122–8. doi: 10.1016/0304-4165(92)90108-7. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T, Shirasawa T, Uchida K, Maruyama N. Isolation of cDNA clone encoding rat senescence marker protein-30 (SMP30) and its tissue distribution. Biochim Biophys Acta. 1992;1132:297–305. doi: 10.1016/0167-4781(92)90164-u. [DOI] [PubMed] [Google Scholar]
- 3.Fujita T, Mandel JL, Shirasawa T, Hino O, Shirai T, Maruyama N. Isolation of cDNA clone encoding human homologue of senescence marker protein-30 (SMP30) and its location on the X chromosome. Biochim Biophys Acta. 1995;1263:249–52. doi: 10.1016/0167-4781(95)00120-6. [DOI] [PubMed] [Google Scholar]
- 4.Fujita T, Shirasawa T, Maruyama N. Isolation and characterization of genomic and cDNA clones encoding mouse senescence marker protein-30 (SMP30) Biochim Biophys Acta. 1996;1308:49–57. doi: 10.1016/0167-4781(96)00064-4. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama N, Ishigami A, Kuramoto M, Handa S, Kubo S, Imasawa T, et al. Senescence marker protein-30 knockout mouse as an aging model. Ann N Y Acad Sci. 2004;1019:383–7. doi: 10.1196/annals.1297.068. [DOI] [PubMed] [Google Scholar]
- 6.Laurentino SS, Correia S, Cavaco JE, Oliveira PF, Rato L, Sousa M, et al. Regucalcin is broadly expressed in male reproductive tissues and a new androgen target gene in mammalian testis. Reproduction. 2011 doi: 10.1530/REP-11-0085. in press. [DOI] [PubMed] [Google Scholar]
- 7.Maia CJ, Santos CR, Schmitt F, Socorro S. Regucalcin is expressed in rat mammary gland and prostate and down-regulated by 17beta-estradiol. Molecular and cellular biochemistry Research Support, Non-US Gov’t. 2008;311:81–6. doi: 10.1007/s11010-007-9697-x. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi M, Yamamoto T. Purification of calcium binding substance from soluble fraction of normal rat liver. Chem Pharm Bull (Tokyo) 1978;26:1915–8. doi: 10.1248/cpb.26.1915. [DOI] [PubMed] [Google Scholar]
- 9.Billecke SS, Primo-Parmo SL, Dunlop CS, Doorn JA, La Du BN, Broomfield CA. Characterization of a soluble mouse liver enzyme capable of hydrolyzing diisopropyl phosphorofluoridate. Chem Biol Interact. 1999;119–120:251–6. doi: 10.1016/s0009-2797(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 10.Bublitz C, Lehninger AL. The role of aldonolactonase in the conversion of L-gulonate to L-ascorbate. Biochimica et Biophysica Acta. 1961;47:288–97. [Google Scholar]
- 11.Shimokawa N, Yamaguchi M. Molecular cloning and sequencing of the cDNA coding for a calcium-binding protein regucalcin from rat liver. FEBS Lett. 1993;327:251–5. doi: 10.1016/0014-5793(93)80998-a. [DOI] [PubMed] [Google Scholar]
- 12.Kondo Y, Ishigami A, Kubo S, Handa S, Gomi K, Hirokawa K, et al. Senescence marker protein-30 is a unique enzyme that hydrolyzes diisopropyl phosphorofluoridate in the liver. FEBS Lett. 2004;570:57–62. doi: 10.1016/j.febslet.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborti S, Bahnson BJ. Crystal structure of human senescence marker protein 30: insights linking structural, enzymatic, and physiological functions. Biochemistry. 2010;49:3436–44. doi: 10.1021/bi9022297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita T, Inoue H, Kitamura T, Sato N, Shimosawa T, Maruyama N. Senescence marker protein-30 (SMP30) rescues cell death by enhancing plasma membrane Ca(2+)-pumping activity in Hep G2 cells. Biochem Biophys Res Commun. 1998;250:374–80. doi: 10.1006/bbrc.1998.9327. [DOI] [PubMed] [Google Scholar]
- 15.Ishigami A, Fujita T, Handa S, Shirasawa T, Koseki H, Kitamura T, et al. Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-alpha- and Fas-mediated apoptosis. Am J Pathol. 2002;161:1273–81. doi: 10.1016/s0002-9440(10)64404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi M. Role of regucalcin in maintaining cell homeostasis and function (review) Int J Mol Med. 2005;15:371–89. [PubMed] [Google Scholar]
- 17.Yamaguchi M. Role of regucalcin in calcium signaling. Life Sci. 2000;66:1769–80. doi: 10.1016/s0024-3205(99)00602-5. [DOI] [PubMed] [Google Scholar]
- 18.Lehninger AL, Ul Hassan M. Enzymatic formation of ascorbic acid in rat liver extracts. J Biol Chem. 1956;223:123–38. [PubMed] [Google Scholar]
- 19.Winkelman J, Lehninger AL. Aldono- and uronolactonases of animal tissues. J Biol Chem. 1958;233:794–9. [PubMed] [Google Scholar]
- 20.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 21.Kondo Y, Inai Y, Sato Y, Handa S, Kubo S, Shimokado K, et al. Senescence marker protein 30 functions as gluconolactonase in L-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc Natl Acad Sci U S A. 2006;103:5723–8. doi: 10.1073/pnas.0511225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi M. Regucalcin and metabolic disorders: osteoporosis and hyperlipidemia are induced in regucalcin transgenic rats. Mol Cell Biochem. 2010;341:119–33. doi: 10.1007/s11010-010-0443-4. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi M, Misawa H, Uchiyama S, Morooka Y, Tsurusaki Y. Role of endogenous regucalcin in bone metabolism: bone loss is induced in regucalcin transgenic rats. Int J Mol Med. 2002;10:377–83. [PubMed] [Google Scholar]
- 24.Hasegawa G. Decreased senescence marker protein-30 could be a factor that contributes to the worsening of glucose tolerance in normal aging. Islets. 2010;2:258–60. doi: 10.4161/isl.2.4.12157. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa G, Yamasaki M, Kadono M, Tanaka M, Asano M, Senmaru T, et al. Senescence marker protein-30/gluconolactonase deletion worsens glucose tolerance through impairment of acute insulin secretion. Endocrinology. 2010;151:529–36. doi: 10.1210/en.2009-1163. [DOI] [PubMed] [Google Scholar]
- 26.Ishigami A, Kondo Y, Nanba R, Ohsawa T, Handa S, Kubo S, et al. SMP30 deficiency in mice causes an accumulation of neutral lipids and phospholipids in the liver and shortens the life span. Biochem Biophys Res Commun. 2004;315:575–80. doi: 10.1016/j.bbrc.2004.01.091. [DOI] [PubMed] [Google Scholar]
- 27.Park H, Ishigami A, Shima T, Mizuno M, Maruyama N, Yamaguchi K, et al. Hepatic senescence marker protein-30 is involved in the progression of nonalcoholic fatty liver disease. J Gastroenterol. 2010;45:426–34. doi: 10.1007/s00535-009-0154-3. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi M, Igarashi A, Uchiyama S, Sawada N. Hyperlipidemia is induced in regucalcin transgenic rats with increasing age. Int J Mol Med. 2004;14:647–51. [PubMed] [Google Scholar]
- 29.Ishigami T, Fujita T, Simbula G, Columbano A, Kikuchi K, Ishigami A, et al. Regulatory effects of senescence marker protein 30 on the proliferation of hepatocytes. Pathol Int. 2001;51:491–7. doi: 10.1046/j.1440-1827.2001.01238.x. [DOI] [PubMed] [Google Scholar]
- 30.Maia C, Santos C, Schmitt F, Socorro S. Regucalcin is under-expressed in human breast and prostate cancers: Effect of sex steroid hormones. J Cell Biochem. 2009;107:667–76. doi: 10.1002/jcb.22158. [DOI] [PubMed] [Google Scholar]
- 31.Ishigami A, Handa S, Maruyama N, Supakar PC. Nuclear localization of senescence marker protein-30, SMP30, in cultured mouse hepatocytes and its similarity to RNA polymerase. Biosci Biotechnol Biochem. 2003;67:158–60. doi: 10.1271/bbb.67.158. [DOI] [PubMed] [Google Scholar]
- 32.Misawa H, Yamaguchi M. The gene of Ca2+-binding protein regucalcin is highly conserved in vertebrate species. Int J Mol Med. 2000;6:191–6. doi: 10.3892/ijmm.6.2.191. [DOI] [PubMed] [Google Scholar]
- 33.Shimokawa N, Isogai M, Yamaguchi M. Specific species and tissue differences for the gene expression of calcium-binding protein regucalcin. Mol Cell Biochem. 1995;143:67–71. doi: 10.1007/BF00925928. [DOI] [PubMed] [Google Scholar]
- 34.Gomi K, Hirokawa K, Kajiyama N. Molecular cloning and expression of the cDNAs encoding luciferin-regenerating enzyme from Luciola cruciata and Luciola lateralis. Gene. 2002;294:157–66. doi: 10.1016/s0378-1119(02)00764-3. [DOI] [PubMed] [Google Scholar]
- 35.Day JC, Bailey MJ. Structure and evolution of the luciferin-regenerating enzyme (LRE) gene from the firefly Photinus pyralis. Insect Mol Biol. 2003;12:365–72. doi: 10.1046/j.1365-2583.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 36.Gomi K, Kajiyama N. Oxyluciferin, a luminescence product of firefly luciferase, is enzymatically regenerated into luciferin. J Biol Chem. 2001;276:36508–13. doi: 10.1074/jbc.M105528200. [DOI] [PubMed] [Google Scholar]
- 37.Goto SG. Expression of Drosophila homologue of senescence marker protein-30 during cold acclimation. J Insect Physiol. 2000;46:1111–20. doi: 10.1016/s0022-1910(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima Y, Natori S. Identification and characterization of an anterior fat body protein in an insect. J Biochem. 2000;127:901–8. doi: 10.1093/oxfordjournals.jbchem.a022685. [DOI] [PubMed] [Google Scholar]
- 39.Gupta SD, Chaudhuri CR, Chatterjee IB. Incapability of L-ascorbic acid synthesis by insects. Arch Biochem Biophys. 1972;152:889–90. doi: 10.1016/0003-9861(72)90287-1. [DOI] [PubMed] [Google Scholar]
- 40.Kramer KJ, Seib PA. Ascorbic acid and the growth and development of insects. Adv Chem Ser. 1982:275–91. [Google Scholar]
- 41.Prosser CL, editor. Environmental and Metabolic Animal Physiology. 4. New York: Wiley-Liss; 1991. [Google Scholar]
- 42.Stone I. Natural history of ascorbic acid in evolution of mammals and primates and its significance for present-day man. Orthomol Psych. 1972;1:82–9. [Google Scholar]
- 43.Chaudhuri CR, Chatterjee IB. L-ascorbic acid synthesis in birds: phylogenetic trend. Science. 1969;164:435–6. doi: 10.1126/science.164.3878.435. [DOI] [PubMed] [Google Scholar]
- 44.Elliot O, Yess NJ, Hegsted DM. Biosynthesis of ascorbic acid in tree shrew and slow loris. Nature. 1966;212:739–40. [Google Scholar]
- 45.Inoue HFT, Kitamura T, Shimosawa T, Nagasawa R, Inoue R, Maruyama N, Nagasawa T. Senescence marker protein-30 (SMP30) enhances the calcium efflux from renal tubular epithelial cells. Clin Exp Nephrol. 1999;3:261–7. [Google Scholar]
- 46.Matsuyama S, Kitamura T, Enomoto N, Fujita T, Ishigami A, Handa S, et al. Senescence marker protein-30 regulates Akt activity and contributes to cell survival in Hep G2 cells. Biochem Biophys Res Commun. 2004;321:386–90. doi: 10.1016/j.bbrc.2004.06.161. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi H, Yamaguchi M. Stimulatory effect of regucalcin on ATP-dependent calcium transport in rat liver plasma membranes. Molecular and Cellular Biochemistry. 1997;168:149–53. doi: 10.1023/a:1006811222806. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi H, Yamaguchi M. Role of regucalcin as an activator of Ca2+-ATPase activity in rat liver microsomes. Journal of Cellular Biochemistry. 1999;74:663–9. [PubMed] [Google Scholar]
- 49.Izumi T, Yamaguchi M. Overexpression of regucalcin suppresses cell death in cloned rat hepatoma H4-II-E cells induced by tumor necrosis factor-alpha or thapsigargin. J Cell Biochem. 2004;92:296–306. doi: 10.1002/jcb.20056. [DOI] [PubMed] [Google Scholar]
- 50.Alikhani M, Alikhani Z, He H, Liu R, Popek BI, Graves DT. Lipopolysaccharides indirectly stimulate apoptosis and global induction of apoptotic genes in fibroblasts. J Biol Chem. 2003;278:52901–8. doi: 10.1074/jbc.M307638200. [DOI] [PubMed] [Google Scholar]
- 51.Izumi T, Yamaguchi M. Overexpression of regucalcin suppresses cell death and apoptosis in cloned rat hepatoma H4-II-E cells induced by lipopolysaccharide, PD 98059, dibucaine, or Bay K 8644. J Cell Biochem. 2004;93:598–608. doi: 10.1002/jcb.20214. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa T, Yamaguchi M. Overexpression of regucalcin suppresses apoptotic cell death in cloned normal rat kidney proximal tubular epithelial NRK52E cells: change in apoptosis-related gene expression. Journal of cellular biochemistry. 2005;96:1274–85. doi: 10.1002/jcb.20617. [DOI] [PubMed] [Google Scholar]
- 53.Sato T, Seyama K, Sato Y, Mori H, Souma S, Akiyoshi T, et al. Senescence marker protein-30 protects mice lungs from oxidative stress, aging, and smoking. Am J Respir Crit Care Med. 2006;174:530–7. doi: 10.1164/rccm.200511-1816OC. [DOI] [PubMed] [Google Scholar]
- 54.Son TG, Zou Y, Jung KJ, Yu BP, Ishigami A, Maruyama N, et al. SMP30 deficiency causes increased oxidative stress in brain. Mech Ageing Dev. 2006;127:451–7. doi: 10.1016/j.mad.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Son TG, Kim SJ, Kim K, Kim MS, Chung HY, Lee J. Cytoprotective roles of senescence marker protein 30 against intracellular calcium elevation and oxidative stress. Arch Pharm Res. 2008;31:872–7. doi: 10.1007/s12272-001-1240-3. [DOI] [PubMed] [Google Scholar]
- 56.Amano A, Aigaki T, Maruyama N, Ishigami A. Ascorbic acid depletion enhances expression of the sodium-dependent vitamin C transporters, SVCT1 and SVCT2, and uptake of ascorbic acid in livers of SMP30/GNL knockout mice. Arch Biochem Biophys. 2010;496:38–44. doi: 10.1016/j.abb.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Arai KY, Sato Y, Kondo Y, Kudo C, Tsuchiya H, Nomura Y, et al. Effects of vitamin C deficiency on the skin of the senescence marker protein-30 (SMP30) knockout mouse. Biochem Biophys Res Commun. 2009;385:478–83. doi: 10.1016/j.bbrc.2009.05.104. [DOI] [PubMed] [Google Scholar]
- 58.Furusawa H, Sato Y, Tanaka Y, Inai Y, Amano A, Iwama M, et al. Vitamin C is not essential for carnitine biosynthesis in vivo: verification in vitamin C-depleted senescence marker protein-30/gluconolactonase knockout mice. Biol Pharm Bull. 2008;31:1673–9. doi: 10.1248/bpb.31.1673. [DOI] [PubMed] [Google Scholar]
- 59.Koike K, Kondo Y, Sekiya M, Sato Y, Tobino K, Iwakami S, et al. Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol-Lung C. 2010;298:L784–L92. doi: 10.1152/ajplung.00256.2009. [DOI] [PubMed] [Google Scholar]
- 60.Kondo Y, Sasaki T, Sato Y, Amano A, Aizawa S, Iwama M, et al. Vitamin C depletion increases superoxide generation in brains of SMP30/GNL knockout mice. Biochem Biophys Res Commun. 2008;377:291–6. doi: 10.1016/j.bbrc.2008.09.132. [DOI] [PubMed] [Google Scholar]
- 61.Sato Y, Kajiyama S, Amano A, Kondo Y, Sasaki T, Handa S, et al. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun. 2008;375:346–50. doi: 10.1016/j.bbrc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Handa S, Maruyama N, Ishigami A. Over-expression of senescence marker protein-30 decreases reactive oxygen species in human hepatic carcinoma Hep G2 cells. Biol Pharm Bull. 2009;32:1645–8. doi: 10.1248/bpb.32.1645. [DOI] [PubMed] [Google Scholar]
- 63.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Draganov DI. Lactonases with organophosphatase activity: structural and evolutionary perspectives. Chem Biol Interact. 2010;187:370–2. doi: 10.1016/j.cbi.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 65.Blum MM, Chen JC. Structural characterization of the catalytic calcium-binding site in diisopropyl fluorophosphatase (DFPase)--comparison with related beta-propeller enzymes. Chem Biol Interact. 2010;187:373–9. doi: 10.1016/j.cbi.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 66.Morton DJ, Barrett-Connor EL, Schneider DL. Vitamin C supplement use and bone mineral density in postmenopausal women. J Bone Miner Res. 2001;16:135–40. doi: 10.1359/jbmr.2001.16.1.135. [DOI] [PubMed] [Google Scholar]
- 67.Gabbay KH, Bohren KM, Morello R, Bertin T, Liu J, Vogel P. Ascorbate synthesis pathway: dual role of ascorbate in bone homeostasis. J Biol Chem. 2010;285:19510–20. doi: 10.1074/jbc.M110.110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uchiyama S, Yamaguchi M. Bone loss in regucalcin transgenic rats: enhancement of osteoclastic cell formation from bone marrow of rats with increasing age. Int J Mol Med. 2004;14:451–5. [PubMed] [Google Scholar]
- 69.Yamaguchi M, Sawada N, Uchiyama S, Misawa H, Ma ZJ. Expression of regucalcin in rat bone marrow cells: involvement of osteoclastic bone resorption in regucalcin transgenic rats. Int J Mol Med. 2004;13:437–43. [PubMed] [Google Scholar]
- 70.Bayard M, Holt J, Boroughs E. Nonalcoholic fatty liver disease. Am Fam Physician. 2006;73:1961–8. [PubMed] [Google Scholar]
- 71.Chuang JH, Wang PW, Tai MH. An adipocentric view of liver fibrosis and cirrhosis. Chang Gung Med J. 2004;27:855–68. [PubMed] [Google Scholar]
- 72.Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141–9. doi: 10.1097/MED.0b013e3283293015. [DOI] [PubMed] [Google Scholar]
- 73.Lv S, Wang JH, Liu F, Gao Y, Fei R, Du SC, et al. Senescence marker protein 30 in acute liver failure: validation of a mass spectrometry proteomics assay. BMC Gastroenterol. 2008;8:17. doi: 10.1186/1471-230X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park JK, Ki MR, Lee HR, Hong IH, Ji AR, Ishigami A, et al. Vitamin C deficiency attenuates liver fibrosis by way of up-regulated peroxisome proliferator-activated receptor-gamma expression in senescence marker protein 30 knockout mice. Hepatology. 2010;51:1766–77. doi: 10.1002/hep.23499. [DOI] [PubMed] [Google Scholar]
- 75.Nakashima C, Yamaguchi M. Overexpression of regucalcin suppresses gene expression of insulin signaling-related proteins in cloned rat hepatoma H4-II-E cells: involvement of insulin resistance. Int J Mol Med. 2007;20:709–16. [PubMed] [Google Scholar]
- 76.Murata T, Shinya N, Yamaguchi M. Expression of calcium-binding protein regucalcin mRNA in the cloned human hepatoma cells (HepG2): stimulation by insulin. Mol Cell Biochem. 1997;175:163–8. doi: 10.1023/a:1006844815743. [DOI] [PubMed] [Google Scholar]
- 77.Nakagawa T, Sawada N, Yamaguchi M. Overexpression of regucalcin suppresses cell proliferation of cloned normal rat kidney proximal tubular epithelial NRK52E cells. Int J Mol Med. 2005;16:637–43. [PubMed] [Google Scholar]
- 78.Yamaguchi M, Daimon Y. Overexpression of regucalcin suppresses cell proliferation in cloned rat hepatoma H4-II-E cells: involvement of intracellular signaling factors and cell cycle-related genes. J Cell Biochem. 2005;95:1169–77. doi: 10.1002/jcb.20490. [DOI] [PubMed] [Google Scholar]
- 79.Tsurusaki Y, Yamaguchi M. Overexpression of regucalcin modulates tumor-related gene expression in cloned rat hepatoma H4-II-E cells. J Cell Biochem. 2003;90:619–26. doi: 10.1002/jcb.10652. [DOI] [PubMed] [Google Scholar]
- 80.Tsurusaki Y, Yamaguchi M. Role of regucalcin in liver nuclear function: binding of regucalcin to nuclear protein or DNA and modulation of tumor-related gene expression. Int J Mol Med. 2004;14:277–81. [PubMed] [Google Scholar]
- 81.Subramaniam S. The Biology Workbench--a seamless database and analysis environment for the biologist. Proteins [Editorial] 1998;32:1–2. [PubMed] [Google Scholar]