Abstract
Interleukin-2 (IL-2) is a pleiotropic cytokine that drives T-cell growth, augments NK cytolytic activity, induces the differentiation of regulatory T cells, and mediates activation-induced cell death. Along with IL-4, IL-7, IL-9, IL-15, and IL-21, IL-2 shares the common cytokine receptor γ chain, γc, which is mutated in humans with X-linked severe combined immunodeficiency. Herein, we primarily focus on the recently discovered complex roles of IL-2 in broadly modulating T cells for T helper cell differentiation. IL-2 does not specify the type of Th differentiation that occurs; instead, IL-2 modulates expression of receptors for other cytokines and transcription factors, thereby either promoting or inhibiting cytokine cascades that correlate with each Th differentiation state. In this fashion, IL-2 can prime and potentially maintain Th1 and Th2 differentiation as well as expand such populations of cells, whereas it inhibits Th17 differentiation but also can expand Th17 cells.
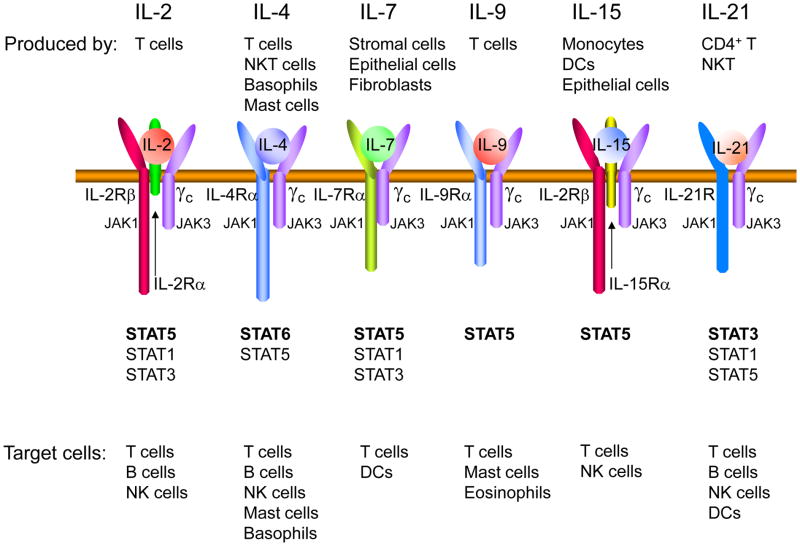
Interleukin-2 (IL-2) was discovered in 1976 as a T-cell growth factor activity in the supernatants of activated T cells [1]. IL-2 is a 15.5 kDa type 1 four α-helical bundle cytokine [2] produced primarily by CD4+ T cells following their activation by antigen. IL-2 was the first type 1 cytokine cloned and the first cytokine for which a receptor component was cloned. Three different IL-2 receptor chains exist that together generate low, intermediate, and high affinity IL-2 receptors [2]. The ligand-specific IL-2 receptor α chain (IL-2Rα, CD25, Tac antigen), which is expressed on activated but not non-activated lymphocytes, binds IL-2 with low affinity (Kd ~ 10−8 M); the combination of IL-2Rβ (CD122) and IL-2Rγ (now denoted as the common cytokine receptor γ chain,γc, or CD132) together form an IL-2Rβ/γc complex mainly on memory T cells and NK cells that binds IL-2 with intermediate affinity (Kd ~ 10−9 M); and when all three receptor chains are co-expressed on activated T cells and Treg cells, IL-2 is bound with high affinity (Kd ~ 10−11 M) [2]. For the high affinity receptor, the three dimensional structure of the quaternary complex supports a model wherein IL-2 initially bind IL-2Rα, then IL-2Rβ is recruited, and finally γc [3,4]. The intermediate and high affinity receptor forms are functional, transducing IL-2 signals. IL-2Rβ is also a key part of the IL-15 receptor, whereas γc is an essential component shared by the receptors for IL-2, IL-4, IL-7, IL-9,IL-15, and IL-21 [5](Figure 1). γc is encoded by the gene, IL2RG, that is mutated in humans with X-linked severe combined immunodeficiency (XSCID) [6**] and physically recruits JAK3, which when mutated also causes an XSCID-like T−B+NK− form of SCID [7,8]. In XSCID and JAK3-deficient SCID, the lack of signaling by IL-7 and IL-15, respectively, explains the lack of T and NK cell development [5], whereas defective signaling by IL-4 and IL-21 together explain the non-functional B cells and hypogammaglobulinemia found in this disease [9]. IL-2 itself primarily acts on lymphoid populations, including T [10], B [11], and NK [12] cells, but in addition, it can exert functional effects on other hematopoietic lineages, including, for example, neutrophils [13](reviewed in [2])(Table 1).
Figure 1.
γc-family cytokines. Shown are the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. These cytokines each activate STAT proteins through phosphorylation of JAK1 and JAK3. The principal STAT protein activated by each cytokine is in bold. DC, dendritic cell; NK cell, natural killer cell; NKT cell, natural killer T cell. STAT5 refers collectively to STAT5A and STAT5B, which are closely-related tandem head-to-head genes.
Table 1.
Main Biological functions of IL-2
| Cell type | Primary activities |
|---|---|
| CD4+T cells | Induces proliferation and survival Promotes activation-induced cell death (AICD) Required for Thl, Th2, and Treg differentiation but represses Thl7 differentiation |
| CD8+ T cells | Induces differentiation and expansion of effector cells Augments cytolytic activity Promotes generation and proliferation of memory CD8+ T cells |
| B cells | Enhances antibody secretion Promotes proliferation |
| NK cells | Promotes proliferation Augments cytokine production Enhances cytolytic activity |
| Neutrophils | Augments cytokine production |
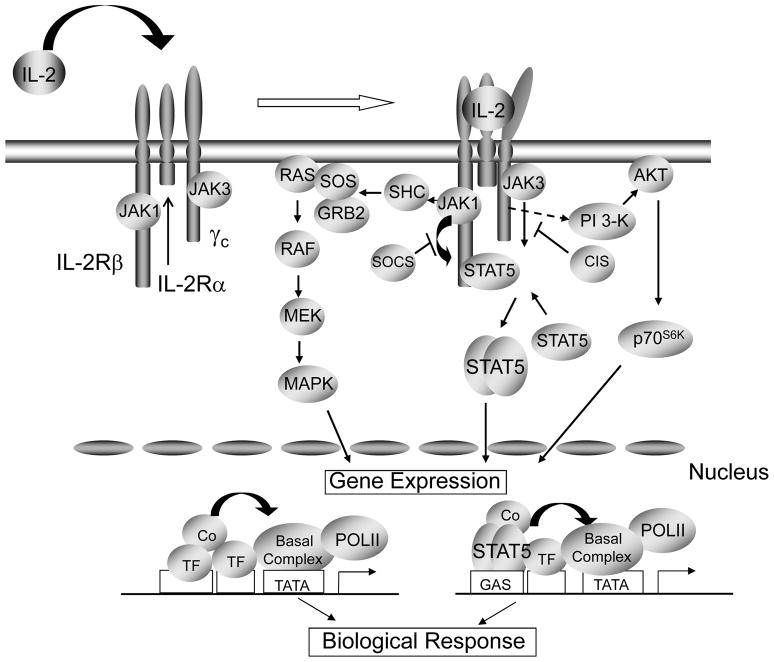
IL-2 signals via the heterodimerization of the IL-2Rβ and γc cytoplasmic domains [14*,15*], which leads to the activation of at least three major signaling pathways: phosphoinositol 3-kinase (PI 3-K)/AKT, Ras-MAP kinase, and JAK-STAT pathways, with JAK1 and JAK3, and principally STAT5A and STAT5B being the JAKs and STATs used, although STAT3 and STAT1 can also be activated by IL-2 (Figure 2). Together, these three signaling pathways mediate cell growth, survival, activation-induced cell death (AICD), and differentiation [2,16,17].
Figure 2.
IL-2 signals by activating three principal signaling pathways, the SHC/RAS/MAP kinase pathway, JAK1/JAK3/STAT5 pathway, and PI 3-K/AKT/p70 S6 kinase pathway.
During primary immune responses, naïve CD4+ T cells can differentiate into a range of effector T cells based on the actions of key cytokines and expression of critical transcription factors [18–22]. For example, IL-12 and STAT4 together with T-bet promote differentiation into Th1 cells, which produce IFN-γ and are important for host defense to intracellular pathogens such as Listeria monocytogenes and Leishmania major, viruses, and pathogenic inflammatory diseases [21]; IL-4/STAT6 and GATA3 promote differentiation into Th2 cells, which produce IL-4, IL-5, and IL-13 and participate in controlling humoral immunity to extracellular parasites and allergic inflammatory responses [18,22]; and TGF-β/IL-6 and IL-23/IL-21/STAT3 and RORγt together promote differentiation into Th17 cells, which produce IL-17A, IL-17F, and IL-22 and are involved not only in host defense to bacteria and fungal diseases, but also play key roles in autoimmune diseases, including multiple sclerosis, psoriasis, autoimmune uveitis, insulin-dependent diabetes, rheumatoid arthritis, and Crohn’s disease [19,20]. In the presence of TGF-β, IL-2 promotes the differentiation of naïve CD4+ T cells into regulatory T cells (Treg cells) to eliminate autoreactive T cells and promote self-tolerance [23], whereas IL-2 also promotes the differentiation of CD8+ T cells into effector and memory cytolytic T lymphocytes (CTL) upon antigen stimulation [24*,25*,26]. Herein, we review the critical roles of IL-2 in regulating Th differentiation, underscoring its broad contributions within effector T cell biology.
IL-2 broadly modulates cytokine receptor expression
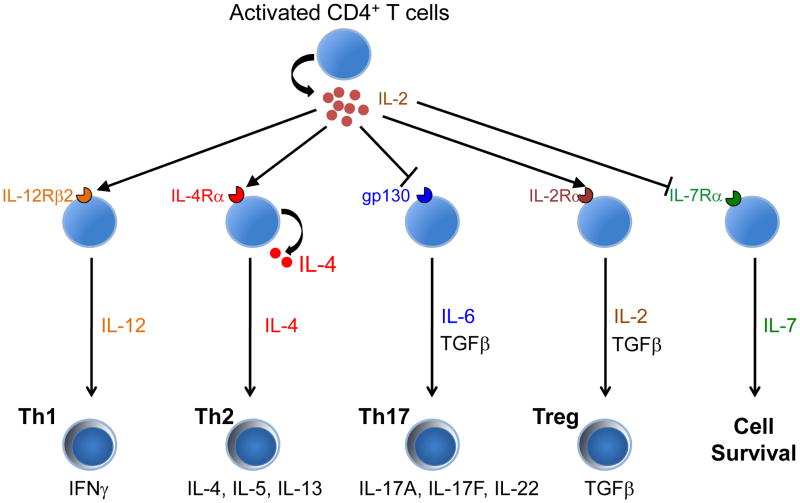
IL-2 is well known to induce expression of both IL-2Rα [27] and IL-2Rβ [28], presumably serving as a positive feedback loop, a strategy utilized by other cytokines as well, such as IL-4, IL-12, and IL-21, which also induce expression of their own receptors [29–31]. More recently, we observed that IL-2 also regulates expression of receptors for heterologous cytokine receptors, inducing IL-4Rα [32**] and IL-12Rβ2 [33**] while repressing IL-6Rα, gp130 [33**], as well as IL-7Rα [34**] (Figure 3). Because IL-7 is a known survival factor, IL-7Rα repression can serve to diminish survival signals, thereby potentially facilitating activation-induced cell death, a process that requires pre-exposure to IL-2, and/or the contraction phase that follows T-cell expansion during a viral response [34**]. The induction of IL-4Rα [32**] and IL-12Rβ2 [33**] instead can facilitate the induction by IL-2 of Th2 and Th1 differentiation, respectively, and repression of gp130 can at least in part explain the inhibition by IL-2 of Th17 differentiation [33**].
Figure 3.
Pleiotropic actions of IL-2 on CD4+ T cell differentiation via its modulation of cytokine receptor expression. IL-2 modulates effector cell differentiation at least in part via regulation of cytokine receptor expression. It promotes Th1 differentiation by inducing IL-12Rβ2 (and IL-12Rβ1), promotes Th2 differentiation by inducing IL-4Rα, inhibits Th17 differentiation by inhibiting gp130 (and IL-6Rα), and drives Treg differentiation by inducing IL-2Rα. IL-2 also potently represses IL-7Rα, which decreases survival signals that normally promote cell survival and memory cell development.
IL-2 and Th2 differentiation
As noted above, IL-4/STAT6 and GATA3 promote differentiation into Th2 cells, which produce IL-4, IL-5, and IL-13 and participate in controlling humoral immunity to extracellular parasites and allergic inflammatory responses [18,22,35]. It was recognized that the presence of IL-2 during the Th2 differentiation process is also important [36], with IL-2 opening chromatin accessibility at the Il4 locus in a STAT5A-dependent fashion [37**]. IL-4Rα is not expressed by naïve T cells and thus must be induced by TCR stimulation upon antigen encounter to allow potent Th2 differentiation. The observation that IL-2 induces IL-4Rα expression led us to hypothesize that IL-2 might play a key role in the early phase of Th2 differentiation to allow cellular responsiveness to IL-4. Although IL-4 is known to induce IL-4Rα expression, IL-2 induces IL-4Rα expression even in Il4−/− T cells and thus in an IL-4-independent fashion [32**]; moreover, the use of Il2−/− and Il4−/− T cells revealed that it is IL-2 rather than IL-4 that mediates TCR-induced IL-4Rα expression [32**]. Induction of IL-4Rα by IL-2 requires STAT5, as shown by studies using Stat5b transgenic mice and mice in which Stat5a and Stat5b were conditionally deleted, and functionally important STAT5 binding sites exist within the Il4ra gene. A genome-wide analysis of STAT5 binding sites by ChIP-Seq during Th2 differentiation revealed that STAT5A and STAT5B bind to key sites in the Il4ra gene within 8 hours of Th2 differentiation. STAT5 proteins also bind, albeit kinetically later, to the Il4-Il13-Il5 Th2 cytokine gene cluster, with occupancy at the DNase I hypersensitivity sites HSII, HSIII, and HSV, as well as at the locus control region (LCR) B and C elements within the adjacent Rad50 gene [32**]. The importance of IL-2-induced IL-4Rα expression in Th2 differentiation was underscored by the observation that retroviral transduction of Il4ra into Il2−/− T cells rescued defective Th2 differentiation in these cells [32**]. Interestingly, IL-7 and IL-15, which like IL-2 activate STAT5A and STAT5B, also can increase IL-4Rα expression, raising the possibility that other cytokines that activate STAT5 might also contribute to Th2 differentiation in vivo. The broad role of STAT5 in Th2 differentiation was further underscored by its binding to the Maf and Gata3 genes [32**]. Overall, these data indicate a key role for IL-2 and potentially other STAT5-dependent cytokines in inducing IL-4Rα expression, thus priming cells for Th2 differentiation and helping to maintain this state.
IL-2 and Th1 differentiation
As noted above, Th1 cells secrete IFNγ to promote the eradication of intracellular pathogens [21,22]. IL-12 via its activation of STAT4 has been established as the key signal for Th1 differentiation, inducing epigenetic changes at the Ifng locus and enhancing IFNγ expression [38]. IL-12 also induces expression of T-bet [39], a master regulator of Th1 differentiation that induces a transcriptionally permissive chromatin structure at the Ifng locus and enhances IL-12Rβ2 expression [40,41]. T-bet inhibits the GATA3 binding to target genes, including the Il4 gene, thus suppressing Th2 differentiation [42]. The induction of T-bet by IFNγ and the subsequent induction of IL-12Rβ2 by T-bet are believed to be key events for Th1 differentiation [43]; however, this model of Th1 differentiation requires partial revision to include the major, recently observed, contributions of IL-2, given the markedly impaired Th1 differentiation in Il2−/− T cells both in vitro and in vivo [33**]. Prior studies indicated that IL-2 could promote IFNγ production [44] and that production of IFNγ expression was cell-cycle dependent [45]. Interestingly, IL-2 also can induce expression of both IL-12Rβ1 and IL-12Rβ2 [33**], although only the latter is diminished in Il2−/− cells, as well as expression of T-bet [33**]. Expression of Il12rb2 and of Tbx21, which encodes T-bet, are STAT5-dependent, with STAT5A and STAT5B binding to key elements within these genes. Impaired Th1 differentiation in mouse Il2−/− T cells can be restored by expression of IL-12Rβ2 [33**], indicating the essential role of IL-2 in driving IL-12Rβ2 expression inTh1 differentiation. In contrast, retroviral transduction of T-bet cannot rescue Th1 differentiation in Il2−/− cells, indicating that IL-2 provides a key signal that T-bet cannot provide [33**]. In this regard, T-bet had comparatively little effect on IL-12Rβ2 expression, suggesting that T-bet could not restore normal IL-12 responsiveness to these cells [33**]. Interestingly, defects related to Th1 differentiation also were observed in Jak3−/− mice, and it was suggested that this resulted from defective IL-2-induced STAT5 binding to Ifng [46]. Thus, IL-2 may make multiple contributions to Th1 differentiation.
IL-2 and Th17 differentiation
In vitro differentiation of Th17 cells is mediated by IL-6 plus TGF-β [19,20]; anti-IFNγ and anti-IL-4 are typically added to block Th1 and Th2 differentiation. Interestingly, IL-2 signaling can diminish Th17 cell generation [47**]. Because IL-6 signals via STAT3 and IL-2 via STAT5, it was proposed that IL-2-induced STAT5 competed for STAT3 binding sites in the Il17a gene locus, inhibiting Il17a transcription [48], although direct inhibition of Il17a transcription by STAT5 was not shown. Two alternative/additional explanations are possible. First, consistent with the ability of IL-6, which signals via IL-6Rα + gp130, to drive Th17 differentiation, IL-2 inhibits expression of both IL-6Rα and gp130, and conversely, the expression of these receptor components and of IL-17A are increased in Il2−/− T cells [33**]. Whereas retroviral transduction of Il6ra did not affect IL-17A production, retroviral transduction of Il6st, which encodes gp130, increased Th17 expression and partially overcame IL-2-induced inhibition of IL-17A [33**], indicating that expression of gp130 was limiting. Nevertheless, IL-2 could still partially inhibit IL-17A expression even when gp130 was constitutively expressed, suggesting the inhibitory actions of IL-2 involved a receptor-independent mechanism of action as well [33**]. In this regard, as noted above, IL-2 induces Tbx21, and retroviral transduction of Tbx21 inhibited Th17 differentiation [33**], consistent with the ability of T-bet to inhibit Runx1-mediated RORγt-dependent transcription [49**]. Interestingly, retroviral transduction of Tbx21 of Th17 cells also augmented IFNγ production, including an increase in IL-17A/IFNγ double producing cells, even though it did not increase IFNγ under Th1 conditions [33**]. In addition to its inhibition of Th17 differentiation, IL-2 can also expand IL-17-producing cells once generated [50*], indicating complex roles of IL-2 in the regulation of IL-17.
IL-2 and Treg differentiation
Treg cells suppress activation of the immune system and maintain immune homeostasis and tolerance to self-antigens. They include natural Treg (nTreg) and induced Treg (iTreg) cells, which are induced from naïve T cells by TCR stimulation in the presence of TGF-β plus IL-2 [51]. Both types of Treg cells express the Foxp3 transcription factorand IL-2Rα (CD25) [52], and IL-2 is required for their normal development [51]. Consistent with this, Il2, Il2ra, or Il2rb deficient mice exhibit severe autoimmunity [53–56], and defective IL-2 production contributes to diminished tolerance and the development of autoimmune diabetes in the NOD mouse [57]. Nevertheless, the way in which IL-2 affects Treg function remains incompletely understood [58].
IL-2 and effector/memory cytolytic T cell differentiation
In addition to its actions on CD4+ cells, IL-2 also promotes the development of naïve CD8+ T cells into effector or memory cytolytic T lymphocytes (CTL) depending on the IL-2/IL-2R signal strength [24*,25*], and IL-2 is crucial for the secondary expansion of memory CD8+ T cells [26]. During viral infection, CD25low cells, which are less sensitive to IL-2, upregulate CD127 and CD62L and give rise to long-lived memory cells, whereas CD25hi cells proliferate more strongly to IL-2, are prone to apoptosis, exhibit a more pronounced effector phenotype, and appear to be terminally differentiated [24*]. Moreover, increasing IL-2/IL-2R signal strength promotes effector CTL differentiation by inducing eomesodermin and perforin expression while inhibiting expression of Bcl6 and IL-7Rα [25*].
Conclusions
IL-2 is a pleiotropic cytokine first identified as a T-cell growth factor that was subsequently shown to have a broad range of other actions as well. IL-2 is now recognized as also important for activation-induced cell death, development of Treg cells, and development of cytotoxic T lymphocytes, as well as for secondary expansion of memory CD8+ T cells, and as detailed herein, for modulating T helper cell differentiation. It is striking that in addition to upregulating IL-2Rα and IL-2Rβ, IL-2 increases IL-4Rα[32**]and IL-12Rβ2 [33**] but decreases gp130 [33**] expression, thus modulating signals by IL-2, IL-4, IL-12, and IL-6, and thereby affecting Th1, Th2, Treg, and Th17 differentiation, underscoring the ability of IL-2 to modulate expression of key cytokine receptors to control responsiveness to a range of cytokines after antigen encounter. Indeed, IL-2 is not a driving force for any type of T helper cell, but instead helps to augment or attenuate the signaling pathway essential for differentiation into various types of T helper cells. Thus, depending on the cytokine milieu after antigen stimulation, IL-2 can function as a master regulator to help broadly influence cell fate decisions, both priming for differentiation and helping to maintain a differentiated state (Figure 3). Beyond its regulation of receptors, IL-2 also critically regulates expression of key transcription factors, such as T-bet. By inducing T-bet, IL-2 not only can promote Th1 differentiation, but it also inhibits Th17 differentiation, given the potent role of Tbx21 as a negative regulator of Runx1-dependent RORγt transcription. In addition to its regulating these Th effector populations, as noted above, IL-2 is vital for development of Treg cells as well, which express IL-2Rα and accordingly can respond to low concentrations of IL-2. Our expanding knowledge of the broad and complex functions of IL-2 family cytokines not only have revealed intricacies of immunoregulation by this cytokine but should additionally provide the molecular basis for designing better immune therapies in the future.
Acknowledgments
This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health. We thank Dr. Rosanne Spolski, NHLBI, for critical comments.
References
- 1.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 2.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc NatlAcad Sci U S A. 2006;103:2788–2793. doi: 10.1073/pnas.0511161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310:1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 5.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 6**.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. Demonstrated that the IL-2 receptor γchain is mutated in humans with X-linked SCID and speculated that the γchain would be shared by multiple cytokine receptors, establishing the XSCID/common γchain connection. [DOI] [PubMed] [Google Scholar]
- 7.Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, Ugazio AG, Johnston JA, Candotti F, O’Shea JJ, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 8.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, Migone TS, Noguchi M, Markert ML, Buckley RH, et al. Mutation of Jak3 in a patient with SCID. essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 10.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackman MA, Tigges MA, Minie ME, Koshland ME. A model system for peptide hormone action in differentiation. interleukin 2 induces a B lymphoma to transcribe the J chain gene. Cell. 1986;47:609–617. doi: 10.1016/0092-8674(86)90625-2. [DOI] [PubMed] [Google Scholar]
- 12.Henney CS, Kuribayashi K, Kern DE, Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981;291:335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- 13.Wei S, Blanchard DK, Liu JH, Leonard WJ, Djeu JY. Activation of tumor necrosis factor-alpha production from human neutrophils by IL-2 via IL-2-R beta. J Immunol. 1993;150:1979–1987. [PubMed] [Google Scholar]
- 14*.Nakamura Y, Russell SM, Mess SA, Friedmann M, Erdos M, Francois C, Jacques Y, Adelstein S, Leonard WJ. Heterodimerization of the IL-2 receptor beta-and gamma-chain cytoplasmic domains is required for signalling. Nature. 1994;369:330–333. doi: 10.1038/369330a0. Demonstrated that heterodimerization of the IL-2R βand γchains is required for signaling. [DOI] [PubMed] [Google Scholar]
- 15*.Nelson BH, Lord JD, Greenberg PD. Cytoplasmic domains of the interleukin-2 receptor beta and gamma chains mediate the signal for T-cell proliferation. Nature. 1994;369:333–336. doi: 10.1038/369333a0. Demonstrated that heterodimerization of the IL-2R βand γchains is required for signaling. [DOI] [PubMed] [Google Scholar]
- 16.Friedmann MC, Migone TS, Russell SM, Leonard WJ. Different interleukin 2 receptor beta-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc Natl Acad Sci U S A. 1996;93:2077–2082. doi: 10.1073/pnas.93.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 18.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 19.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 20.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. Demonstrated the importance of sustained IL-2Rαexpression on terminal-effector differentiation of T cells following viral infection. [DOI] [PubMed] [Google Scholar]
- 25*.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. Demonstrated a complex relationship between IL-2 and inflammation during CTL differentiation, with the induction of Eommesodermin and perforin by IL-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Depper JM, Leonard WJ, Drogula C, Kronke M, Waldmann TA, Greene WC. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985;82:4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel JP, Sharon M, Smith PL, Leonard WJ. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987;238:75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- 29.Renz H, Domenico J, Gelfand EW. IL-4-dependent up-regulation of IL-4 receptor expression in murine T and B cells. J Immunol. 1991;146:3049–3055. [PubMed] [Google Scholar]
- 30.Chang JT, Shevach EM, Segal BM. Regulation of interleukin (IL)-12 receptor beta2 subunit expression by endogenous IL-12: a critical step in the differentiation of pathogenic autoreactive T cells. J Exp Med. 1999;189:969–978. doi: 10.1084/jem.189.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, Zhao K, Leonard WJ. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9:1288–1296. doi: 10.1038/ni.1656. Demonstrated the critical role for IL-2 in IL-4Rαinduction as a priming and maintenance step for Th2 differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12:551–559. doi: 10.1038/ni.2030. Demonstrated that IL-2 broadly regulates Th differentiation by regulating cytokine receptors, including inducing Th1 differentiation via its induction of IL-12Rβand repression of Th17 differentiation, at least in part via its repression of gp130 but also via its induction of the transcription factor T-bet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Xue HH, Kovanen PE, Pise-Masison CA, Berg M, Radovich MF, Brady JN, Leonard WJ. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:13759–13764. doi: 10.1073/pnas.212214999. The first paper to demonstrate down-regulation of IL-7Rαby IL-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunol Rev. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 36.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. Demonstrated that STAT5 plays a critical role in Th2 differentiation, explaining earlier studies that indicated a key role for IL-2 in this process. [DOI] [PubMed] [Google Scholar]
- 38.Chang S, Aune TM. Histone hyperacetylated domains across the Ifng gene region in natural killer cells and T cells. Proc Natl Acad Sci U S A. 2005;102:17095–17100. doi: 10.1073/pnas.0502129102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ylikoski E, Lund R, Kylaniemi M, Filen S, Kilpelainen M, Savolainen J, Lahesmaa R. IL-12 up-regulates T-bet independently of IFN-gamma in human CD4+ T cells. Eur J Immunol. 2005;35:3297–3306. doi: 10.1002/eji.200526101. [DOI] [PubMed] [Google Scholar]
- 40.Hatton RD, Harrington LE, Luther RJ, Wakefield T, Janowski KM, Oliver JR, Lallone RL, Murphy KM, Weaver CT. A distal conserved sequence element controls Ifng gene expression by T cells and NK cells. Immunity. 2006;25:717–729. doi: 10.1016/j.immuni.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 42.Hwang ES, Hong JH, Glimcher LH. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med. 2005;202:1289–1300. doi: 10.1084/jem.20051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reem GH, Yeh NH. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984;225:429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- 45.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 46.Shi M, Lin TH, Appell KC, Berg LJ. Janus-kinase-3-dependent signals induce chromatin remodeling at the Ifng locus during T helper 1 cell differentiation. Immunity. 2008;28:763–773. doi: 10.1016/j.immuni.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. Demonstrated that IL-2 can inhibit Th17 differentiation. [DOI] [PubMed] [Google Scholar]
- 48.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2010;12:96–104. doi: 10.1038/ni.1969. Demonstrated that T-bet inhibits Th17 differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. Demonstrated that IL-2 expands already differentiated Th17 cells. [DOI] [PubMed] [Google Scholar]
- 51.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 52.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 53.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 54.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 56.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 57.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nature Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]