Abstract
This study revealed that in awake chronic spinal cord-injured (SCI) cats reflexes from perigenital skin area to the bladder can be either inhibitory or excitatory. Electrical perigenital stimulation at frequencies between 5 and 7 Hz significantly inhibited large-amplitude rhythmic reflex bladder activity, whereas frequencies between 20 and 40 Hz induced large-amplitude bladder contractions even at low bladder volumes when reflex bladder activity was absent. Both inhibitory and excitatory effects were enhanced as the stimulation intensity increased (5–30 V, 0.2-ms pulse width). During cystometrograms, the inhibitory stimulation (7 Hz) significantly increased the micturition volume threshold 35 ± 13% above the control volume, while the excitatory stimulation (30 Hz) significantly reduced the threshold 21 ± 3%. Mechanical perigenital stimulation applied by repeated light stroking of the perigenital skin with a cotton swab only induced an excitatory effect on the bladder. Both electrical and mechanical perigenital stimuli induced large-amplitude (>30 cmH2O) bladder contractions that were relatively consistent over a range of bladder volumes (10–90% of the capacity). However, the excitatory electrical stimulation only induced bladder contractions lasting on average 42.2 ± 3.9 s, but the mechanical stimulation induced bladder contractions that lasted as long as the stimulation continued (2–3 min). Excitatory electrical or mechanical perigenital stimulation also induced poststimulus voiding. The ability to either inhibit or excite the bladder by noninvasive methods could significantly transform the current clinical management of bladder function after SCI.
Keywords: urinary bladder, electrical stimulation, spinal cord injury
After spinal cord injury (SCI) incontinence occurs frequently due to detrusor overactivity. Meanwhile, the bladder also does not empty well due to detrusor sphincter dyssynergia resulting in a large residual volume of urine. Thus, the management of bladder function after SCI is a challenging task, because it requires inhibition of detrusor overactivity during urine storage and induction of a large-amplitude bladder contraction to empty the bladder (3). Current treatment for bladder dysfunction after SCI has either limited success (49) or requires major invasive spinal surgery to implant stimulating electrodes on spinal roots (6). Intermittent urethral catheterization is the most common method for managing urinary tract dysfunction (10). However, it can lead to frequent bladder infections (25).
In this study in chronic paraplegic cats, we evaluated an alternative method to regulate bladder function involving electrical stimulation of somatic afferent nerves innervating the perigenital region. Perigenital-to-bladder reflexes undergo marked changes during postnatal development and after SCI. In neonatal kittens before the spinobulbospinal micturition reflex is fully developed, the bladder is emptied by the mother cat periodically licking the skin in the perigenital area of the kitten to induce a bladder contraction and voiding (15, 17). This excitatory perigenital-to-bladder reflex is gradually suppressed during development and becomes inhibitory in adult cats as the pontine micturition center takes control of the micturition reflex (2, 12). However, after chronic SCI in adult cats an excitatory perigenital-to-bladder spinal reflex reemerges (16, 40). Whether this reemerged excitatory reflex can trigger a micturition reflex that can completely empty the bladder as in the neonates is unknown. Thus, a further investigation in adult chronic SCI cats to understand the properties of the reemerged excitatory perigenital-to-bladder spinal reflex after SCI is warranted.
The perigenital skin area is innervated by the pudendal nerve (31). Electrical stimulation of the pudendal nerve at low frequencies (1–10 Hz) (41, 47) can inhibit the bladder in adult chronic SCI cats, but at high frequencies (20–30 Hz) can excite the bladder (41). However, the pudendal nerve innervates many areas in the pelvic region including the urethra, anal canal, anal and urethral sphincters, and skin (31). Whether electrical stimulation applied to the perigential skin area can mimic the effect of pudendal nerve stimulation and induce both inhibitory and excitatory effects on the bladder in the chronic SCI cats is uncertain, since the mechanical perigenital stimulation is primarily excitatory after SCI (16, 40).
In humans after chronic SCI, bladder inhibition can also be elicited by electrical stimulation of the dorsal penile/clitoral nerve (a branch of pudendal nerve) using skin electrodes (1, 24, 28, 36, 37, 45, 48), indicating an inhibitory perigenital-to-bladder spinal reflex might also exist in adult chronic SCI cats. An effective, noninvasive method that is able to either inhibit or induce bladder activity would improve the current clinical management of bladder function after SCI. The possibility of using electrical stimulation of the perigential skin area to activate the inhibitory perigenital-to-bladder reflex at one frequency but to activate the reemerged excitatory perigenital-to-bladder reflex at another frequency was investigated in this study in adult chronic SCI cats.
To eliminate possible effects of anesthesia on the perigenital-to-bladder reflexes that may influence voiding efficiency, experiments were performed under awake conditions so that the results would be directly comparable to the voiding in neonates. This condition would also produce results more relevant to the clinical situation.
METHODS
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Spinal cord transection and animal care
Four female adult cats (2.8–3.4 kg) were spinalized under isoflurane anesthesia (2–3% in O2) using aseptic surgical techniques. After a dorsal laminectomy was performed at T9-T10 vertebral level, a local anesthetic (lidocaine 1%) was applied to the surface of the spinal cord and then injected into the cord through the dura. The spinal cord was then cut completely and a piece of gel foam was placed between the cut ends (usually a separation of 2–3 mm). The muscle and skin were sutured and after full recovery from anesthesia, the animal was returned to its cage. Following spinal transection, the bladder was emptied daily by manual expression. If manual expression was not successful, a sterile catheter (3.5 F) was inserted through the urethra to empty the bladder. Ketaprofen (2 mg/kg sc, twice a day for 3 days) and antibiotics (Clavamox, 15–20 mg/kg sc for 7 days) were given following surgery. Experiments were conducted beginning at least 4–5 wk following spinal cord transection. The cats were used for multiple experiments at a maximal frequency of twice per week. After each experiment, the animal was given 150 mg/kg of ampicillin subcutaneously. Bladder infection rarely occurred.
Experimental setup
A sterile double lumen balloon catheter (7 F) was inserted through the urethra into the bladder of the chronic SCI cats without anesthesia. The balloon was distended by 2 ml of air and then positioned at the bladder neck by gently pulling the catheter back. The balloon prevented leakage of the fluid from the bladder. One lumen of the catheter was connected to a pump to infuse the bladder with sterile saline at a rate of 2 ml/min, and the other lumen was connected to a pressure transducer to measure the pressure change in the bladder. As shown in Fig. 1, a pair of sterilized hook electrodes (made from 23-gauge needles) was attached to the skin (~1-mm penetration into the skin with 2–4 mm contact) on the left and right sides of the vagina ~1–1.5 cm from the vaginal opening. A piece of medical tape was applied between the electrode tip and the exposed length of the electrode to fix the electrode in place. The electrodes were soldered to a pair of wires that were connected to a stimulator to deliver electrical stimulation. Due to the complete spinal transection, the animals did not sense either bladder catheterization or electrical stimulation. During the experiment (usually 4–5 h), the animals rested comfortably in a padded animal transport carrier. Since the animal was free to move in the carrier, bladder pressure recordings that were disrupted by the animal’s movements were discarded. At the end of the experiment, the catheter was withdrawn and the electrodes were detached.
Fig. 1.
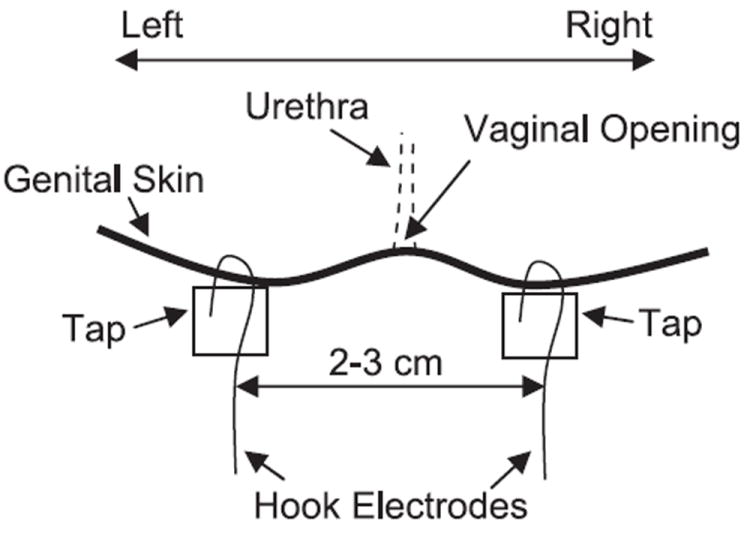
Schematic drawing of the electrode attachment.
Some experiments were conducted to evaluate voiding induced by electrical or mechanical perigenital stimulation. In these experiments, the bladder was not catheterized. The voided volume was collected using a funnel. The animal was lying quietly on a table during the voiding tests.
Stimulation protocol
Uniphasic pulses (0.2-ms pulse width) of different intensities (1–30 V) and frequencies (0.5–50 Hz) were delivered to the perigenital skin area via the attached electrodes using a stimulator (Grass Medical Instruments, S88) with a stimulus isolator (Grass Medical Instruments, SIU5).
In the first group of experiments, the bladder was infused with sterile saline to one of two different volumes: 1) a volume slightly above the micturition threshold to induce large-amplitude (>30 cmH2O), rhythmic reflex bladder contractions (see Figs. 2A and 3A) or 2) a volume slightly below the micturition threshold so that large-amplitude, reflex bladder contractions did not occur (see Fig. 4A). During the large-amplitude rhythmic bladder contractions, electrical perigenital stimulation was applied to determine the effective stimulation parameters to inhibit the bladder. The stimulation duration was longer than the period of at least two bladder contractions to clearly demonstrate an inhibitory effect. The effective stimulation parameters to induce bladder contractions were determined when bladder volume was below micturition threshold. A stimulation duration of 20–50 s was used which was longer than the period of the induced bladder contractions. Different stimulation parameters were tested in a random order but are shown in ascending intensity and/or frequency for clarity.
Fig. 2.
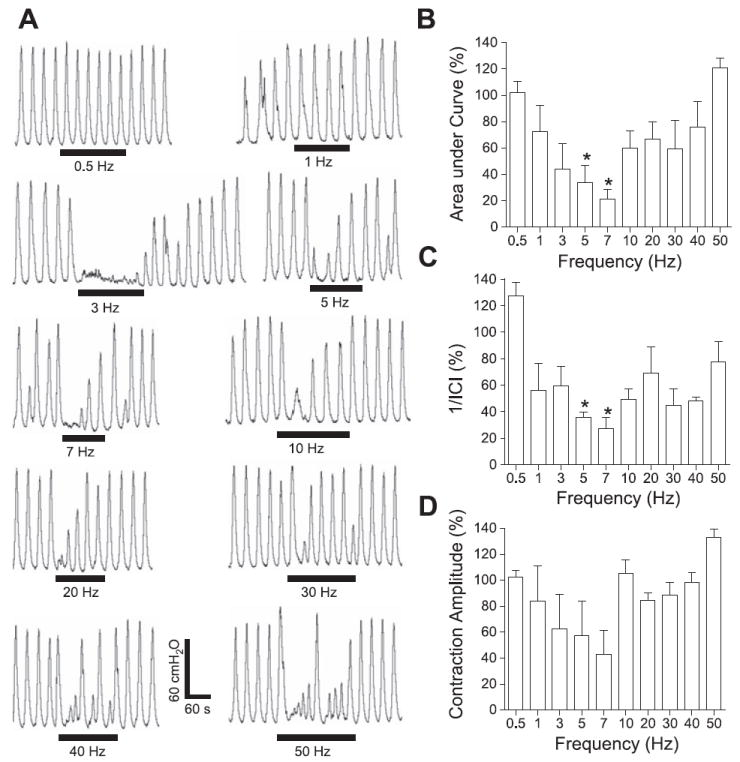
Frequency-dependent inhibitory effect on rhythmic bladder activity induced by electrical perigenital stimulation. A: effect on bladder pressure traces at different stimulation frequencies. The black bars under bladder pressure traces mark the stimulation duration. B: area under bladder pressure curve. C: reciprocal of intercontraction interval (1/ICI). D: average bladder contraction amplitude. Bladder responses during stimulation were normalized to the response before stimulation in B–D. Stimulation: 30 V in A, 5–30 V in B–D; 0.2-ms pulse width. The calibration bars in A apply to all bladder pressure recordings. *Statistical significance (P < 0.05); n = 3.
Fig. 3.
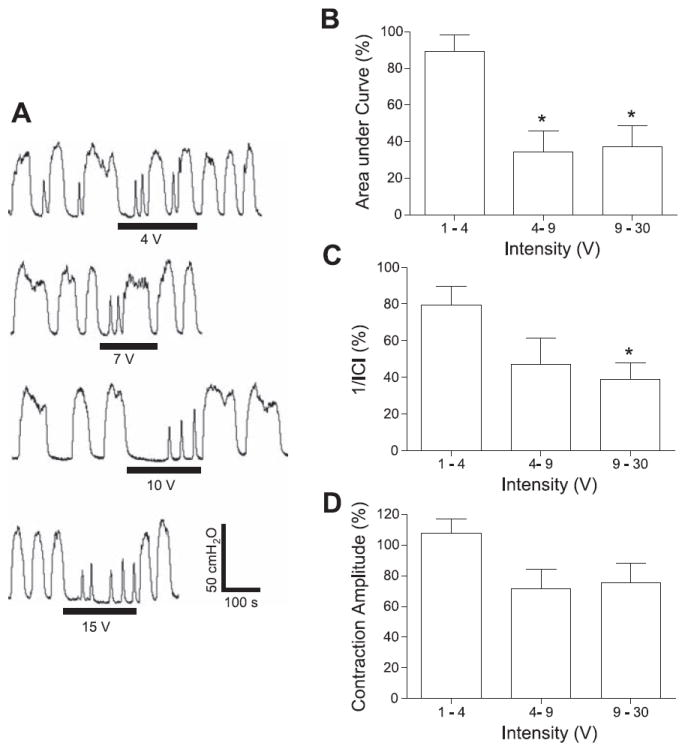
Intensity-dependent inhibitory effect on rhythmic bladder activity induced by electrical perigenital stimulation. A: effect on bladder pressure traces at different intensities. The black bars under bladder pressure traces mark the stimulation duration. B: area under bladder pressure curve. C: reciprocal of ICI (1/ICI). D: average bladder contraction amplitude. Bladder response during stimulation was normalized to the response before stimulation in B–D. Stimulation: 7 Hz; 0.2-ms pulse width. The calibration bars in A apply to all bladder pressure recordings. *Statistical significance (P < 0.05); n = 3.
Fig. 4.
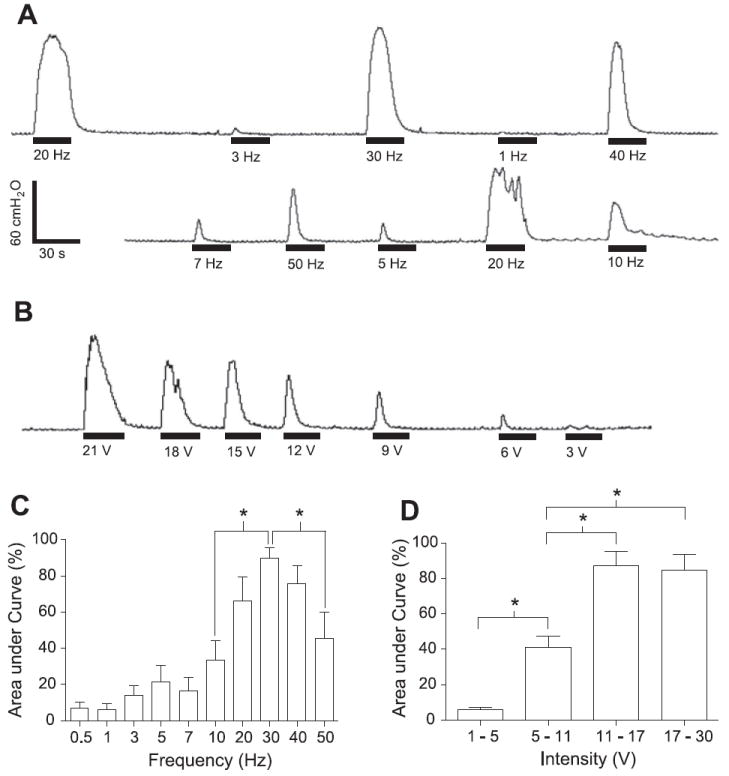
Bladder contractions induced by electrical perigenital stimulation at different frequencies (A) or at different intensities (B). The area under bladder pressure curve is dependent on both stimulation frequency (C) and intensity (D). Stimulation: 15 V in A, 15–30 V in C; 30 Hz in B and D; 0.2-ms pulse width. The black bars under bladder pressure traces mark the stimulation duration. The response was normalized to the maximal response during each trial in C–D. The calibration bars in A apply to bladder pressure recordings in both A and B. *Statistical significance (P < 0.05); n = 4.
In the second group of experiments, the most effective stimulation frequencies identified during isovolumetric recordings (7 Hz for inhibition, but 30 Hz for excitation) were tested during a cystometrogram (CMG; see Fig. 5A) which consisted of a slow infusion of saline (2 ml/min) starting with an empty bladder to determine functional bladder capacity and examine bladder reflex activity during filling. Two or three control CMGs were performed without stimulation to obtain the control values and evaluate the reproducibility. Then, either inhibitory or excitatory perigenital stimulation was applied during the CMG to evaluate the inhibitory or excitatory effects by measuring the change in bladder capacity. Stimulation and infusion were stopped after occurrence of the first micturition reflex contraction, which was defined as the first large-amplitude (>30 cmH2O), long-duration (>20 s) reflex bladder contraction accompanied by hindlimb stepping movements. Previous studies (40, 43) showed that hindlimb stepping movement was a useful marker for the occurrence of a micturition reflex in awake chronic SCI cats. Bladder capacity is defined as the volume threshold for inducing a micturition reflex during a CMG. A control CMG was performed at the end of the test to confirm the recovery of the micturition reflex. The bladder was emptied after each CMG and a 5- to 10-min rest period was inserted between CMGs to allow the bladder reflexes to recover.
Fig. 5.
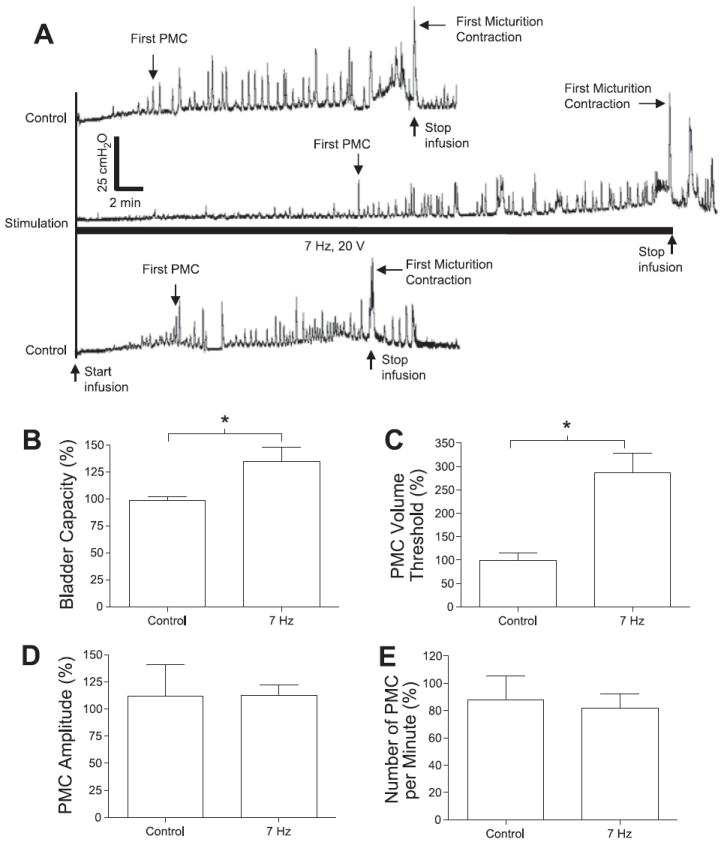
Inhibitory effect of electrical perigenital stimulation on bladder activity during cystometrograms (CMGs). A: bladder pressure traces. The initial empty bladder was infused with saline at 2 ml/min. Control: no stimulation. Stimulation: 7 Hz; 20 V; 0.2-ms pulse width. The black bar under bladder pressure trace marks the stimulation duration. B: bladder capacity was significantly increased by electrical perigenital stimulation at 7 Hz. C: volume threshold to induce the first premicturition contraction (PMC) was also significantly increased. D and E: PMC amplitude and number of PMCs per minute were not changed. Responses in B–E were normalized to the measurements during first control CMG. Stimulation in B–E: 5–30 V; 0.2-ms pulse width. The calibration bars in A apply to all bladder pressure recordings. *Statistical significance (P < 0.05); n = 4.
In the third group of experiments, the ability of the excitatory electrical perigenital stimulation (30 Hz) to induce bladder contractions at different bladder volumes was evaluated. Short periods (20–50 s) of stimulation were applied during the CMGs at intervals representing 4- to 8-ml increments in the infused volume. Similar tests were also performed using mechanical perigenital stimulation that is known to be excitatory in chronic SCI cats (40). The mechanical perigenital stimulation was performed by repeated light stroking (2–3 times/s for 20–50 s, stroke length 2–3 cm) of the perigenital skin area using a cotton swab. The bladder contractions induced by electrical or mechanical perigenital stimulation were compared.
Excitatory electrical (30 Hz) and mechanical perigenital stimuli were also used to induce voiding in the awake chronic SCI cats. Voiding induced by electrical perigenital stimulation was tested in three cats (2 times in each cat). The bladder was first infused to its capacity using a urethral catheter. Then, the catheter was withdrawn and several minutes were allowed for any spontaneous voiding to occur. If no spontaneous voiding occurred or after it was finished, a short-duration (10–20 s) electrical perigenital stimulation was repeatedly applied to induce voiding. Stimulation was continued until voiding stopped. Then, the bladder was emptied by manual expression to measure residual volume. If manual expression failed, a sterile urethral catheter (3.5 F) was used to empty the bladder. Voiding tests were also performed using repeated, short-duration (10–20 s) mechanical perigenital stimulation with the overnight residual bladder volume as the initial bladder volume.
Data analysis
For the analysis of rhythmic bladder activity, the area under the bladder pressure curve, the intercontraction interval (ICI), and the average bladder contraction amplitude were measured during the electrical stimulation and were normalized to the measurements during the same time period before the stimulation. The contraction frequency is represented as 1/ICI because ICI was an infinite value when complete bladder inhibition occurred. For the bladder contractions induced by electrical stimulation at a bladder volume below the capacity, the areas under the induced bladder pressure curves were measured and normalized to the maximal measurement during each experimental trial. Small-amplitude (5–30 cmH2O), short-duration (<20 s) premicturition contractions (PMCs) also occurred during CMGs before the large-amplitude micturition contraction in chronic SCI cats (40). The bladder capacity, the volume threshold to induce the first PMC, the average PMC amplitude, and the number of PMCs per minute were measured and normalized to the measurements during the first control CMG. The variability of control CMGs was evaluated by comparing the measurements from the repeated control CMGs to the first control CMG. The amplitudes of bladder contractions induced by electrical or mechanical perigenital stimulation during a CMG were compared at different bladder volumes that were normalized to the bladder capacity in each experiment, and then grouped into bins representing 10% increments in bladder volume. For the voiding tests, the voiding efficiency was calculated as the voided volume divided by the total volume (voided volume + residual volume). Repeated measurements in the same animal during different experiments were averaged. The normalized data from different animals are presented as means ± SE. One-sample Student’s t-test and paired Student’s t-test were used to detect statistical significance (P < 0.05) except in two instances as indicated in the text where unpaired Student’s t-test was used. Linear regression analysis (95% confidence interval) and ANOVA analysis were used to determine whether the amplitude of bladder contractions induced by perigenital stimulation increased as the bladder volume increased.
RESULTS
Reflex bladder activity in awake chronic SCI cats
In awake chronic SCI cats, infusion of saline into the bladder at a rate of 2 ml/min when the bladder neck was blocked with a balloon catheter produced an immediate small increase in baseline bladder pressure (3–8 cmH2O) and later three types of reflex bladder activity: 1) low-amplitude (5–30 cmH2O), transient (10- to 20-s duration) increases in bladder pressure (termed PMCs; see Fig. 5A) that occurred in the absence of hindlimb movements, 2) large-amplitude (30–100 cmH2O, 20- to 100-s duration) increases in bladder pressure (micturition contractions; see Fig. 5A) that were accompanied by rhythmic alternating movements of the hindlimbs resembling stepping movements (40, 43), and 3) large-amplitude rhythmic isovolumetric contractions (1–3/min, 30–100 cmH2O in amplitude, 20- to 100-s duration; see Figs. 2A and 3A) that persisted after the bladder infusion was stopped at the end of a CMG when the bladder volume was above the micturition threshold (20–120 ml) and the first micturition contraction was induced (see Figs. 5A and 6A). The rhythmic hindlimb movements were also elicited by manual compression of the bladder when attempting to express urine during daily nursing care or during voiding induced by tactile stimulation of the perigenital region. The association of somatic reflexes with voiding has also been reported in previous studies (40, 43) and is a useful marker for the occurrence of a micturition reflex. Because some experiments were performed with the urethral outlet closed which prevents elimination of the bladder contents, the simultaneous occurrence of a large-amplitude bladder contraction and hindlimb movements was used as an indicator of the first micturition reflex during the CMG (see Figs. 5A and 6A). The cystometric parameters were relatively constant in the same animal over the course of many experiments during a several week period.
Fig. 6.
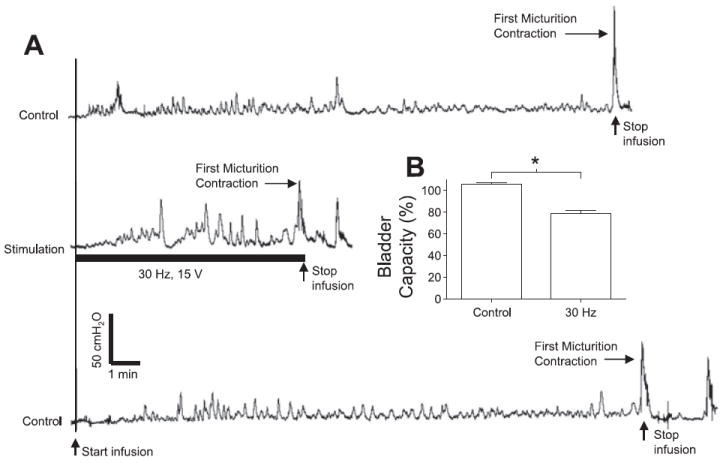
Excitatory effect of electrical perigenital stimulation on bladder activity during CMGs. A: bladder pressure traces. The initial empty bladder was infused with saline at 2 ml/min. Control: no stimulation. Stimulation: 30 Hz, 15 V, 0.2-ms pulse width. The black bar under bladder pressure trace marks the stimulation duration. B: bladder capacity was significantly reduced by electrical perigenital stimulation at 30 Hz. Bladder capacity was normalized to the capacity measured in the first control CMG. Stimulation: 10–30 V; 0.2-ms pulse width. The calibration bars in A apply to all bladder pressure recordings. *Statistical significance (P < 0.05); n = 4.
Inhibitory perigenital-to-bladder spinal reflex
The inhibitory effect of electrical perigenital stimulation on large-amplitude, rhythmic reflex bladder activity in awake chronic SCI cats was dependent on stimulation frequency (0.5–50 Hz; Fig. 2A). At stimulation frequencies of 5 and 7 Hz, the electrical perigenital stimulation significantly (P < 0.05) reduced the area under the bladder contraction curves (70–80%; Fig. 2B) and decreased the frequency (1/ICI) of the rhythmic bladder contractions (70–80%; Fig. 2C), but not the average contraction amplitude (P > 0.05; Fig. 2D) compared with the bladder activity before the stimulation.
The inhibition of large-amplitude, rhythmic reflex bladder activity at a stimulation frequency of 7 Hz was also dependent on stimulation intensity (Fig. 3A). At an intensity above 4 V, the stimulation significantly (P < 0.05) reduced the area under the bladder contraction curves (70%; Fig. 3B). It also significantly (P < 0.05) decreased the frequency of the rhythmic bladder contractions at an intensity above 9 V (60%; Fig. 3C), but the average contraction amplitude was not decreased significantly at any stimulation intensity (P > 0.05; Fig. 3D).
Excitatory perigential-to-bladder spinal reflex
Although a bladder excitatory effect induced by electrical perigenital stimulation could not be observed during the large rhythmic bladder contractions (Figs. 2-3), it became obvious when bladder volume was below micturition threshold and the large rhythmic bladder contractions were absent (Fig. 4, A and B). The excitatory perigenital-to-bladder reflex also depended on the electrical stimulation frequency and intensity. Large-amplitude (>30 cmH2O), long-duration (>20 s) bladder contractions were induced by stimulation at frequencies between 20 and 40 Hz (Fig. 4A). This excitatory effect was diminished as the stimulation intensity decreased (Fig. 4B). Electrical stimulation at 30 Hz produced bladder contractions significantly (P < 0.05) larger than those produced at frequencies of 10 or 50 Hz (Fig. 4C). The threshold intensity was ~5 V, but at least 11 V was required for a 30-Hz stimulation to induce a maximal bladder contraction (Fig. 4D).
Micturition volume threshold modulated by perigenital stimulation
The threshold bladder volume (i.e., bladder capacity) to induce a micturition reflex contraction was significantly (P < 0.05) increased 35 ± 13% above control capacity by electrical perigenital stimulation at the optimal frequency (7 Hz) for inhibiting rhythmic contractions (Fig. 5, A and B). Stimulation at this frequency also significantly (P < 0.05) increased (187 ± 42% above control value) the bladder volume necessary to induce the first PMC (Fig. 5, A and C). However, the average amplitude and the frequency of PMCs (Fig. 5, D and E) and the amplitude of the first micturition contraction (see Fig. 5A) were not significantly (P > 0.05) changed by the stimulation. Conversely, electrical perigenital stimulation at the optimal frequency (30 Hz) for inducing bladder excitation significantly (P < 0.05) reduced bladder capacity 21 ± 3% below the control capacity (Fig. 6, A and B).
Influence of bladder volume on the excitatory perigenital-to-bladder spinal reflex
The reflex bladder contractions evoked by electrical or mechanical stimulation of the perigenital region were elicited at different times during bladder filling to evaluate the influence of bladder volume on the reflex response. As shown in Fig. 7A when short periods (20 s) of electrical perigenital stimulation (15 V, 30 Hz) were repeatedly applied after 4-ml increments in bladder volume during a CMG, the stimulation induced large-amplitude (>30 cmH2O) relatively consistent bladder contractions over a range of bladder volumes. Similarly, mechanical perigenital stimulation (repeatedly stroking the perigenital skin with a cotton swab at a rate of 2–3 times/s) could also induce large bladder contractions of relatively consistent amplitude at increasing bladder volumes (Fig. 7B). The average amplitude of the bladder contractions induced by either electrical (30 Hz) or mechanical stimulation (Fig. 7, C and D) was not significantly influenced by the bladder volume (P > 0.05, ANOVA analysis and linear regression analysis, n = 3).
Fig. 7.
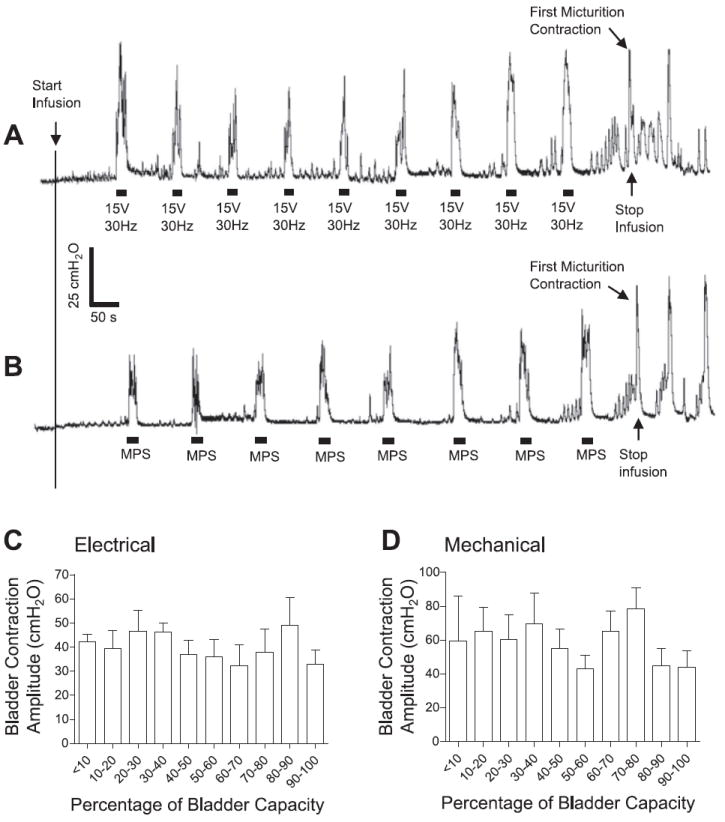
Bladder contractions induced by electrical (A and C) or mechanical (B and D) perigenital stimulation during CMGs. Electrical stimulation: 30 Hz; 15 V in A, 11–30 V in C; 0.2-ms pulse width. Mechanical perigenital stimulation (MPS): repeatedly stroking (2–3 times/s) the perigenital skin with a cotton swab. The black bars under bladder pressure traces mark the stimulation duration. The calibration bars apply to bladder pressure recordings in both A and B. Saline infusion was started with the bladder empty. Infusion rate: 2 ml/min; n = 3 for C and D.
Voiding induced by perigenital stimulation
In awake chronic SCI cats, continuous electrical (30 Hz) or mechanical perigenital stimulation released fluid from the bladder. However, the voiding was inefficient probably due to detrusor sphincter dyssynergia, and usually only a small amount of bladder volume was released at the end of stimulation (i.e., a poststimulus voiding). Therefore, short periods (10–20 s) of electrical or mechanical perigential stimulation were repeatedly applied (3- to 6-s interval). This released small amounts of fluid (1–10 ml) at the end of every period of stimulation. Usually 5–10 periods of stimulation over 1–5 min were sufficient to elicit the maximal bladder emptying.
Voiding induced by mechanical perigenital stimulation was tested using the overnight residual volume as the initial bladder volume. Total voided volume and the residual volume were measured in three cats over a 2-mo period (Fig. 8A) together with the average amplitude of micturition contractions induced by bladder distension or by mechanical perigenital stimulation (Fig. 8B). The daily residual bladder volumes detected by manual expression or by emptying using a urethral catheter were relatively large ranging from 20 to 80 ml (see Fig. 8A). Meanwhile, frequent incontinent episodes resulting in the release of small amount of urine were also observed in these animals. Similar spontaneous bladder activity was also reported in a recent study (35). Voiding efficiencies induced by mechanical perigenital stimulation in these cats were very different (cat 1: 6.7 ± 3.2%, cat 2: 43.2 ± 7.7%, cat 3: 95.0 ± 3.3%) with an average voiding efficiency of 48.3 ± 25.6% in the three animals (30–32 tests per cat). This may be due to the fact that mechanical perigenital stimulation generated the smallest bladder pressure (38.8 ± 2.5 cmH2O) in cat 1, whereas it induced the largest bladder pressure (88.1 ± 8.8 cmH2O) in cat 3 (Fig. 8B). In cat 1 the bladder pressure induced by mechanical perigenital stimulation was significantly (P < 0.05, unpaired t-test) smaller than the bladder pressure (64.7 ± 4.8 cmH2O) during the distention-induced micturition reflex (see Fig. 8B). It is also noteworthy that the efficiency of mechanical perigenital stimulation to induce voiding changed in the same animal over time (Fig. 8A).
Fig. 8.
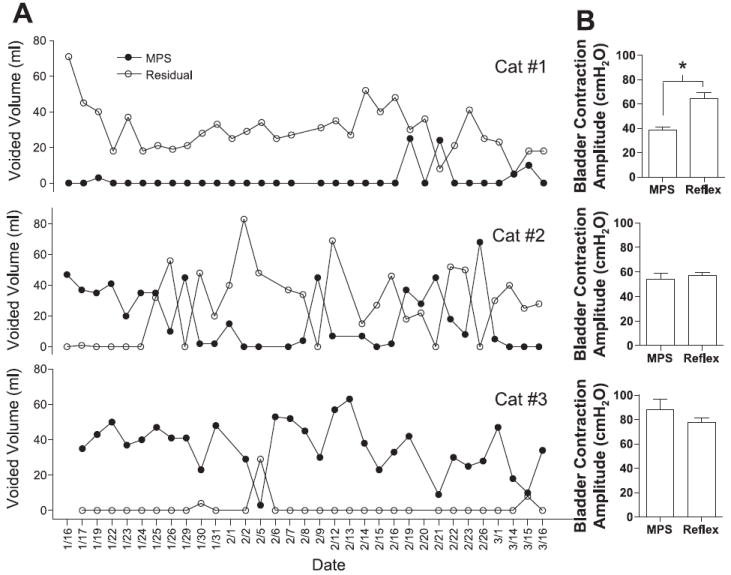
A: comparison of voided volumes induced by MPS with the residual volumes following MPS during a 2-mo period in 3 cats. B: average bladder contraction pressure induced by MPS under isovolumetric conditions or by distention-induced micturition reflex (Reflex) is also shown for each cat.
Voiding induced by electrical stimulation was performed by first infusing the bladder to the capacity. After the urethral catheter was withdrawn, spontaneous voiding only occurred in one cat (cat 2) with a voiding efficiency ranging from 60 to 70% (2 tests). No spontaneous voiding was observed in the other two cats (cats 1 and 3). Repeated, short-duration electrical perigenital stimulation (30 Hz, 30 V, 0.2 ms) was then tested to empty the bladder. The initial bladder volumes ranged from 21 to 116 ml. Voiding efficiency ranged from 30 to 100% with an average of 83.3 ± 10.1% (n = 3), which was not correlated with the initial volume or the voiding efficiency induced by mechanical perigenital stimulation in each cat. There was no significant difference (P < 0.05) between the voiding efficiencies induced by mechanical or electrical perigenital stimulation.
Comparison between electrical and mechanical perigential stimulation
Under isovolumetric conditions with bladder volume below the micturition threshold, electrical stimulation (30 Hz, 20–30 V) induced significantly larger bladder contractions (P < 0.05, unpaired t-test) compared with those induced by mechanical stimulation in cats 1 and 3, but not in cat 2 (Fig. 9). Electrical stimulation produced poststimulus voiding in all cats tested and generated isovolumetric bladder contraction pressures greater than 50 cmH2O (Fig. 9). But the mechanical stimulation generated isovolumetric bladder contraction pressures less than 50 cmH2O in cat 1 (Fig. 9) and often failed to induce poststimulus voiding in this cat (Fig. 8A).
Fig. 9.
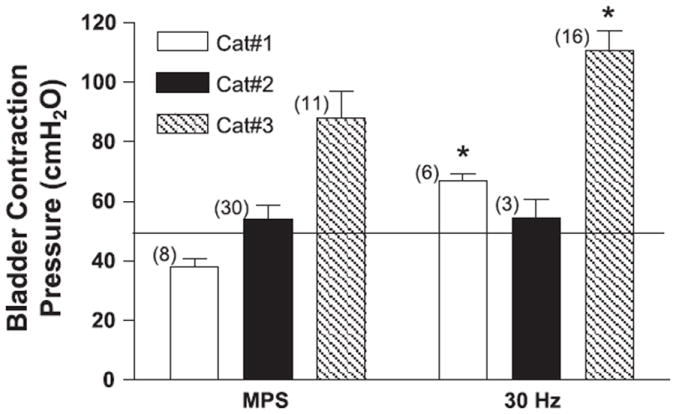
Comparison of average bladder contraction pressure induced by MPS or 30-Hz electrical perigenital stimulation (20–30 V) in 3 cats under isovolumetric conditions when bladder volume was below micturition threshold. The thin line marks the bladder pressure of 50 cmH2O. The numbers of tests are indicated in the parentheses. *Statistical significance (P < 0.05).
At the bladder volumes below micturition threshold, the 30-Hz electrical stimulation induced isovolumetric bladder contractions that lasted 42.2 ± 3.9 s on average (Fig. 10A), but the mechanical stimulation could induce isovolumetric bladder contractions that were maintained for as long as the stimulation was continued (Fig. 10B). The maximal mechanical stimulation duration tested was ~3 min. At the bladder volumes above micturition threshold when large rhythmic bladder contractions were occurring, electrical stimulation at 7 Hz inhibited bladder (Fig. 10C), but mechanical stimulation slightly facilitated the bladder contractions evidenced by a longer contraction duration compared with the bladder activity before the stimulation (Fig. 10D). However, this facilitation was not observed during the 30-Hz electrical stimulation (see Fig. 2A). Neither mechanical nor 30-Hz electrical stimuli significantly increased the amplitudes of the rhythmic bladder contractions (see Figs. 10D and 2A).
Fig. 10.
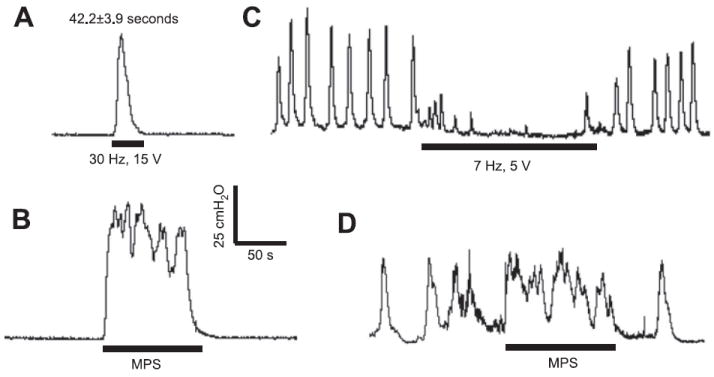
Comparison of perigenital-to-bladder reflexes induced by electrical (A and C) or mechanical (B and D) perigenital stimulation under isovolumetric conditions. A and B: bladder volume was below micturition threshold. C and D: bladder volume was above micturition threshold and large rhythmic bladder contractions were occurring. The calibration bars in B apply to all bladder pressure recordings in A–D.
DISCUSSION
This study revealed that in awake chronic SCI cats electrical stimulation of afferent nerves in the perigenital area could elicit either an inhibitory or an excitatory effect on the bladder depending on the frequency of stimulation. The inhibitory effect was maximal at a stimulation frequency of 5–7 Hz (Figs. 2 and 5), whereas the excitatory effect was maximal at 30 Hz (Figs. 4 and 6). Both the excitatory electrical perigenital stimulation (30 Hz) and the mechanical perigenital stimulation induced large bladder contractions that were not dependent on bladder volume (Fig. 7). Poststimulus voiding was also induced by the two types of excitatory perigenital stimuli (Fig. 8). However, the properties of the perigenital-to-bladder reflex induced by electrical or mechanical stimulation were significantly different (see Figs. 9-10) indicating that either different afferent pathways were activated or that the same afferent pathways were activated by these two stimuli in very different ways.
Comparison of the effects of mechanical and electrical perigenital stimulation on isovolumetric bladder activity
Mechanical stimulation only induced an excitatory effect on bladder, but electrical stimulation could induce either an excitatory or an inhibitory effect (see Fig. 10). The excitatory effect induced by mechanical stimulation lasted as long as the stimulation continued, but the electrical stimulation only induced short-duration bladder contractions (Fig. 10, A and B). These differences may be caused by the fact that afferent nerves are activated asynchronously by mechanical stimulation, but they are activated synchronously by each electrical pulse. The frequency of afferent firing can be selected by electrical stimulation, but mechanical stimulation may activate afferent nerves with a large range of firing frequencies. Electrical stimulation could activate both mechano- and nonmechano-sensitive afferent nerves from both skin and muscles, whereas mechanical stimulation mainly activates the mechano-sensitive afferent nerves from the skin.
Similar to the mechanical stimulation, the excitatory electrical stimulation also induced large-amplitude bladder contractions at both low and high bladder volumes (see Fig. 7), indicating that the excitatory perigenital-to-bladder reflexes are independent of afferent input from the bladder. Electrical stimulation at 30 Hz probably activated the reemerged, excitatory perigenital-to-bladder spinal reflex in chronic SCI cats, indicating that the average firing frequency of the afferent nerves activated by mechanical stimulation might be 30 Hz. A previous study (27) showed that light constant pressure on the clitoris of cat produced sustained afferent firing of pudendal nerve pathway with a maximal frequency of 40 Hz. In addition to directly activating the excitatory perigenital-to-bladder spinal reflex (Figs. 4 and 7A), the electrical stimulation could also facilitate the micturition reflex induced by bladder distension and reduce the micturition volume threshold during CMGs (Fig. 6). The direct excitatory effect on the bladder lasted less than a minute (see Fig. 10A), but the facilitatory effect during CMGs could persist for many minutes (see Fig. 6). Thus, it is possible that the 30-Hz electrical stimulation induces both excitatory and inhibitory effects. The excitatory effect may be dominant initially but then suppressed by an inhibitory effect that turns off the perigenital-to-bladder reflex. However, the facilitatory effect on the micturition reflex pathway induced by bladder distention seems to be insensitive to the later inhibition. This suggests that different spinal interneuronal pathways or mechanisms may be involved in the direct excitatory perigenital-to-bladder reflex and the facilitatory effect on the bladder-to-bladder reflex.
Possible mechanisms of frequency-dependent effects induced by electrical stimulation
At the same stimulation intensity, the electrical perigenital stimulation is inhibitory to the bladder at 5–7 Hz (Fig. 2), but it becomes excitatory at 20–40 Hz (Fig. 4). This raises the possibility that the same population of afferent nerves can induce different responses at different frequencies or that different populations of afferent nerves with the same electrical threshold can produce inhibition and excitation. The frequency selection of perigenital-to-bladder spinal reflexes must occur in the spinal cord. One possible explanation is that the afferent firing at different frequencies triggers the release of different neurotransmitters at the first-order synapses between the afferent neurons and the spinal interneurons resulting in either an inhibitory or an excitatory effect on the bladder activity. Another possible explanation is that the spinal interneuronal networks for bladder inhibition or excitation are optimally tuned at different frequencies. Afferent firing at 30 Hz is optimally transmitted through the excitatory spinal neural network, but 5–7 Hz is optimal for the inhibitory network (21). Frequency tuning of spinal neural networks involved in bladder function was also evident in previous studies in cats (13, 19, 20, 23, 30). For example, the maximal inhibition of the bladder mediated via the hypogastric nerve could be obtained when the pudendal afferent pathway was stimulated at 5 Hz, whereas the spinal inhibition via pelvic nerve could be optimally activated at frequencies 5–10 Hz (23, 30). Recurrent inhibition in sacral parasympathetic pathways to the bladder was optimally tuned at frequencies of 15–40 Hz (14, 20), whereas the pelvic-to-hypogastric reflex was maximally activated at the frequency of 0.5 Hz (19).
Voiding induced by perigenital stimulation in chronic SCI cats
Although both mechanical and electrical (30 Hz) stimulation induced large bladder contractions at a low bladder volume (Fig. 7), they failed to induce voiding during the stimulation in adult chronic SCI cats, probably due to a coactivation of urethral sphincter detrusor sphincter dyssynergia. However, a small amount of fluid release occurred at the end of each short period of stimulation (i.e., poststimulus voiding) presumably due to the ability of the urethral sphincter striated muscle to relax faster than the smooth muscle of the bladder after the stimulation (43). The poststimulus voiding required a high bladder pressure (greater than 50 cmH2O, see Figs. 8-9). The higher the bladder pressure induced by mechanical stimulation, the higher the voiding efficiency that was produced (see Fig. 8). However, high pressure voiding is not clinically useful since it could cause autonomic dysreflexia and kidney problems in long-term application (10, 44). In contrast, mechanical stimulation of the perigenital region is utilized by the mother cats to completely empty the bladder of neonatal kittens indicating that coactivation of the urethral sphincter is probably not induced in the neonates. Similar differences have also been noted in neonatal and SCI rats (29).
Possible afferent and efferent pathways involved in perigenital-to-bladder reflexes
Since the skin and muscles around the perigenital area are innervated by the pudendal nerve (31), the afferent limb of the perigenital-to-bladder reflex is probably in the pudendal nerve. It has been suggested that stimulation of pudendal afferents could inhibit bladder activity via activation of the hypogastric nerve when bladder volume was low, and via spinal inhibition of the parasympathetic efferent pathways to the bladder when bladder volume was high (22, 23). A previous study (41) in anesthetized chronic SCI cats also showed the involvement of both hypogastric and pelvic efferent nerves in the inhibitory pudendal-to-bladder spinal reflex. The inhibitory perigenital-to-bladder reflex demonstrated in this study in awake chronic SCI cats might involve the same efferent pathways (hypogastric and pelvic) as revealed previously.
The efferent pathway in the excitatory perigenital-to-bladder reflex must involve the pelvic nerve. However, whether the facilitatory effect of perigenital afferent input on micturition reflex involves both pelvic and hypogastric nerves is still uncertain. Perigenital stimulation could either enhance the pelvic nerve efferent activity or suppress the hypogastric nerve efferent inhibitory activity to facilitate the micturition reflex. Further studies are needed to identify the efferent pathways involved in the facilitation of micturition reflex induced by perigenital afferent activation.
Somato-visceral interactions
When the excitatory electrical (30 Hz) or mechanical perigenital stimulation (somatic) induced large-amplitude (>30 cmH2O) bladder contractions at different bladder volumes (see Fig. 7), the stimulation also triggered bilateral hindlimb stepping movements. The stepping movements also occurred during the large-amplitude (>30 cmH2O), long-duration (>20 s), distention-induced micturition reflexes, but not during the small PMCs (Figs. 5 and 6), indicating that they were closely associated with the activation of the micturition reflex and that the stepping movements were not directly activated by pudendal afferent input independent of bladder activity. This idea is further supported by the fact that the excitatory 30-Hz electrical perigenital stimulation did not induce the hindlimb movements during CMGs until a micturition reflex occurred (see Fig. 6). The hindlimb stepping movement was a useful marker to indicate the occurrence of a micturition reflex in awake chronic SCI cats (40, 43). This observation also indicates that a full bladder may be one of the mechanisms that triggers the occurrence of leg spasticity in SCI humans.
Inhibition of a C-fiber afferent-mediated spinal micturition reflex
After SCI reflex micturition is changed from an Aδ-fiber afferent-mediated spinobulbospinal reflex to a C-fiber afferent-mediated spinal reflex (8, 9, 16, 18). Previous studies (18, 33) showed that the bladder C-fiber reflex can survive an acute spinalization at a low thoracic level and can be strongly inhibited by stimulation of dorsal penile/clitoris nerve (a branch of pudendal nerve). The results in this study further indicate that the C-fiber-mediated micturition reflex in awake chronic SCI cats can be inhibited by the stimulation applied to the perigenital skin area. Naloxone (an opioid antagonist) facilitates the C-fiber micturition reflex in chronic SCI cats (43) and in acute SCI cats (18), but it does not alter the inhibition of the bladder C-fiber reflex induced by stimulation of the dorsal clitoris nerve (34), indicating that the opioid peptides might not be involved in the inhibitory pudendal-to-bladder spinal reflex, leaving GABA or glycine as potential inhibitory transmitters (11, 18).
Neuroplasticity of perigenital-to-bladder reflex after SCI
The perigenital-to-bladder reflex induced by mechanical stimulation exhibited significant plasticity after SCI (Table 1). The excitatory perigenital-to-bladder reflex, which exists in neonatal kittens (15, 17), disappears during development and is replaced by an inhibitory perigenital-to-bladder reflex in adult cats (12, 15, 17). After SCI, the mechanical stimulation-induced, excitatory perigenital-to-bladder reflex reemerges (see Figs. 7-10) and the inhibitory reflex disappears (13, 16, 20, 28). This reflex plasticity indicates a significant reorganization occurring in the spinal cord after chronic SCI.
Table 1.
Comparison of perigenital-to-bladder reflexes induced by mechanical and electrical stimulation in spinal intact and chronic SCI cats
The effect of electrical perigenital stimulation on bladder reflexes in awake spinal intact cats has not been determined, making it impossible to evaluate the plasticity of this reflex after chronic SCI. However, in anesthetized spinal intact cats intravaginal electrical stimulation at a frequency of 5–10 Hz inhibited bladder activity (22, 30). Furthermore, dorsal penile/clitoris nerve stimulation at 10 Hz also inhibited both Aδ-fiber-mediated (26) and C-fiber-mediated (33) pelvic-to-pelvic micturition reflexes in spinal intact cats, indicating that electrical stimulation of the perigenital skin area at these frequencies might also be inhibitory (see Table 1). An excitatory effect of electrical perigenital stimulation on bladder activity in spinal intact cats might also occur since pudendal nerve stimulation in a range of frequencies (1–40 Hz) induced bladder contractions (4, 5, 21, 32, 39) (see Table 1). The perigenital-to-bladder reflex induced by electrical stimulation in spinal intact cats needs to be further investigated to fully understand the neuroplasticity of this reflex after SCI.
Potential clinical application
Our current study in awake chronic SCI animals tested the hypothesis that frequency-dependent activation of the inhibitory or excitatory pudendal-to-bladder spinal reflex could be achieved by applying electrical stimulation to the perigenital skin area. A previous study (47) using restrained, awake, chronic SCI cats only investigated the inhibitory effect on the bladder by inserting fine wire electrodes percutaneously to stimulate the pudendal nerve. Bladder excitation by perineal stimulation was also investigated previously in spinal dogs (46). Our study further showed that both inhibitory and excitatory bladder responses could be induced in chronic SCI cats by electrical perigenital stimulation at different frequencies. Clinical tests using suprapubic tapping and jabbing could only induce bladder contractions in ~50% of the SCI subjects (7) and manual anorectal stimulation inhibited bladder activity (38). But these clinical tests activated very different afferent pathways than perigenital stimulation. Currently, a systematic clinical investigation of the frequency-dependent bladder responses to perigenital electrical stimulation in SCI subjects is still not available. Although a recent study in chronic SCI cats (35) showed that intraspinal micro-stimulation might be applicable to restore bladder function after SCI, a noninvasive clinical approach to either excite or inhibit the bladder activity will still be very useful and could be envisioned based on the results presented in our study.
Acknowledgments
GRANTS
This study is supported by National Institutes of Health Grants RO1-DK-068566 and RO1-DK-077783, and by Christopher and Dana Reeve Foundation.
References
- 1.Andre R, Schmid DM, Curt A, Knapp PA, Schurch B. Afferent fibers of the pudendal nerve modulate sympathetic neurons controlling the bladder neck. Neurourol Urodyn. 2003;22:597–601. doi: 10.1002/nau.10134. [DOI] [PubMed] [Google Scholar]
- 2.Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaivas JG. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982;127:958–963. doi: 10.1016/s0022-5347(17)54147-6. [DOI] [PubMed] [Google Scholar]
- 4.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 5.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol. 2006;577:115–126. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brindley GS. The first 500 sacral anterior root stimulator implants: general description. Paraplegia. 1994;32:795–805. doi: 10.1038/sc.1994.126. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas DD, Kelly E, Mayo ME. Manual stimulation of reflex voiding after spinal cord injury. Arch Phys Med Rehabil. 1985;66:459–462. [PubMed] [Google Scholar]
- 8.Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol Regul Integr Comp Physiol. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- 10.Consortium for Spinal Cord Medicine. Bladder management for adults with spinal cord injury: a clinical practice guideline for health-care providers. 2006 www.pva.org/site/DocServer/Bladder.WEB.pdf?docID=1101. [PMC free article] [PubMed]
- 11.de Groat WC. The effects of glycine, GABA and strychnine on sacral parasympathetic preganglionic neurons. Brain Res. 1970;18:542–544. doi: 10.1016/0006-8993(70)90137-x. [DOI] [PubMed] [Google Scholar]
- 12.de Groat WC. Excitation and inhibition of sacral parasympathetic neurons by visceral and cutaneous stimuli in the cat. Brain Res. 1971;33:499–503. doi: 10.1016/0006-8993(71)90125-9. [DOI] [PubMed] [Google Scholar]
- 13.de Groat WC. Nervous control of the urinary bladder of the cat. Brain Res. 1975;87:201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- 14.de Groat WC. Mechanisms underlying recurrent inhibition in the sacral parasympathetic outflow to the urinary bladder. J Physiol. 1976;257:503–513. doi: 10.1113/jphysiol.1976.sp011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Groat WC. Plasticity of bladder reflex pathways during postnatal development. Physiol Behav. 2002;77:689–692. doi: 10.1016/s0031-9384(02)00919-8. [DOI] [PubMed] [Google Scholar]
- 16.de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 17.de Groat WC, Douglas JW, Glass J, Simonds W, Weimer B, Werner P. Changes in somato-vesical reflexes during postnatal development in the kitten. Brain Res. 1975;94:150–154. doi: 10.1016/0006-8993(75)90884-7. [DOI] [PubMed] [Google Scholar]
- 18.de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990;30:S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- 19.de Groat WC, Lalley PM. Reflexes firing in the lumbar sympathetic outflow to activation of vesical afferent fibers. J Physiol. 1972;226:289–309. doi: 10.1113/jphysiol.1972.sp009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groat WC, Ryall RW. Recurrent inhibition in sacral parasympathetic pathways to the bladder. J Physiol. 1968;196:579–591. doi: 10.1113/jphysiol.1968.sp008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groat WC, Ryall RW. Reflex to sacral parasympathetic neurons concerned with micturition in the cat. J Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fall M, Erlandson BE, Carlsson CA, Lindstrom S. The effect of intravaginal electrical stimulation on the feline urethra and urinary bladder: neuronal mechanisms. Scand J Urol Nephrol. 1978;44:19–30. [PubMed] [Google Scholar]
- 23.Fall M, Lindstrom S. Electrical stimulation: a physiologic approach to the treatment of urinary incontinence. Urol Clin North Am. 1991;18:393–407. [PubMed] [Google Scholar]
- 24.Hansen J, Media S, Nohr M, Biering-Sorensen F, Sinkjaer T, Rijkhoff NJM. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol. 2005;173:2035–2039. doi: 10.1097/01.ju.0000158160.11083.1b. [DOI] [PubMed] [Google Scholar]
- 25.Jamil F. Toward a catheter free status in neurogenic bladder dysfunction: a review of bladder management options in spinal cord injury (SCI) Spinal Cord. 2001;39:355–361. doi: 10.1038/sj.sc.3101132. [DOI] [PubMed] [Google Scholar]
- 26.Jiang CH, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol. 1999;517:599–605. doi: 10.1111/j.1469-7793.1999.0599t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawatani M, Tanowitz M, de Groat WC. Morphological and electrophysiological analysis of the peripheral and central afferent pathways from the clitoris of the cat. Brain Res. 1994;646:26–36. doi: 10.1016/0006-8993(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 28.Kirkham APS, Shah NC, Knight SL, Shah PJR, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord. 2001;39:420–428. doi: 10.1038/sj.sc.3101177. [DOI] [PubMed] [Google Scholar]
- 29.Kruse MN, de Groat WC. Consequences of spinal cord injury during the neonatal period on micturition reflexes in the rat. Exp Neurol. 1994;125:87–92. doi: 10.1006/exnr.1994.1010. [DOI] [PubMed] [Google Scholar]
- 30.Lindstrom S, Fall M, Carlsson CA, Erlandson BE. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol. 1983;129:405–410. doi: 10.1016/s0022-5347(17)52127-8. [DOI] [PubMed] [Google Scholar]
- 31.Martin WD, Fletcher RF, Bradley WE. Innervation of feline perineal musculature. Anat Rec. 1974;180:15–29. doi: 10.1002/ar.1091800104. [DOI] [PubMed] [Google Scholar]
- 32.Mazieres L, Jiang C, Lindstrom S. Bladder parasympathetic response to electrical stimulation of urethral afferents in the cat. Neurourol Urodyn. 1997;16:471–472. [Google Scholar]
- 33.Mazieres L, Jiang CH, Lindstrom S. The C fibre reflex of the cat urinary bladder. J Physiol. 1998;513:531–541. doi: 10.1111/j.1469-7793.1998.531bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazieres L, Jiang CH, Lindstrom S. Recurrent inhibition of the bladder C fiber reflex in the cat and its response to naloxone. J Physiol. 2006;575:603–615. doi: 10.1113/jphysiol.2006.112995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pikov V, Bullara L, McCreery DB. Intraspinal stimulation for bladder voiding in cats before and after chronic spinal cord injury. J Neural Eng. 2007;4:356–368. doi: 10.1088/1741-2560/4/4/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Previnaire JG, Soler JM, Perrigot M. Is there a place for pudendal nerve maximal electrical stimulation for the treatment of detrusor hyperreflexia in spinal cord injury patients? Spinal Cord. 1998;36:100–103. doi: 10.1038/sj.sc.3100440. [DOI] [PubMed] [Google Scholar]
- 37.Previnaire JG, Soler JM, Perrigot M, Boileau G, Delahaye H, Schumacker P, Vanvelcenaher J, Vanhee JL. Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength. Paraplegia. 1996;34:95–99. doi: 10.1038/sc.1996.17. [DOI] [PubMed] [Google Scholar]
- 38.Rodriquez AA, Awad E. Detrusor muscle and sphincter response to anorectal stimulation in spinal cord injury. Arch Phys Med Rehabil. 1979;60:269–272. [PubMed] [Google Scholar]
- 39.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244:137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 40.Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp Neurol. 2006;199:427–437. doi: 10.1016/j.expneurol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal cord-injured cats. Exp Neurol. 2006;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26:570–577. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 43.Thor KB, Roppolo JR, de Groat WC. Naloxone induced micturition in unanesthetized paraplegic cats. J Urol. 1983;129:202–205. doi: 10.1016/s0022-5347(17)51984-9. [DOI] [PubMed] [Google Scholar]
- 44.Vaidyanathan S, Soni BM, Singh G, Oo T, Hughes PL, Mansour P. Flawed trail of micturition in cervical spinal cord injury patients: guidelines for trial of voiding in men with tetraplegia. Spinal Cord. 2003;41:667–672. doi: 10.1038/sj.sc.3101519. [DOI] [PubMed] [Google Scholar]
- 45.Vodusek DB, Light JK, Libby JM. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodyn. 1986;5:381–389. [Google Scholar]
- 46.Walter JS, Wheeler JS, Robinson CJ, Wurster RD. Surface stimulation techniques for bladder management in the spinal dog. J Urol. 1989;141:161–165. doi: 10.1016/s0022-5347(17)40632-x. [DOI] [PubMed] [Google Scholar]
- 47.Walter JS, Wheeler JS, Robinson CJ, Wurster RD. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol Urodyn. 1993;12:241–253. doi: 10.1002/nau.1930120306. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler JS, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992;147:100–103. doi: 10.1016/s0022-5347(17)37145-8. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura N, Smith CP, Chancellor MB, de Groat WC. Pharmacologic and potential biologic interventions to restore bladder function after spinal cord injury. Curr Opin Neurol. 2000;13:677–681. doi: 10.1097/00019052-200012000-00011. [DOI] [PubMed] [Google Scholar]


