Abstract
Type 2 diabetes in youth was almost unheard of only two decades ago. However, tracking the recent dramatic rise in childhood obesity, type 2 diabetes has become increasingly prevalent. Thus, there is an urgent need for high-quality clinical trials to increase in-depth knowledge about pathophysiology, optimal treatment, and prevention. We therefore systematically reviewed published and ongoing clinical trials of type 2 diabetes in children and adolescents. The results demonstrate that (i) few randomized clinical trials have been completed and published in children with type 2 diabetes; (ii) ongoing trials in type 1 diabetes clearly outnumber trials in type 2 diabetes; and (iii) recruitment and enrollment into the latter trials are challenging, however once achieved, drop-out rates are not excessively high. We conclude that type 2 diabetes in youth is an important but difficult new field of clinical research, and we discuss the existing barriers to successful recruitment, conduct, and support of these clinical trials.
Keywords: adolescent, clinical trials, pediatric, type 2 diabetes, youth
The incidence of type 2 diabetes in adolescents and young adults continues to rise at an alarming rate, and much remains to be learned about etiology and pathophysiology in order to develop effective prevention and treatment approaches. Data from the SEARCH For Diabetes in Youth study showed that in the year 2002, 3700 youths were newly diagnosed with type 2 diabetes in the USA, approximately 25% of the annual incidence of type 1 diabetes in children and adolescents (1). Rates of type 2 diabetes were as high as 49.4 per 100 000 among 15- to 19-yr-old adolescent minority populations. In all ethnic groups, females were significantly more affected than males. Although a wide array of treatment options is available for type 2 diabetes in adults, only two medications, insulin and metformin, are approved for use in children and adolescents with this condition.
Clinical trials in children with type 1 diabetes were initiated soon after the discovery of insulin in 1921 (2, 3) and have markedly improved our knowledge about the immunologic and metabolic characteristics of this condition. However, interventional and prospective long-term clinical studies in pediatric type 2 diabetes are sparse. One reason is certainly the low prevalence of this disease until about 20 yr ago. Another issue is the fact that recruitment for these clinical trials remains challenging. An example is the necessity to prolong the predicted recruitment period for the large, multicenter Treatment Options for Type 2 Diabetes in Adolescents and Youth trial (TODAY) from 3 to 4 yr in order to complete enrollment of 750 children and adolescents nationwide (4). Our own experience has been similar: a study at the National Institutes of Health (5) has recruited only 11 subjects in a 2.5-yr period. At Texas Children’s Hospital, nine subjects have been recruited in 2 yr for another study (6), despite having a total population of about 300 children and adolescents with type 2 diabetes and diagnosing approximately 75 youths with new onset type 2 diabetes per year. Motivated by these difficulties, we reviewed the literature to examine the current status of completed and ongoing clinical trials in children and adolescents with type 2 diabetes and explored existing hurdles and obstacles to successful enrollment into these clinical study protocols.
Methods
Literature review
We conducted a systematic review of the literature using PubMed, EMBASE, and Web of Science to identify relevant reports about interventional trials involving children and adolescents with type 2 diabetes. Search criteria consisted of combinations of the key words ‘diabetes’, ‘type 2’, ‘clinical trials’, ‘pediatric’, ‘adolescent’, and ‘youth’. Inclusion criteria required that (i) articles be published in peer-reviewed journals in the English language; (ii) recruited subjects included children (ages 0–18 yr) with physician-diagnosed type 2 diabetes; and (iii) interventional clinical trials had a minimum of two observations or visits.
Abstracted data included method of diagnosis, intervention, number of patients per treatment group, whether randomization had taken place, outcome measures, total number of study visits, study duration, retention rate, and authors’ comments regarding recruitment and/or retention.
Ongoing clinical trials
Registered ongoing and completed clinical trials in children with type 2 diabetes were identified through clinicaltrials.gov. Searches were conducted using the search phrases ‘type 2 diabetes and adolescent’ and ‘type 2 diabetes and pediatric’. The last search was performed on 17 November 2009. For comparison, additional searches for trials in children with type 1 diabetes were conducted using the same phrases, replacing the key words ‘type 2 diabetes’ with ‘type 1 diabetes’. Data were abstracted and categorized according to the patient enrollment criteria, study type (interventional vs. observational), status of study (e.g., completed, currently recruiting, and not yet open for recruitment), and whether results were published. Ongoing interventional trials in children with type 2 diabetes were further categorized according to the type of intervention (e.g., pharmacological vs. behavioral), the specific intervention being implemented, age range of study population, duration of study (e.g., start date and estimated end date), and number of study sites (single vs. multicenter).
Results
Literature review
One hundred and fifty journal articles were retrieved from our literature search. Only five published reports of prospective interventional trials were found [four randomized (7–10) and one non-randomized (11)] (Table 1).
Table 1.
Published interventional studies in adolescents with T2DM
| Author, yr | Intervention | N | Study population | Randomized | Outcome measures | Study visits | Duration of study | Retention (%) | Comment on recruitment and/or retention |
|---|---|---|---|---|---|---|---|---|---|
| Jones et al., 2002 (7) | 1–2 g metformin Placebo |
42 40 |
All T2DM | Yes (double blind) | Mean change in FPG, HbA1c, weight, BMI, lipid, stimulated C-peptide | 2 | 16 wk | 75.7 | 481 screened; 82 randomized; 6 (14.3%) subjects on metformin and 4 (10.0%) on placebo discontinued during the double-blind period |
| Sellers and Dean, 2004 (11) | 70/30 insulin* | 18 | All T2DM | No (open label) | Mean change in HbA1c | 6 | ≤16 wk therapy and 12 months follow-up | Not reported | No data available on completion |
| FDA, 2004† | 500 mg metformin 2.5 mg glyburide 1.25/250 mg Met/Gly |
54 49 57 |
All T2DM | Yes (double blind) | Mean change in HbA1c | 2 | 26 wk | Not reported | 167 enrolled; 160 ITT |
| Gottschalk et al. 2007 (8) | 1–8 mg glimepiride 1–2 g metformin |
132 131 |
All T2DM | Yes (single blind) | Mean change in HbA1c and fasting SMBG | 6 | 24 wk | 80 | 536 screened; 285 randomized; 263 ITT; 210 patients completed the study |
| Gellar et al., 2009 (10) | 1 high GI meal and 1 low GI meal | 11 | T2DM (5) IGT (6) |
Yes (cross-over) | Mean blood glucose and blood glucose variability | 2 | 4 d | 100 | Due to difficult recruitment, expansion of inclusion criteria to include IGT |
| Malloy et al., 2009 (9) | 2.5 μg exenatide 5.0 μg exenatide Placebo |
13 | All T2DM | Yes (cross-over) | Pharmacokinetics and safety profile; glucose, insulin, and glucagon measurements | 3 | 5 wk | 92 | 13 randomized; 12 completed |
T2DM, type 2 diabetes mellitus; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; BMI, body mass index; FDA, Food and Drug Administration; ITT, intention to treat; SMBG, self-monitored blood glucose; GI, glycemic index; IGT, impaired glucose tolerance.
Insulin was initiated at a starting dose of 0.5 U/kg/day in two doses. Insulin dose was titrated to maintain blood glucose in the target range of 4–7 mmol/L. Mean maximum insulin dose was 0.76 U/kg/day reached at 2.3 wk.
Unpublished data available from the FDA.
The first clinical trial in children and adolescents with type 2 diabetes was published by Jones et al. (7). In this randomized, double-blind, placebo-controlled, multicenter trial, metformin vs. placebo were administered for 16 wk and the effects on blood glucose control were examined. Recruitment for this study involved screening of 481 subjects in order to randomize 82 who met eligibility criteria. Of these 82 participants, only 21 (26%) completed the 16-wk double-blind treatment, although the majority of those who discontinued study drug did so due to hyperglycemia (30 subjects) or early study unblinding (20 subjects). Data from this study served as the basis for Food and Drug Administration (FDA) approval of metformin in the pediatric age range.
The second study identified, published in 2004, was a non-randomized pilot study evaluating short-term insulin therapy in 18 adolescents with type 2 diabetes (11). The intervention consisted of up to 16 wk (mean 8.7 ± 4.3 wk) of insulin therapy and a minimum of 12-month follow-up off insulin. Mean change in hemoglobin A1c was measured at the start and end of insulin therapy and at 3, 6, 9, and 12 months off insulin. No information on recruitment or retention rates was available in this report.
In 2007, Gottschalk et al. (8) published a 24-wk, randomized, single-blind, comparative study evaluating glimepiride vs. metformin as monotherapy. Of 536 patients screened, 241 did not meet inclusion criteria. Of 285 subjects who were randomized to glimepiride or metformin therapy, 210 (80%) completed the study. Both drugs had a similar beneficial effect on hemoglobin A1c.
In 2009, Gellar et al. (10) published a small trial in five youths with type 2 diabetes. Though the intervention was safe and non-invasive (test meals with varying glycemic indices), recruitment was described as difficult and the inclusion criteria were adjusted to include six patients with impaired glucose tolerance (Nansel TR, personal communication). Also in 2009, Malloy et al. (9) published a placebo-controlled, cross-over trial assessing the pharmacokinetics, pharmacodynamics, and tolerability of single doses of exenatide (2.5 and 5.0 μg) vs. placebo. Thirteen patients were enrolled and 12 completed the study.
Review of ongoing clinical trials
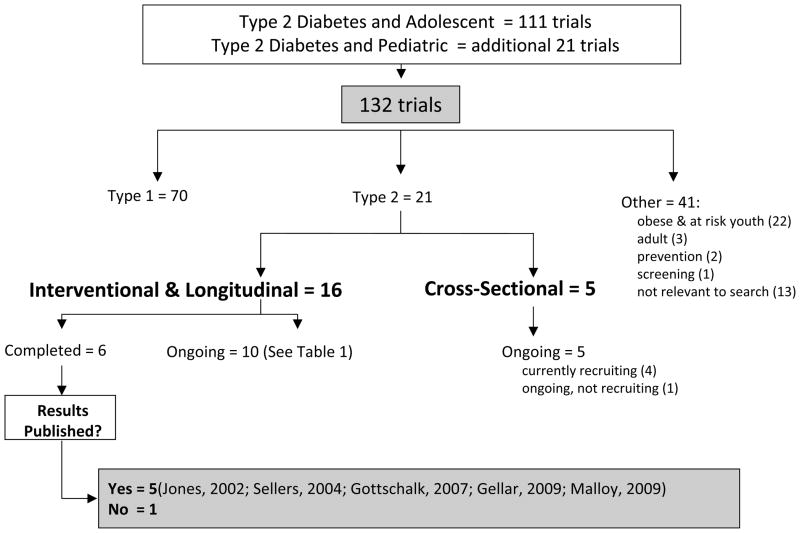
One hundred and thirty-two registered studies were identified from the search for clinical trials in children and adolescents with type 2 diabetes in clinicaltrials.gov (Fig. 1). Of these, only 21 (16%) were actually trials involving children or adolescents with type 2 diabetes. Seventy studies involved patients with type 1 diabetes only; 22 involved obese and youth at risk for type 2 diabetes; 3 involved the screening and prevention of type 2 diabetes; 3 evaluated adults with type 2 diabetes; and 13 were judged to be irrelevant to type 2 diabetes in children.
Fig. 1.
ClinicalTrials.gov: Search results for trials in adolescents with type 2 diabetes.
Of the 21 trials in children and adolescents with type 2 diabetes, 16 were interventional and 5 were cross-sectional. Six of 16 interventional trials were classified as completed and 10 were ongoing. Of these 10 ongoing studies, 7 were actively recruiting, 2 were no longer recruiting, and 1 was not yet open for recruitment. Six of these 10 trials had a pharmacological (5, 6, 12–15) and 4 a behavioral intervention (16–19) (Table 2). Of these, one ongoing interventional trial has thus far resulted in the publication of a pilot study (20), but no results of the other clinical trials are presently available.
Table 2.
Ongoing clinical trials of type 2 diabetes in youth
| Title | Intervention | Age (yr) | Start date of study | Duration of intervention | Multicenter |
|---|---|---|---|---|---|
| Pharmacological intervention | |||||
| Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY)* | Metformin 1000 mg bid Met + rosiglitazone 4 mg bid Met + intensive lifestyle intervention |
10–17 | May 2004 | Yes | |
| Effect of Short-Term Beta-Cell Rest in Adolescents and Young Adults with Type 2 Diabetes Mellitus | Metformin, Diet, and Exercise Insulin and Metformin, Diet, and Exercise |
12–25 | March 2007 | 12 Months | No |
| Safety and Efficacy of Exenatide as Monotherapy and Adjunctive Therapy to Oral Antidiabetic Agents in Adolescents with Type 2 Diabetes | Exenatide (5, 10 μg) Placebo |
10–16 | May 2008 | Yes | |
| A Study to Assess the Pharmacokinetics, Safety and Tolerability of Sitagliptin in Adolescents | Sitagliptin (50, 100, and 200 mg) | 10–17 | August 2008 | Yes | |
| Safety of Liraglutide in Pediatric Patients with Type 2 Diabetes | Liraglutide (0.3–1.8 or 0.6–1.8 mg) | 10–45 | July 2009 | Yes | |
| The Effect of Byetta and Symlin on Post-meal Meal Blood Sugar Levels in Children with Type 2 Diabetes | Exenatide 5 μg Pramlintide 60 μg |
12–21 | July 2009 | No | |
| Behavioral intervention | |||||
| Situational Problem Solving in Adolescents with Type 2 Diabetes: Enhancing a Randomized Controlled Trial (RCT) | Participation in a comprehensive disease management program | 12–19 | June 2009 | No | |
| Study of Intensive, Home-Based Family Therapy to Improve Illness Management in Youth with Diabetes | 60 min home-based, family psychotherapy sessions, two to three times a week for 6 months | 10–17 | March 2007 | No | |
| Personalized Exercise for Adolescents with Diabetes | 2-h education session 6-wk personal exercise program |
12–19 | August 2007 | No | |
| Partners for Better Health in Adolescent Type 2 Diabetes: the Buddy Study | Weekly contact with volunteer health worker (buddy) × 6 months vs. conventional follow-up | 12–20 | October 2009 | 6 Months | No |
Ongoing, not recruiting.
Published results were found for five of six completed interventional trials (Table 1) (7–11). For the study without published results, results had been submitted to the FDA (21). The study was a pharmaceutical company-sponsored trial and enrolled 167 patients aged 9–16 yr with type 2 diabetes. It was a randomized, controlled, double-blind, 26-wk trial with three treatment arms: glyburide/metformin (1.25/250 mg), glyburide (2.5 mg), and metformin (500 mg) titrated for glucose control. Results showed that the combination of metformin and glyburide produced an average decrease in hemoglobin A1c of 0.86%, which was not statistically superior to either metformin or glyburide alone.
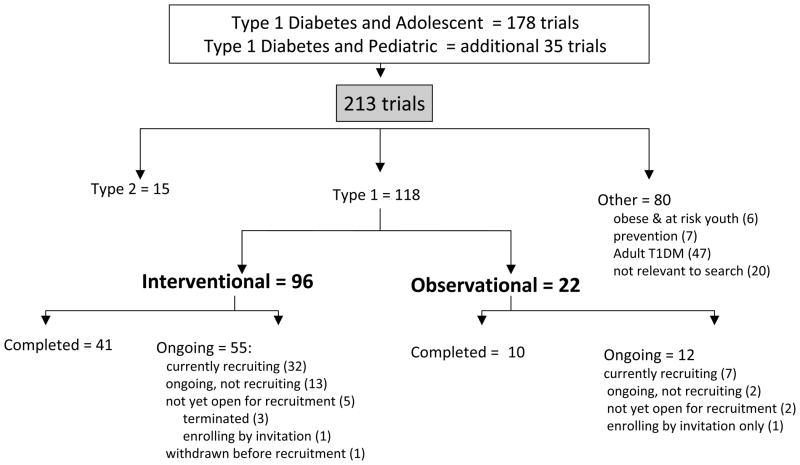
Clinicaltrials.gov was also searched for registered studies in children and adolescents with type 1 diabetes, and a total of 213 ongoing clinical trials were found (Fig 2). Of these, 118 (55%) were actually studies in children with type 1 diabetes. Fifteen were trials in children with type 2 diabetes, 47 evaluated adults with type 1 diabetes, 6 involved obese and at risk youth, 7 were prevention trials, and 20 were not relevant to type 1 diabetes. Of 118 clinical trials involving children with type 1 diabetes, 96 were interventional and 22 were observational trials.
Fig. 2.
ClinicalTrials.gov: Search results for trials in adolescents with type 1 diabetes.
Ongoing trials in children with type 1 diabetes exceeded the number of studies in type 2 diabetes over fivefold. Of the identified studies in clinicaltrials.gov, there were nearly eight times as many completed and five times as many ongoing interventional trials in type 1 compared to type 2 diabetes.
Discussion
A number of informative reports concerning pediatric patients with type 2 diabetes have been published in the form of case reports or as cross-sectional and epidemiological studies (22–30). However, only 381 patients have been described in five published interventional studies to date. Once these patients were enrolled in clinical trials, retention rates were satisfactory, ranging from 75 to 100% (7–10). Thus, recruitment of adolescents with type 2 diabetes into existing studies appears to be the most challenging predicament.
The challenge begins before study recruitment, with up to 60% of patients dropping out of routine clinical care within 2 yr (31). The demographics of type 2 diabetes in youth may explain why so many patients fail to participate in both clinical and research-oriented medical care: its highest incidence occurs in 15- to 19-yr-old adolescents, disproportionately affecting minority populations. Furthermore, a positive family history of obesity and diabetes may influence the reaction to diagnosis and perceptions of illness.
Adolescents as a group are generally less adherent than younger pediatric and adult cohorts to oral treatment regimens and study visits. This has been documented for adolescent patients enrolled in cancer and HIV trials (32–34). Self-reported reasons for non-compliance included forgetfulness, non-availability of medication (32), jobs and busy schedules (32, 34) as well as less developed concepts of illness, less perceived vulnerability, higher levels of denial, and less cohesive future orientation (33). Many adolescents may also leave home to attend college or live independently while others may have parents who are absent or apathetic to their child’s condition. Patients report that major facilitators to research participation are positive peer and family influences, program incentives (e.g., money and school credit), spending time with friends, commitment, and personal gain (34).
In contrast to individuals with type 1 diabetes, patients with type 2 diabetes frequently have a strong family history of the disease and share many high-risk features. Recently, Pinhas-Hamiel et al. (35) reported that first-degree family members of adolescent patients (aged 11–17 yr) with type 2 diabetes, including healthy siblings, shared many or all the following high-risk features: marked central obesity, poor dietary habits, and a sedentary lifestyle. The strong hereditary component of type 2 diabetes may present a significant barrier to participation in clinical research, particularly if relatives with the disease are not sufficiently concerned about their own disease management and are not supportive in the management of the affected adolescent. Furthermore, unless a high degree of independence has been achieved, lifestyle interventions of diet and exercise often need to target the entire family in order to be effective in the adolescent.
Historically, racial and ethnic minority populations have been underrepresented in clinical research, and individuals with type 2 diabetes, who often belong to minority groups, have lower odds of research participation compared to age-matched individuals with type 1 diabetes (36). Individuals who consent to clinical research tend to be more trusting of the medical system and possess a greater understanding of the research conducted (37). Mistrust of medical research generated by the Tuskegee syphilis experiments endures among some African American communities today and permeates into other racial and ethnic minority groups’ perceptions of clinical research. Furthermore, decisions regarding Hispanic and Native American youth are often made by the family and tribal elders, respectively. An acute awareness of the dynamics of daily life, the distinct subculture to which these groups subscribe (e.g., movies, songs, and advertisements), is thus expected to increase interest and facilitate involvement of minority adolescents in clinical research.
Lower socioeconomic status and lack of a powerful political and medical lobby are additional challenges affecting youth with type 2 diabetes. In contrast, children with type 1 diabetes benefit from support by organizations such as the Juvenile Diabetes Research Foundation (JDRF), which is the largest charitable funder and advocate of type 1 diabetes research in the world (38). Since its founding in 1970, JDRF has awarded over 1.3 billion dollars to research, including more than 156 million dollars in the 2008 fiscal year. No comparable private funding organization exists to promote research in youth with type 2 diabetes.
Finally, several studies in adolescent type 2 diabetes report that a large percentage of screened patients do not meet eligibility criteria. This may be due in part to the high prevalence of obesity-related comorbidities, such as hypertension, hyperlipidemia, non-alcoholic fatty liver disease, menstrual irregularities, and obstructive sleep apnea, which result in failure to meet strict inclusion or exclusion criteria (39, 40).
How might we improve recruitment and retention of adolescent patients in clinical studies? Studies outside the field of pediatric diabetes offer some promising strategies. One group demonstrated that widely dispersed advertisements in community-specific newspapers, using culturally sensitive language and graphics, captured higher response rates than did flyers send to churches and beauty salons (41). Villarruel et al. (34) showed that a combination of incentives (money and school credit for participation), flexible program start times, continued contact with project staff including more frequent reminders, increasing the interactive components of follow-up, and recognizing the importance of potential mobility limitations among adolescents and their families contributed to a reduction in risky sexual behavior in Latino youth. Finally, we suggest that more should be done to find young patients in the places where they are most comfortable – at their local clinics, in their neighborhoods, and at their schools. Rather than asking patients to come to us, perhaps it is time that we, as physicians and investigators, go to them.
In summary, many demographic and disease-specific variables represent barriers to active participation in much-needed clinical research. Ongoing clinical studies, such as the TODAY trial (14, 42), are expected to shed light on important questions, such as whether the natural history and treatment response of youths with type 2 diabetes is different compared to the well-characterized condition of type 2 diabetes in older adults. Specifically, the time course of deterioration of beta-cell function (27, 29) and the regenerative capacity of beta-cells in these young patients needs to be studied (43). Efforts taking into account the specific psychological and socioeconomic situation of affected children and adolescents may lead to improved patient recruitment and the successful conduct of clinical research in the field.
Acknowledgments
We would like to thank Mary E Ryan, MLS, in the National Institutes of Health Library for her support.
References
- 1.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 2.Boyd JD, Jackson RL. Levels of control in the treatment of diabetes mellitus. JAMA. 1938;111:906–909. [Google Scholar]
- 3.Jackson RL, Boyd JD, Smith TE. Stabilization of the diabetic child. Am J Dis Child. 1940;59:332–241. [Google Scholar]
- 4.Zietler PS. Children and adolescents with type 2 diabetes: characteristics of the TODAY study participants at baseline. LWPES/ESPE 8th Joint meeting; New York. 2009. [Google Scholar]
- 5.Effect of Short-Term Beta-Cell Rest in Adolescents and Young Adults with Type 2 Diabetes Mellitus. (available from http://clinicaltrials.gov/ct2/show/NCT00445627)
- 6.The Effect of Byetta and Symlin on Post-meal Meal Blood Sugar Levels in Children with Type 2 Diabetes (T2DM) (available from http://clinicaltrials.gov/ct2/show/NCT00950677)
- 7.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25:89–94. doi: 10.2337/diacare.25.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care. 2007;30:790–794. doi: 10.2337/dc06-1554. [DOI] [PubMed] [Google Scholar]
- 9.Malloy J, Capparelli E, Gottschalk M, Guan X, Kothare P, Fineman M. Pharmacology and tolerability of a single dose of exenatide in adolescent patients with type 2 diabetes mellitus being treated with metformin: a randomized, placebo-controlled, single-blind, dose-escalation, crossover study. Clin Ther. 2009;31:806–815. doi: 10.1016/j.clinthera.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Gellar L, Nansel TR. High and low glycemic index mixed meals and blood glucose in youth with type 2 diabetes or impaired glucose tolerance. J Pediatr. 2009;154:455–458. doi: 10.1016/j.jpeds.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellers EA, Dean HJ. Short-term insulin therapy in adolescents with type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17:1561–1564. doi: 10.1515/jpem.2004.17.11.1561. [DOI] [PubMed] [Google Scholar]
- 12.A Study to Assess the Pharmacokinetics, Safety and Tolerability of Sitagliptin in Adolescents. (available from http://clinicaltrials.gov/ct2/show/NCT00730275)
- 13.Safety and Efficacy of Exenatide as Monotherapy and Adjunctive Therapy to Oral Antidiabetic Agents in Adolescents with Type 2 Diabetes. (available from http://clinicaltrials.gov/ct2/show/NCT00658021)
- 14.Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) (available from http://clinicaltrials.gov/ct2/show/NCT00081328)
- 15.Safety of Liraglutide in Pediatric Patients with Type 2 Diabetes. (available from http://clinicaltrials.gov/ct2/show/NCT00943501)
- 16.Situational Problem Solving in Adolescents with Type 2 Diabetes: Enhancing a Randomized Controlled Trial. (available from http://clinicaltrials.gov/ct2/show/NCT00889785)
- 17.Study of Intensive, Home-Based Family Therapy to Improve Illness Management in Youth with Diabetes. (available from http://clinicaltrials.gov/ct2/show/NCT00372814)
- 18.Personalized Exercise for Adolescents with Diabetes. (available from http://clinicaltrials.gov/ct2/show/NCT00686283)
- 19.Partners for Better Health in Adolescent Type 2 Diabetes: The Buddy Study. NIH Study T-DK-0413. (available from http://clinicaltrials.gov/ct2/show/NCT01007266)
- 20.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 2009;32:2184–2186. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A Research Study to Determine the Safety and Efficacy of Glucovance Compared to Metformin and Glyburide in Children and Adolescents with Type 2 Diabetes. (available from http://clinicaltrials.gov/ct2/show/NCT00035542)
- 22.Gottschalk M, Danne T, Fuerst-Recktenwald S. Ethnic origin is unrelated to autoimmunity and residual pancreatic function in 471 youth with clinically diagnosed type 2 diabetes. Pediatr Diabetes. 2009;10:240–247. doi: 10.1111/j.1399-5448.2008.00489.x. [DOI] [PubMed] [Google Scholar]
- 23.Shield JP, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with type 2 diabetes. Arch Dis Child. 2009;94:206–209. doi: 10.1136/adc.2008.143313. [DOI] [PubMed] [Google Scholar]
- 24.Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31:1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer-Davis EJ, Dabelea D, Lamichhane AP, et al. Breast-feeding and type 2 diabetes in the youth of three ethnic groups: the SEARCh for diabetes in youth case-control study. Diabetes Care. 2008;31:470–475. doi: 10.2337/dc07-1321. [DOI] [PubMed] [Google Scholar]
- 26.Tfayli H, Bacha F, Gungor N, Arslanian S. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes. 2009;58:738–744. doi: 10.2337/db08-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad R, Gungor N, Arslanian S. Progression from normal glucose tolerance to type 2 diabetes in a young girl: longitudinal changes in insulin sensitivity and secretion assessed by the clamp technique and surrogate estimates. Pediatr Diabetes. 2005;6:95–99. doi: 10.1111/j.1399-543X.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 28.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. 2005;28:638–644. doi: 10.2337/diacare.28.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gungor N, Arslanian S. Progressive beta cell failure in type 2 diabetes mellitus of youth. J Pediatr. 2004;144:656–659. doi: 10.1016/j.jpeds.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 30.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. Beta-cell function across the spectrum of glucose tolerance in obese youth. Diabetes. 2005;54:1735–1743. doi: 10.2337/diabetes.54.6.1735. [DOI] [PubMed] [Google Scholar]
- 31.Reinehr T, Schober E, Roth CL, Wiegand S, Holl R. Type 2 diabetes in children and adolescents in a 2-year follow-up: insufficient adherence to diabetes centers. Horm Res. 2008;69:107–113. doi: 10.1159/000111814. [DOI] [PubMed] [Google Scholar]
- 32.Tebbi CK. Treatment compliance in childhood and adolescence. Cancer. 1993;71:3441–3449. doi: 10.1002/1097-0142(19930515)71:10+<3441::aid-cncr2820711751>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 33.Tamaroff MH, Festa RS, Adesman AR, Walco GA. Therapeutic adherence to oral medication regimens by adolescents with cancer. II. Clinical and psychologic correlates. J Pediatr. 1992;120:812–817. doi: 10.1016/s0022-3476(05)80257-4. [DOI] [PubMed] [Google Scholar]
- 34.Villarruel AM, Jemmott LS, Jemmott JB, Eakin BL. Recruitment and retention of Latino adolescents to a research study: lessons learned from a randomized clinical trial. J Spec Pediatr Nurs. 2006;11:244–250. doi: 10.1111/j.1744-6155.2006.00076.x. [DOI] [PubMed] [Google Scholar]
- 35.Pinhas-Hamiel O, Standiford D, Hamiel D, Dolan LM, Cohen R, Zeitler PS. The type 2 family: a setting for development and treatment of adolescent type 2 diabetes mellitus. Arch Pediatr Adolesc Med. 1999;153:1063–1067. doi: 10.1001/archpedi.153.10.1063. [DOI] [PubMed] [Google Scholar]
- 36.Liese AD, Liu L, Davis C, et al. Participation in pediatric epidemiologic research: the SEARCH for Diabetes in Youth Study experience. Contemp Clin Trials. 2008;29:829–836. doi: 10.1016/j.cct.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tait AR, Voepel-Lewis T, Malviya S. Participation of children in clinical research: factors that influence a parent’s decision to consent. Anesthesiology. 2003;99:819–825. doi: 10.1097/00000542-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Juvenile Diabetes Research Foundation International. 2008 Annual Report. 2008. [Google Scholar]
- 39.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–1831. doi: 10.1016/S0140-6736(07)60821-6. [DOI] [PubMed] [Google Scholar]
- 40.Sellers EA, Blydt-Hansen TD, Dean HJ, Gibson IW, Birk PE, Ogborn M. Macroalbuminuria and renal pathology in First Nation youth with type 2 diabetes. Diabetes Care. 2009;32:786–790. doi: 10.2337/dc08-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staffileno BA, Coke LA. Recruiting and retaining young, sedentary, hypertension-prone African American women in a physical activity intervention study. J Cardiovasc Nurs. 2006;21:208–216. doi: 10.1097/00005082-200605000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Zeitler P, Epstein L, Grey M, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]