Abstract
Polymeric micelles of single and mixed poloxamines (Tetronic) were evaluated regarding their ability to host the antiglaucoma agent ethoxzolamide (ETOX) for topical ocular application. Three highly hydrophilic varieties of poloxamine (T908, T1107 and T1307) and a medium hydrophilic variety (T904), possessing a similar number of propylene oxide units but different contents in ethylene oxide, were chosen for the study. The critical micellar concentration and the cloud point of mixed micelles in 0.9 per cent NaCl were slightly greater than the values predicted from the additive rule, suggesting that the co-micellization is hindered. Micellar size ranged between 17 and 120 nm and it was not altered after the loading of ETOX (2.7–11.5 mg drug g–1 poloxamine). Drug solubilization ability ranked in the order: T904 (50-fold increase in the apparent solubility) > T1107 ≅ T1307 > T908. Mixed micelles showed an intermediate capability to host ETOX but a greater physical stability, maintaining almost 100 per cent drug solubilized after 28 days. Furthermore, the different structural features of poloxamines and their combination in mixed micelles enabled the tuning of drug release profiles, sustaining the release in the 1–5 days range. These findings together with promising hen's egg test-chorioallantoic membrane biocompatibility tests make poloxamine micelles promising nanocarriers for carbonic anhydrase inhibitors in the treatment of glaucoma.
Keywords: polymeric micelles, ethoxzolamide, poloxamine, ocular delivery, carbonic anhydrase inhibitor solubilization, poly(ethylene oxide)-poly(propylene oxide) block copolymer
1. Introduction
Carbonic anhydrase inhibitor (CAI) drugs represent an important option for the treatment of glaucoma owing to their efficacy in decreasing the rate of aqueous humour secretion. Carbonic anhydrases are metalloenzymes present in the anterior uvea of the eye that catalyse the conversion of CO2 to bicarbonate and proton [1]. The use of CAIs, namely sulphonamides such as acetazolamide, as a way to lower the intraocular pressure (IOP) in the treatment of glaucoma is mostly centred in the oral route. Unfortunately, the oral administration of CAIs is associated with relevant systemic adverse effects such as depression, fatigue, gastrointestinal irritation, metabolic acidosis, metallic taste, loss of libido and paresthaesia [2] owing to the ubiquitous distribution of carbonic anhydrases in all the tissues of the body. Thus, the topical administration of CAIs to the eye may notably decrease these side effects and be more patient compliant. However, the development of suitable ophthalmic formulations of CAIs has to face up to their limited aqueous solubility, particularly in the case of those that have adequate log p-values to pass through the cornea [3,4].
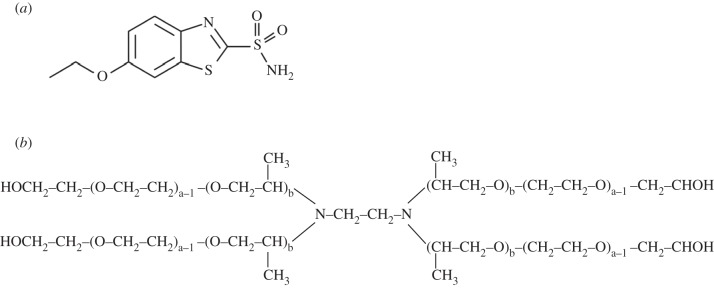
Ethoxzolamide (ETOX) is a hydrophobic CAI (log p = 2.08) with a high activity against carbonic anhydrases and a favourable corneal permeability (100 times greater than the most popular one, acetazolamide) [5–7]. ETOX structure (scheme 1a) has been used as the building block for the design of new molecules, such as dorzolamide and brinzolamide [8,9], which were approved as the first topical CAIs [3,10]. Another hydrophilic derivative, 6-hydroxyethoxzolamide, has shown efficient corneal penetration and ocular hypotensive effects in albino rabbit eyes [11]. The 6-amino-2-benzothiazolesulphonamide formulated as topical gel reduced the pressure in human eyes, but not when prepared as suspension [12]. These findings confirmed that the poor solubilization limits corneal penetration. On the other hand, it was shown that ETOX can be solubilized by forming inclusion complexes with cyclodextrin derivatives and that topically active formulations which combine ETOX and timolol can be thus prepared [13,14]. More recently, biomimetic contact lenses capable of loading ETOX and of controlling the drug release were prepared with the intention of prolonging the contact time between the drug and the eye and enhancing the ocular bioavailability [15].
Scheme 1.
(a) Chemical structure of ethoxzolamide and (b) general structure of poloxamine.
Polymeric micelles of block copolymers are a potentially valuable tool to overcome some limitations involved in CAIs formulation for ophthalmic application. The core–shell structure may notably enhance the apparent aqueous solubility, leading to a greater concentration gradient favourable for diffusion, and also provide sustained release since the dilution factor in the lachrymal fluid is less than in other administration routes [16]. Polymeric micelles have been shown to be suitable ocular carriers for anti-inflammatory drugs [17] or even plasmids and genes [18,19]. Transcorneal permeation studies through excised rabbit cornea indicated that non-steroidal anti-inflammatory drug formulations in polymeric micelles can enhance about twofold drug permeation compared with that of an aqueous suspension of the same concentration because the dissolution step is overcome [17]. Recent studies have demonstrated that poloxamine micelles are able to host relatively hydrophobic drugs and to increase their apparent solubility and stability [20,21]. As opposed to the linear counterparts (poloxamers) that are only thermoresponsive, poloxamines (four arms of poly(ethylene oxide)-poly(propylene oxide) connected through an ethylenediamine group) are appealing amphiphiles owing to the greater chemical versatility and dually pH- and temperature-responsive behaviour [22]. Furthermore, some block copolymers and particularly certain varieties of poloxamers and poloxamines are able to block the P-glycoprotein efflux pumps and enhance drug penetration in different tumour cells [23–25]. Such a feature may also contribute to increase the corneal penetration of drugs by inhibition of the P-glycoprotein present in the corneal tissue [26].
The aim of this work was to elucidate the potential of single and mixed polymeric micelles of branched poly(ethylene oxide)-poly(propylene oxide) (PEO–PPO) block copolymers of the poloxamine family (Tetronic) as nanocarriers suitable for the ocular delivery of ETOX (scheme 1). The self-associative behaviour of mixtures of these X-shaped copolymers in 0.9 per cent NaCl has been explored in detail to gain an insight into the potential performance of these systems for localized ocular delivery. ETOX solubilization, micelle stability, tissue irritability and in vitro ETOX release were also evaluated. To the best of our knowledge, this is the first study evaluating the performance of polymeric micelles for the encapsulation of CAIs in the treatment of glaucoma.
2. Material and methods
2.1. Materials
ETOX was from Sigma-Aldrich Chemicals (Madrid, Spain). Tetronic 904 (T904, Mw 6700, 40% PEO, 15 EO and 17 PO units per arm), 908 (T908, Mw 25 000, 80% PEO, 114 EO and 21 PO units per arm), 1107 (T1107, Mw 15 000, 70% PEO, 60 EO and 20 PO units per arm) and 1307 (T1307, Mw 18 000, 70% PEO, 72 EO and 23 PO units per arm) were from BASF (New Milford, CT, USA). Purified water was obtained by reverse osmosis (resistivity > 18.2 MΩ cm; MilliQ, Millipore, Spain). Others reagents were of analytical grade.
2.2. Preparation of single and mixed polymeric micelles
Solutions of T904, T908, T1107 and T1307 at 10% (w/v) were prepared by adding the copolymer to cold 0.9 per cent NaCl aqueous medium and stored for 24 h at 25°C before the assays. Mixed micelles of T904 : T1107 and T904 : T1307 were prepared by mixing the earlier-prepared solutions at 75 : 25, 50 : 50 and 25 : 75 weight ratios. Mixed micelles of T1107 : T1307 were prepared at a 50 : 50 weight ratio. In all cases, the overall copolymer concentration was 10 per cent.
2.3. Critical micellar concentration
Copolymer solutions (0.001–10%) were filtered through cellulosic membranes of 0.22 µm (Westboro, MA, USA) and equilibrated at 25°C before measurement of size distribution by means of dynamic laser scattering (DLS) employing a Zetasizer Nano-Zs (Malvern Instruments, Worcestershire, UK) fitted with a He–Ne (633 nm) laser and a digital correlator at a scattering angle of θ = 173° to the incident beam. The experiments were carried out in triplicate. Critical micellar concentration (CMC) of single and mixed poloxamine solutions was estimated as the concentration at which a jump in the scattering intensity versus poloxamine concentration plot occurred [27].
2.4. Cloud point
Glass tubes containing 2 ml of micellar system (10%) without drug were immersed in an oil bath at room temperature. Then, the temperature was progressively increased at 1°C min–1 until an abrupt change in the visual appearance of the system from clear to turbid was observed [28]. Assays were carried out in duplicate.
2.5. Micellar solubilization of ethoxzolamide
Solutions of single and mixed polymeric micelles (5 ml) were transferred into vials containing ETOX in excess (8 mg) and kept under magnetic stirring at 25 ± 1°C for 72 h. Then, the solutions were filtered through polytetrafluoroethylene membranes of 0.45 µm pore size (Sartorius, Goettingen, Germany) to remove the insoluble drug. The concentration of the dissolved drug was spectrophotometrically quantified at 304 nm (CARY 1E UV-Vis, Varian, Palo Alto, CA, USA) using a calibration curve obtained with ETOX ethanolic solutions (5–20 mg l−1). Solubility factors (fS) were calculated as follows:
| 2.1 |
where Sa and SSAq represent the ETOX apparent solubility in the micellar systems and the intrinsic solubility in 0.9 per cent NaCl (22.84 mg l–1), respectively.
The binding constant (K) to aggregation number (N) ratio of ETOX-micellar systems was estimated from:
| 2.2 |
where Sa and SSAq maintain the same meaning as above, and Ct and Ccmc refer to the total and critical micellar copolymer concentrations. All concentrations are given in molarity.
2.6. Kinetic stability of drug-containing micelles
ETOX-loaded micellar systems were stored at 25°C and monitored over 28 days [27]. Aliquots (50 µl) were diluted in ethanol (2950 µl) and the absorbance recorded at 304 nm in order to quantify the amount of ETOX remaining in solution. At the same time points, the hydrodynamic diameter (Dh) and the polydispersion index (PDI) of the micelles were recorded by DLS using the same operational conditions described earlier. All measurements were carried out in triplicate.
2.7. Hen's egg test-chorioallantoic membrane assay
Fertilized broiler chicken eggs (not older than 3 days; Avirojo, Pontevedra, Spain) were incubated with the large end upwards in an Ineltec CCSP0150 climatic chamber (Tona, Barcelona, Spain) at 37 ± 0.3°C and 60 ± 2% relative humidity. Eggs were rotated (five times per day) for 8 days to prevent the attachment of the embryo to one side of the egg. Then, the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM)-recommended test method protocol was followed [29]. The upper part of the eggshell (air cell) was removed using a Dremel 300 equipped with a rotary saw (Breda, The Netherlands). The intact inner membrane was moistened with 0.9 per cent NaCl solution and the eggs were placed in the climatic chamber for a maximum of 30 min. The 0.9 per cent NaCl solution was sucked out and the inner membrane was removed with forceps. A micellar solution (300 µl at 25°C) was placed on the chorioallantoic membrane and the irritation potential (haemorrhage, vascular lysis and coagulation) was monitored for 5 min. The experiments were carried out in triplicate. Negative (0.9% NaCl solution) and positive (0.1 N NaOH) controls were tested under the same conditions. Irritation scores (IS) were calculated from the time (in seconds) at which hemorrhage (H), lysis (L) or coagulation (C) started, as follows [29]:
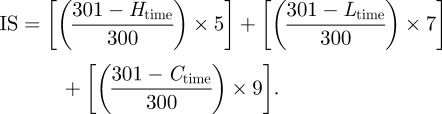 |
2.3 |
According to the IS values, the materials can be classified as non-irritating (0–0.9), weakly irritating (1–4.9), moderately irritating (5–8.9) or severely irritating (9–21) [29].
2.8. In vitro release studies
ETOX release from the loaded micellar systems was studied using Franz diffusion cells fitted with cellulose dialysis membranes (MWCO 3500, Spectrum Laboratory, Rancho Dominguez, CA, USA) previously soaked in distilled water (30 min) and phosphate saline buffer, according to a technique described elsewhere [30–32]. The diffusion area was 0.785 cm2. Isotonic phosphate saline buffer (7 ml, pH 7.4) containing 0.3 per cent sodium dodecylsulphate was used as the receptor medium in order to ensure sink conditions. The donor compartment was filled with 500 µl of the drug-loaded micellar systems and covered to prevent evaporation. The receptor solution was stirred with a magnetic bar and maintained at 32 ± 0.5°C throughout the experiment. This temperature mimics the ocular environment. The ETOX concentration in the receptor solution was monitored over time by ultraviolet spectrophotometry at 304 nm (Agilent 8453, Waldbronn, Germany) by taking 700 µl samples at pre-established time points. The same volume was replaced with a fresh buffer medium (700 µl). Assays were carried out in triplicate.
3. Results and discussion
3.1. Self-aggregation of poloxamines
The present work explored the capacity of three highly hydrophilic varieties (T908 with hydrophilic-lipophilic balance (HLB) > 24, and T1107 and T1307 with HLB 18–23) and a medium hydrophilic variety (T904 with HLB 12–18) of poloxamine and their combinations to form single or mixed polymeric micelles as a nanotechnology platform suitable for encapsulation of the CAI-drug ETOX towards the topical treatment of glaucoma. Previous studies showed that to produce mixed poloxamer/poloxamer polymeric micelles both copolymers need to present two main features: (i) hydrophobic blocks of similar molecular weight and (ii) different hydrophilic/hydrophobic balance [33,34]. These two premises were taken into account when choosing the poloxamine varieties included in the study. Our hypothesis was that co-micellization of T1107 and T1307 with the more hydrophobic derivative T904 would lead to the large encapsulation extents characteristic of T904 and, at the same time, obtain drug-loaded micelles with improved physical stability compared with those of T904 [35,36]. Poloxamine was previously mixed with poloxamer to enhance the physical stability of efavirenz-loaded micelles [27]. Nevertheless, since poloxamines are not linear but behave as two PEO–PPO–PEO triblocks linked through an ethylenediamine central group [24], some steric hindrance in the co-micellization process could be anticipated when two varieties of poloxamines are mixed. We also included in the study the highly hydrophilic T908 variety (despite its very limited ability to solubilize related drugs [21]) as a representative of high HLB in order to elucidate the role of this parameter on the solubilization of a drug so hydrophobic as ETOX in single micelles. Mixed micelles of T904 and T908 were not tested owing to problems encountered obtaining reproducible results, probably owing to the huge dissimilarity in the length of the PEO chains, and require further detailed analysis.
The micellization process of single poloxamines has been previously evaluated in detail in water, HCl medium and other aqueous salt solutions (e.g. NaCl and Na2SO4) [21,37,38]. It should be noticed that these block copolymers are quite sensitive to the physicochemical conditions of the dispersant medium, particularly the pH and the ionic strength, which lead to changes in the protonation extent of the ethylenediamine central group and consequently alter the hydrophobic interactions that govern the self-assembly phenomena [23,39]. The CMC of single and mixed systems in 0.9 per cent NaCl aqueous medium was determined by DLS (table 1) and compared with the CMC value predicted according to the following expression [40]:
| 3.1 |
where X1 and X2 are the molar fractions of the components 1 and 2, and CMC1 and CMC2 the CMC values of components 1 and 2, respectively.
Table 1.
Experimental and predicted CMC and cloud point (CP) values for single and mixed systems in 0.9% NaCl at 25°C. (The coefficient of variation of repeated measurements of CMC values was below 10% in all cases and the error in the experimental CP data was ±1°C.)
| copolymers | experimental CMC (mM) | theoretical CMC (mM) | experimental CP (°C) | theoretical CP (°C) |
|---|---|---|---|---|
| T904 | 0.75 | — | 65 | — |
| T908 | 0.24 | — | 97 | — |
| T1107 | 0.40 | — | 105 | — |
| T1307 | 0.33 | — | 100 | — |
| T1107 : T1307 (50 : 50) | 0.49 | 0.37 | 99 | 102 |
| T904 : T1107 (25 : 75) | 0.52 | 0.50 | 97 | 84 |
| T904 : T1307 (25 : 75) | 0.47 | 0.45 | 93 | 80 |
| T904 : T1107 (50 : 50) | 0.65 | 0.59 | 86 | 74 |
| T904 : T1307 (50 : 50) | 0.61 | 0.56 | 87 | 72 |
| T904 : T1107 (75 : 25) | 0.77 | 0.67 | 76 | 68 |
| T904 : T1307 (75 : 25) | 0.63 | 0.66 | 71 | 68 |
The CMC values of single poloxamine systems (table 1) were in the same order of magnitude of those previously obtained in HCl 10 mM using the pyrene fluorescence technique [21]. The greater the molecular weight of the copolymer, the lower the CMC. This behaviour was less dependent on the HLB. The slight decrease of CMC in NaCl with respect to HCl would stem from the salting out induced by Na+ ions. Then, the analysis focused on the mixed micelles. In general, CMC data were similar to those theoretically predicted, though slight differences were observed. Positive and negative deviations from ideality point out an unfavourable and a favourable mixing process, respectively. When favourable interactions are strong, co-micellization is improved and the experimental CMC value is smaller than the theoretical one. The greater the difference between the theoretical and the experimental value, the more favoured or disfavoured the co-micellization. T1107 : T1307 (50 : 50) micelles showed a CMC value that was greater than that of each copolymer separately, suggesting that the co-micellization was hindered. It is worth stressing that T1107 and T1307 display similar HLB values and, therefore, this is against the rule that the generation of mixed micelles require two components with different HLB [33,34].
The mixture of T904 with growing proportions of T1107 or T1307 led to a gradual decrease of the CMC of pure T904 from 0.75 to 0.47 mM (T904 : T1307 25 : 75) and 0.52 mM (T904 : T1107 25 : 75). These findings would suggest that the aggregation is primarily driven by the micellization of T1107 and T1307 (two copolymers that show relatively low CMC), followed by the incorporation of T904 into the core of the initially formed micelles. This hypothesis was previously formulated for poloxamer/poloxamine mixed micelles studied by electron spin resonance [27]. On the other hand, all CMC values remained greater than those shown by single T1107 and T1307, suggesting that the addition of T904 to the T1107 and T1307 systems has a slight to moderate detrimental effect on the self-aggregation of the highly hydrophilic counterparts. Interestingly, T904 : T1107 (75 : 25) micelles showed a CMC value of 0.77 mM, this value being the greatest of all the systems under evaluation and greater than the one shown by pure T904. These results indicate that the formation of these mixed micelles is strongly hindered, which is in agreement with previous reports on positive deviations for F127 : P85 and F127 : P123 mixed micelles and negative deviations for F88 : P123 [40].
The turbidity of 10 per cent micellar solutions was monitored as a function of temperature with the aim to establish the cloud point (CP) and the effect of NaCl on the aggregation process [28]. As expected from its smaller HLB, T904 evidenced phase separation at a much lower temperature (65°C) than that of the more hydrophilic poloxamines (97–105°C; table 1). Remarkably, the CP of 10 per cent T904 in 0.9 per cent NaCl was 10°C lower than the value measured in phosphate–citrate buffer solution of pH 5 [27]. All systems showed one single CP, revealing the formation of mixed micelles [41]. In addition, values of mixed micelles were always smaller than those of the pure hydrophilic poloxamine, probably owing to the generation of a more hydrophobic system upon the addition of T904. These findings were in full agreement with data reported elsewhere [27].
On the other hand, with the exception of T1107 : T1307 (50 : 50) that displayed an experimental value slightly smaller than that theoretically calculated, experimental CP values of mixed micelles were higher than the theoretical ones (estimated as for the CMC). The CP is associated with the inter-micellar interactions in the binary system and it is expected to differ from that of the single micelles. These results constitute further evidence that, even if taking place, the co-micellization of poloxamine mixtures is a quite disfavoured process. Interestingly, T904 : T1307 (75 : 25) showed an experimental CMC value smaller than the theoretical one, suggesting a favoured micellization. Conversely, in the CP assay, a hindered aggregation was revealed by the slightly greater experimental CP. This discrepancy probably stemmed from experimental deviations.
3.2. Micellar size
Size of single and mixed micelles was recorded before and after the loading with ETOX (table 2). Multimodal distributions were observed in all the cases except for T904 and its combinations with T1107 and T1307 at a 75 : 25 weight ratio. In the case of ETOX-free micelles, main size fractions in the 17–120 nm range (peak 2) correspond to polymeric micelles, while the other ones belong to the smaller unimers or dimers (4–7 nm, peak 1) [21]. Larger size fractions in pure T1107 and T904-containing mixed micelles were probably related to the presence of aggregates generated by the secondary aggregation of drug-loaded micelles over time (more than 200 nm, peak 3). In the case of T908, this fraction was also found in drug-free systems. It is worth stressing that the percentage of unimers/dimers in samples of pure T1107, T1307 and T908 ranged between 25 and 46 per cent and it was substantially greater than that in T904 (7%). This phenomenon relied on the incomplete micellization of hydrophilic PEO–PPO block copolymers at 25°C [20].
Table 2.
Micellar size (Dh), size distribution and polydispersity index (PDI) of (A) ETOX-free polymeric micelles, (B) ETOX-loaded poloxamine micelles at day 0 and (C) ETOX-loaded poloxamine micelles stored at 25°C over 28 days. (The final copolymer concentration was 10%.)
| copolymers | peak 1 |
peak 2 |
peak 3 |
PDI | |||
|---|---|---|---|---|---|---|---|
| Dh (nm) | % | Dh (nm) | % | Dh (nm) | % | ||
| (A) T904 | — | — | 22.7 (0.3) | 100.0 | — | — | 0.39 (0) |
| (B) T904 | — | — | 17.3 (0.1) | 100.0 | — | — | 0.27 (0.02) |
| (C) T904 | 3.8 (0.6) | 7.0 | 16.9 (1.3) | 93.0 | — | — | 0.30 (0.13) |
| (A) T908 | 4.8 (0.1) | 45.3 | 65.4 (3.5) | 18.7 | 545.5 (76.7) | 36.0 | 0.40 (0.01) |
| (B) T908 | 5.1 (0) | 38.3 | 107.7 (13.1) | 32.6 | 349.5 (38.5) | 29.1 | 0.24 (0.03) |
| (C) T908 | 4.6 (0) | 53.5 | 56.1 (6.7) | 29.0 | 418.4 (31.2) | 17.5 | 0.46 (0.01) |
| (A) T1107 | 5.5 (0.1) | 25.0 | 52.4 (0.5) | 75.0 | — | — | 0.73 (0.04) |
| (B) T1107 | 5.8 (0.2) | 22.1 | 69.8 (3.4) | 77.9 | — | — | 0.81 (0.04) |
| (C) T1107 | 4.5 (0) | 37.2 | 28.7 (10.9) | 8.6 | 264.0 (43.1) | 54.2 | 0.63 (0.03) |
| (A) T1307 | 5.7 (0.1) | 27.9 | 54.9 (2.9) | 72.1 | — | — | 0.68 (0.06) |
| (B) T1307 | 5.6 (0.1) | 19.4 | 47.7 (1.5) | 80.6 | — | — | 0.84 (0.01) |
| (C) T1307 | 5.3 (0.1) | 23.3 | 50.4 (0.7) | 76.7 | — | — | 0.38 (0.07) |
| (A) T1107 : T1307 (50 : 50) | 5.3 (0.1) | 27.3 | 43.6 (0.8) | 72.7 | 0.66 (0.04) | ||
| (B) T1107 : T1307 (50 : 50) | 5.4 (0.1) | 30.5 | 47.8 (0.2) | 69.5 | — | — | 0.55 (0.03) |
| (C) T1107 : T1307 (50 : 50) | 5.4 (0) | 25.5 | 46.8 (0.3) | 74.5 | — | — | 0.42 (0.05) |
| (A) T904 : T1107 (50 : 50) | 6.4 (0.6) | 29.7 | 30.7 (4.4) | 70.3 | — | — | 0.52 (0.01) |
| (B) T904 : T1107 (50 : 50) | 4.8 (0.3) | 18.5 | 22.8 (1.2) | 81.5 | — | — | 0.27 (0.15) |
| (C) T904 : T1107 (50 : 50) | 3.7 (0.2) | 11.9 | 21.0 (1.7) | 52.5 | 326.6 (42.6) | 35.6 | 0.59 (0.02) |
| (A) T904 : T1307 (50 : 50) | 5.6 (0.2) | 22.3 | 28.7 (0.3) | 77.7 | — | — | 0.49 (0.06) |
| (B) T904 : T1307 (50 : 50) | 5.5 (0.3) | 27.4 | 20.2 (0.5) | 72.6 | — | — | 0.26 (0.04) |
| (C) T904 : T1307 (50 : 50) | 4.7 (0.1) | 21.0 | 21.7 (2.1) | 59.6 | 222.6 (74.7) | 19.4 | 0.45 (0.21) |
| (A) T904 : T1107 (25 : 75) | 5.7 (0.2) | 27.7 | 42.5 (1.3) | 72.3 | — | — | 0.52 (0.02) |
| (B) T904 : T1107 (25 : 75) | 5.9 (0.1) | 27.8 | 38.4 (0.8) | 72.2 | — | — | 0.47 (0.01) |
| (C) T904 : T1107 (25 : 75) | 5.3 (0.2) | 22.3 | 36.6 (0.9) | 77.7 | — | — | 0.43 (0.17) |
| (A) T904 : T1307 (25 : 75) | 5.9 (0.2) | 30.3 | 39.0 (1.7) | 69.7 | — | — | 0.49 (0.02) |
| (B) T904 : T1307 (25 : 75) | 6.2 (0.3) | 32.3 | 39.2 (1.4) | 67.7 | — | — | 0.49 (0.02) |
| (C) T904 : T1307 (25 : 75) | 5.5 (0.1) | 26.6 | 36.1 (1.9) | 73.4 | — | — | 0.31 (0.09) |
| (A) T904 : T1107 (75 : 25) | — | — | 20.1 (0.1) | 100.0 | — | — | 0.29 (0.01) |
| (B) T904 : T1107 (75 : 25) | — | — | 20.0 (0.1) | 100.0 | — | — | 0.28 (0.01) |
| (C) T904 : T1107 (75 : 25) | 5.3 (0.4) | 16.1 | 23.0 (0.7) | 83.9 | 0.43 (0.02) | ||
| (A) T904 : T1307 (75 : 25) | — | — | 19.1 (0.1) | 100.0 | — | — | 0.29 (0.01) |
| (B) T904 : T1307 (75 : 25) | — | — | 18.3 (0.5) | 100.0 | — | — | 0.27 (0.01) |
| (C) T904 : T1307 (75 : 25) | 5.2 (0.3) | 16.7 | 21.4 (1.4) | 83.3 | — | — | 0.30 (0.11) |
T904 micelles were smaller (approx. 22 nm) than those of the other poloxamines (52–65 nm) owing to the lower molecular weight. The size of mixed micelles depended on the relative composition. In general, the greater the T904 content, the smaller the size. For example, T904 : T1107 and T904 : T1307 (75 : 25) micelles displayed sizes similar to those of pure T904. Conversely, the size of 25 : 75 mixed systems was approximately 40 nm, while 50 : 50 showed an intermediate value. Incorporation of ETOX (in the amounts discussed below) did not cause relevant changes in the micellar size distribution. These findings indicate that ETOX does not induce micellization as previously shown for efavirenz [39] and only a clear size increase from 65.4 and 52.4 nm to 107.7 and 69.8 nm was observed, respectively, for single T908 and T1107 micelles at day 0 (table 2).
3.3. Ethoxzolamide solubilization
ETOX displays a relatively high melting point of 189°C, revealing the presence of strong solute–solute interactions. To efficiently encapsulate the drug within polymeric micelles, the drug–core interaction needs to be stronger than the solute–solute ones. All poloxamine micelles led to sharp increases in drug solubility (table 3), at least one order of magnitude compared with ETOX aqueous solubility (22.8 mg l–1). T904 solely micelles increased up to 50 times the apparent solubility of the drug. The amount of drug hosted by the micelles referred to 1 g of polymer ranged between 2.7 mg for T908 to 11.5 mg for T904 (table 3). Solubilization ability ranked in the order: T904 > T1107 ≅ T1307 > T908. This means that the presence of a large PEO arm in the poloxamine molecule does not favour the drug encapsulation process, but hinders it to a certain extent, as indicated by the lowest amount of ETOX per hydrophobic block that was loaded by T908 (table 3). The apparent solubility clearly shows the opposite trend of the HLB values of the poloxamines; namely, the lower the HLB, the greater the increase in ETOX apparent solubility is. Nevertheless, it is interesting to note that when the amount of ETOX solubilized is referred to the content in PPO, the copolymer varieties that exhibit greater solubilization capability were those with intermediate HLB, i.e. with sufficiently larger PPO blocks to host the drug molecules, namely T1307 and T1107. In fact, the binding constant to aggregation number ratio (K/N), estimated from equation (2.2) [42], was larger for these latter varieties. For a precise estimation of the binding constant, it is necessary to know the aggregation number N. The value of N remains practically constant in a large range of concentrations (from the CMC to about 100 times greater) [42]. Using the values of N for poloxamine micelles in HCl 10 mM previously reported [21], the binding constant K would be much larger for T904 (49 260) compared with that of T908 (21 001), T1107 (16 439) and T1307 (25 482), owing to the fact that each micelle of T904 is formed by a greater number of unimers (14) than those of T908 (7), T1107 (4) and T1307 (5). It is probably because the N values are still larger in NaCl 0.9 per cent than in HCl 10 mM because of a lower degree of protonation of the ethylendiamine group [43], but the trend in the K values should be quite similar.
Table 3.
Apparent solubility (Sa), solubility factor (fS) of ETOX in micellar systems, binding constant to aggregation number ratio (K/N) for drug-saturated 10% poloxamine solutions in 0.9% NaCl, after 72 h at 25°C, and ETOX remaining solubilized (expressed as percentage referred to the initial Sa value) after 28 days of storage.
| copolymers | Sa (mg ml−1) | Sa at 28 days (%) | ETOX/polymer (mg g–1) | fS | ETOX/hydrophobic block (mg g–1) | K/N |
|---|---|---|---|---|---|---|
| T904 | 1.16 (0.11) | 94.9 (4.1) | 11.5 (1.07) | 50.68 (4.68) | 19.26 (1.78) | 3518 |
| T908 | 0.28 (0.02) | 82.6 (12.1) | 2.73 (0.21) | 11.97 (0.90) | 13.65 (1.03) | 3000 |
| T1107 | 0.61 (0.02) | 94.6 (3.1) | 6.14 (0.27) | 26.93 (1.17) | 20.46 (0.89) | 4109 |
| T1307 | 0.63 (0.07) | 90.1 (7.5) | 6.32 (0.70) | 27.70 (2.98) | 22.36 (0.03) | 5096 |
| T1107 : T1307 (50 : 50) | 0.44 (0.01) | 100.0 (5.4) | 4.37 (0.12) | 19.16 (0.50) | 14.56 (0.38) | 3255 |
| T904 : T1107 (25 : 75) | 0.65 (0.01) | 100.0 (2.4) | 6.46 (0.12) | 28.36 (0.54) | 17.24 (0.33) | 3350 |
| T904 : T1307 (25 : 75) | 0.71 (0.01) | 100.0 (3.9) | 7.10 (0.01) | 30.97 (0.04) | 18.83 (0.02) | 4057 |
| T904 : T1107 (50 : 50) | 0.85 (0.01) | 95.4 (4.0) | 8.45 (0.11) | 37.07 (0.48) | 18.78 (0.24) | 3575 |
| T904 : T1307 (50 : 50) | 0.86 (0.02) | 94.5 (6.1) | 8.57 (0.19) | 37.57 (0.82) | 19.04 (0.41) | 3812 |
| T904 : T1107 (75 : 25) | 0.80 (0.01) | 100.0 (0.3) | 7.96 (0.05) | 34.89 (0.21) | 15.15 (0.09) | 2819 |
| T904 : T1307 (75 : 25) | 0.82 (0.02) | 100.0 (5.7) | 8.16 (0.18) | 35.80 (0.80) | 15.54 (0.34) | 2925 |
Mixed micelles did not show a synergistic solubilization as previously shown with efavirenz [27], but improved the physical stability of the system with respect to ETOX-loaded T904 single micelles. Both DLS measurements of micellar size and size distribution (table 2) and spectrophotometric determination of ETOX solubilized (table 3) indicated that drug-loaded micelles are quite stable over 28 days in 0.9 per cent NaCl, remaining encapsulated 82–94% ETOX in single micelles and nearly 100 per cent ETOX in the mixed micelles. Therefore, ETOX formulations in poloxamine micelles could be envisioned as an aqueous solution with a ‘shelf life’ longer than one month. It has been previously shown that 10 per cent T904, T908, T1107 and T1307 micellar solutions enhance simvastatin solubility by factors of 8.5, 2.4, 4.7 and 21, respectively, and protect the labile lactone group from hydrolysis [21]. T904 micelles increased the solubility of the anti-HIV drug efavirenz by 7930.5-fold [27]. The solubility factors achieved for ETOX are intermediate, though closer to those of simvastatin. These findings confirm that the encapsulation ability of a specific micellar system is governed by the features of both the nanocarrier components and the drug, making predictions difficult.
Even though these solubility extents were smaller than those previously reported by Loftsson et al. [13] with 12.5 per cent hydroxypropyl-β-cyclodextrin/0.1 per cent hydroxypropyl methylcellulose, they would be appropriate for the topical treatment of IOP. Moreover, since PEO–PPO block copolymers at greater concentration forming thermo-responsive gel-like to gel matrices [44], these nanocarriers could be employed as a technology platform for the production of ETOX-loaded viscous systems where the drug is completely soluble within the micelles and the contact with the ocular mucosa is extended.
3.4. Hen's egg test-chorioallantoic membrane assay
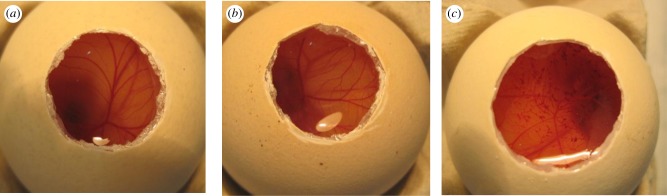
T1107 is a US Food and Drug Administration-approved component of multipurpose solutions for cleaning/storage of contact lenses usually at concentrations of 1 per cent [45]. Since poloxamine micellar solutions contain 10 per cent copolymer concentration, the micellar compatibility with eye tissues was characterized. Namely, the potential ocular irritancy was evaluated according to the hen's egg test-chorioallantoic membrane (HET-CAM) test following the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods–ICCVAM protocol [29]. The hen's embryo, more precisely the chorioallantoic membrane, is as an alternative to the Draize eye rabbits test for the evaluation of ocular formulations. The HET-CAM is a simple, fast and cheap test that provides measurable indices of the biocompatibility of a material [46,47]. The micellar systems tested did not induce haemorrhage, lysis or coagulation both before and after being loaded with ETOX (figure 1a). Thus the IS of all micelles was 0.0, as occurred for the negative control (0.9% NaCl; figure 1b). By contrast, the positive control caused an IS of 19.7 ± 0.1 (figure 1c), fulfilling the requirement for an acceptable test. Thus, this biocompatibility screening test indicates that poloxamine formulations may have good tolerance when used as ocular formulations.
Figure 1.
HET-CAM test: (a) 10% drug-loaded T904 micelles, (b) negative control (0.9% NaCl) and (c) positive control (0.1N NaOH). (Online version in colour.)
3.5. In vitro release studies
A diffusion test was carried out in order to gain an insight into the ETOX release profile from the poloxamine micelles (figure 2). Although all micellar systems provided sustained release, remarkable differences were observed depending on the variety of the poloxamine. Figures 3 and 4 show detailed plots of ETOX release profiles. In the case of the single micelles, T908 provided the fastest release (77.1% in 24 h), followed by T1107 (47.8% in 24 h), T1307 (39.6% in 24 h) and T904 (32.1% in 24 h). This behaviour indicates that the greater the hydrophilicity of the copolymers, the smaller the capacity of the micelles to retain the drug within the core. Interestingly, mixed micelles showed intermediate release rates, introducing an additional feature that enables us to fine tune the release rate. Since each formulation has a different load of ETOX, the diffusion coefficients were estimated for a more precise comparison. Thus, the Higuchi equation was applied to the first 60 per cent drug released and plotted versus the square root of time [48]:
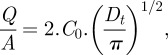 |
3.2 |
where Q/A is the amount of ETOX released per unit area, C0 is the initial ETOX concentration in the micellar system and D is the drug diffusion coefficient through the micelle. The diffusion coefficients are reported in table 4. It is interesting to note that these coefficients were greater for single T1107 and T1307 micelles than for T904 (those with the highest loading) and T908 (those with the lowest loading). These results suggest that the release profile is governed by a combination of parameters that include the micellar HLB and the ETOX concentration gradient between the micelles and the release medium. Mixed micelles of T1107 and T1307 decreased the D values from 2.3 × 10−9 to 1.6 × 0−9 cm2 s–1. In the case of mixed micelles of T904 with T1107 or T1307, the decrease in D values was even more remarkable, particularly when the content in T904 was below 50 per cent. ETOX diffusion coefficients progressively increased as the proportion of T904 in the mixed micelles was raised. These results follow an unexpected trend and would rely on a more substantial hindrance of the co-micellization process of T1107 and T1307 when greater T904 contents are used. D values of ETOX from polymeric micelles were smaller than those previously established in similar assays for acetazolamide forming part or not part of a cyclodextrin complex [13]. These findings would stem from the fact that owing to their remarkably greater hydrodynamic diameter, micelles do not cross the dialysis membrane and serve as reservoirs, making the drug release process more sustained. If applied ocularly, polymeric micelles can remain in the apical zone of the eye or slowly penetrate inside the cornea, releasing the drug over time, as previously found for other micellar carriers [49]. In other words, once formulated in poloxamine micelles, the drug could be absorbed either in its free form or encapsulated in the carrier. In addition, a more sustained release would enable a much better fine tuning of the release profile.
Figure 2.
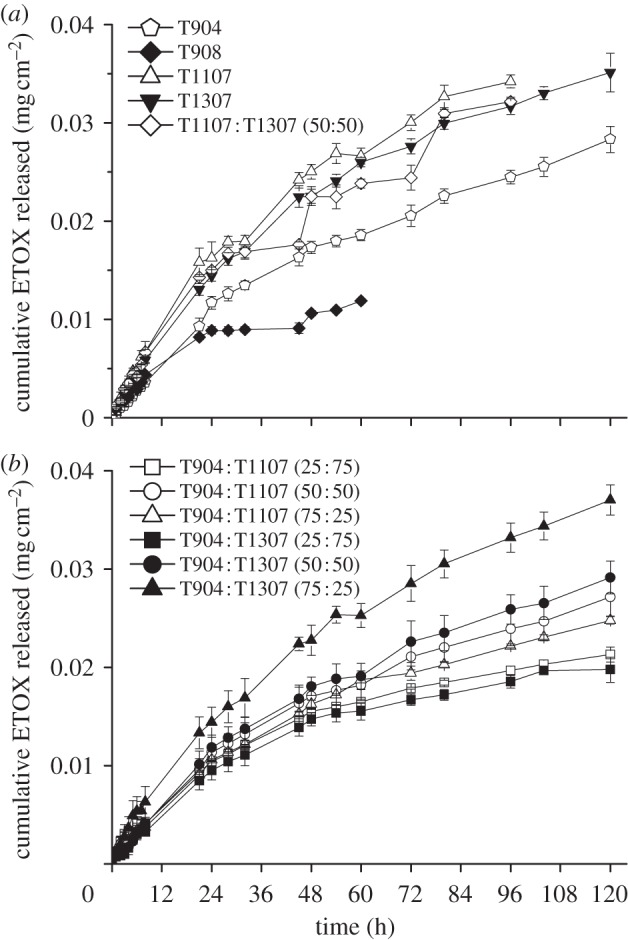
ETOX release profiles in isotonic phosphate saline buffer medium (pH 7.4) from (a) single and (b) mixed poloxamine micellar systems, at 32°C. Mean values and standard deviations (n = 3).
Figure 3.
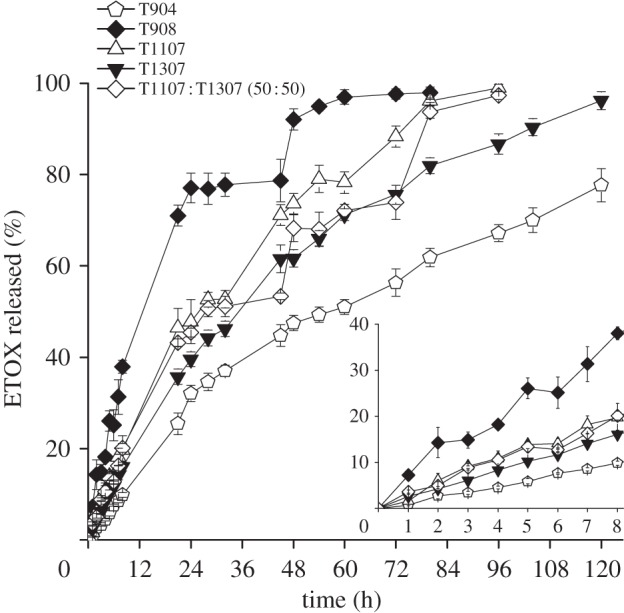
ETOX release (%) profiles in isotonic phosphate saline buffer medium (pH 7.4) from single poloxamine micelles and T1107 : T1037 mixed micelles, at 32°C. The insert shows the first 8 h release pattern.
Figure 4.
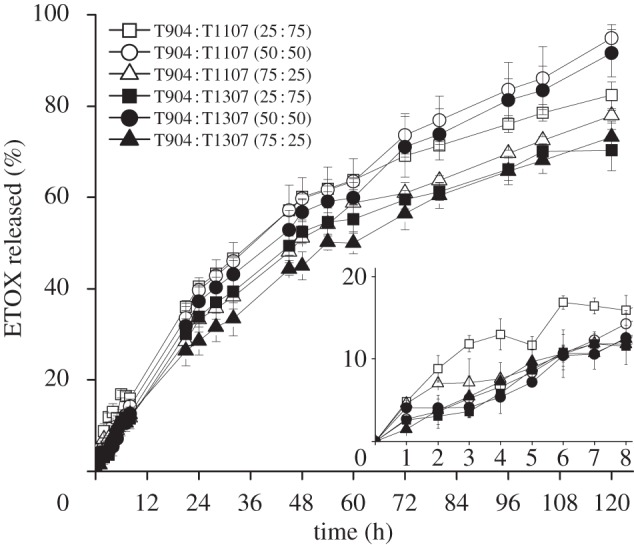
ETOX release (%) profiles in isotonic phosphate saline buffer medium (pH 7.4) from mixed T904 : T1107 and T904 : T1307 micellar formulations, at 32°C. The insert shows the first 8 h release pattern. Open squares, T904 : T1107 (25 : 75); open circles, T904 : T1107 (50 : 50); open triangles, T904 : T1107 (75 : 25); filled squares, T904 : T1307 (25 : 75); filled circles, T904 : T1307 (50 : 50); filled triangles, T904 : T1307 (75 : 25).
Table 4.
Results of diffusion coefficients (D) obtained from the Higuchi equation. (Means values, and in parentheses standards deviations (n = 3).)
| copolymers | D cm2 s–1 (×10−9) | r2 |
|---|---|---|
| T904 | 1.500 (0.106) | 0.9811 |
| T908 | 0.061 (0.006) | 0.9391 |
| T1107 | 2.330 (0.314) | 0.9533 |
| T1307 | 2.410 (0.165) | 0.9693 |
| T1107 : T1307 (50 : 50) | 1.630 (0.098) | 0.9590 |
| T904 : T1107 (25 : 75) | 0.510 (0.006) | 0.9839 |
| T904 : T1307 (25 : 75) | 0.592 (0.059) | 0.9807 |
| T904 : T1107 (50 : 50) | 0.889 (0.160) | 0.9758 |
| T904 : T1307 (50 : 50) | 1.290 (0.205) | 0.9311 |
| T904 : T1107 (75 : 25) | 0.926 (0.016) | 0.9802 |
| T904 : T1307 (75 : 25) | 4.870 (0.426) | 0.9874 |
More hydrophilic CAI drugs, such as acetazolamide, may not be so able to be loaded into micelles as ETOX is and thus it would be more difficult to achieve sustained delivery. Prolonged release on the corneal surface of hydrophilic drugs might be better achieved by means of contact lenses or hydrogel films that bear functional moieties suitable for interacting with the drug molecules [15]. Thus, compared with the biomimetic/imprinted contact lenses [15,50], poloxamine micelles may offer higher initial levels of ETOX at the lachrymal fluid; the greater concentration gradient facilitating the passing through the cornea of this high permeability drug. Subsequent application of ETOX-medicated contact lenses may enable the attainment of long-term drug levels. A future work will be to evaluate the possibilities of incorporating to the contact lenses the drug loaded into poloxamine micelles.
4. Conclusion
The encapsulation and release of ETOX from poloxamine micelles were investigated, to our knowledge, for the first time. T904 only micelles increased drug solubility up to 50 times and the combination of T904 and T1107 or T1307 provided mixed micelles with higher solubilization capability than those of T1107 or T1307 alone. Incorporation of ETOX did not modify the micellar size and size distribution and the resultant systems passed the HET-CAM ocular irritancy test. Furthermore, co-micellization of poloxamines of different hydrophilicity led to more physically stable systems that sustained ETOX release more efficiently than micelles of each single component. In summary, although an unfavourable mixing process was observed, the co-micellization of poloxamines bearing similar number of PO but different of EO units at various weight ratios improves the stability of drug-loaded micelles and enables the tuning of drug loading and release, being an useful tool to adapt the release profile to specific requirements.
Acknowledgments
Work supported by MICINN (SAF2011-22771), Xunta de Galicia (10CSA203013PR) and FEDER (Spain) and Fundação para Ciência e Tecnologia (FCT, Praxis grant SFRH/BD/40947/2007, Portugal). Authors thank ‘Red iberoamericana de nuevos materiales para el diseño de sistemas avanzados de liberación de fármacos en enfermedades de alto impacto socioeconómico’ (RIMADEL) of CYTED. BASF Corporation (Amanda Hinckley and Mark Tapio) are thanked for providing poloxamine (Tetronic) samples, respectively.
References
- 1.Supuran C. T. 2010. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 20, 3467–3474 10.1016/j.bmcl.2010.05.009 (doi:10.1016/j.bmcl.2010.05.009) [DOI] [PubMed] [Google Scholar]
- 2.Supuran C. 2008. Carbonic anhydrase: novel therapeutic applications for inhibitors and activators. Nature 7, 168–181 10.1038/nrd2467 (doi:10.1038/nrd2467) [DOI] [PubMed] [Google Scholar]
- 3.Prausnitz M. R., Noonan J. S. 1998. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharm. Sci. 87, 1479–1488 10.1021/js9802594 (doi:10.1021/js9802594) [DOI] [PubMed] [Google Scholar]
- 4.Shirasaki Y. 2008. Molecular design for enhancement of ocular penetration. J. Pharm. Sci. 97, 2462–2496 10.1002/jps.21200 (doi:10.1002/jps.21200) [DOI] [PubMed] [Google Scholar]
- 5.Loftsson T., Hreinsdôttir D. 2006. Determination of aqueous solubility by heating and equilibration: a technical note. AAPS PharmSciTech 7, E29–E32 10.1208/pt070104 (doi:10.1208/pt070104) [DOI] [PubMed] [Google Scholar]
- 6.Maren T., Jankowska L., Sanyal G., Edelhauser F. H. 1983. The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors and their effect on aqueous humor secretion. Exp. Eye Res. 36, 457–479 10.1016/0014-4835(83)90041-6 (doi:10.1016/0014-4835(83)90041-6) [DOI] [PubMed] [Google Scholar]
- 7.Eller M. G., Schoenwald R. D., Dixson J. A., Segarra T., Barfknecht C. F. 1985. Topical carbonic anhydrase inhibitors. III. Optimization model for corneal penetration of ethoxzolamide analogues. J. Pharm. Sci. 74, 155–160 10.1002/jps.2600740210 (doi:10.1002/jps.2600740210) [DOI] [PubMed] [Google Scholar]
- 8.Lippa E. A. 1991. Topical carbonic anhydrase inhibitors. In The carbonic anhydrases: cellular physiology and molecular genetics (eds Dodgson S. J., Tashian R. E., Gros G., Carter N. D.), pp. 171–181 New York, NY: /London, UK: Plenum Press [Google Scholar]
- 9.Supuran C. T., Scozzafava A., Casini A. 2003. Carbonic anhydrase inhibitors. Med. Res. Rev. 23, 146–189 10.1002/med.10025 (doi:10.1002/med.10025) [DOI] [PubMed] [Google Scholar]
- 10.Schoenwald R. D., Eller M. G., Dixson J. A., Barfknecht C. F. 1984. Topical carbonic anhydrase inhibitors. J. Med. Chem. 27, 810–812 10.1021/jm00372a020 (doi:10.1021/jm00372a020) [DOI] [PubMed] [Google Scholar]
- 11.Tous S. S., Nasser K. A. E. 1992. Acetazolamide topical formulation and ocular effect. STP Pharma. Sci. 2, 125–131 [Google Scholar]
- 12.Lewis R. A., Schoenwald R. D., Eller M. G., Barfknecht C. F., Phelps C. D. 1984. Ethoxzolamide analogue gel. A topical carbonic anhydrase inhibitor. Arch. Ophthalmol. 102, 1821–1824 [DOI] [PubMed] [Google Scholar]
- 13.Loftsson T., Fririksdóttir H., Thórisdóttir S., Stefánsson E., Sigurardóttir A. M., Gumundsson Ö., Sigthórsson T. 1994. 2-hydroxypropyl-[beta]-cyclodextrin in topical carbonic anhydrase inhibitor formulations. Eur. J. Pharm. Sci. 1, 175–180 10.1016/0928-0987(94)90001-9 (doi:10.1016/0928-0987(94)90001-9) [DOI] [Google Scholar]
- 14.Loftsson T., Frithriksdottir H., Stefansson E., Thorisdottir S., Guthmundsson O., Sigthorsson T. 1994. Topically effective ocular hypotensive acetazolamide and ethoxyzolamide formulations in rabbits. J. Pharm. Pharmacol. 46, 503–504 10.1111/j.2042-7158.1994.tb03835.x (doi:10.1111/j.2042-7158.1994.tb03835.x) [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro A., Veiga F., Santos D., Torres-Labandeira J. J., Concheiro A., Alvarez-Lorenzo C. 2011. Bioinspired imprinted PHEMA-hydrogels for ocular delivery of carbonic anhydrase inhibitor drugs. Biomacromolecules 12, 701–709 10.1021/bm101562v (doi:10.1021/bm101562v) [DOI] [PubMed] [Google Scholar]
- 16.Nagarwal R. C., Kant S., Singh P. N., Maiti P., Pandit J. K. 2009. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J. Control. Release 136, 2–13 10.1016/j.jconrel.2008.12.018 (doi:10.1016/j.jconrel.2008.12.018) [DOI] [PubMed] [Google Scholar]
- 17.Gupta A. K., Madan S., Majumdar D. K., Maitra A. 2000. Ketorolac entrapped in polymeric micelles: preparation, characterisation and ocular anti-inflammatory studies. Int. J. Pharm. 209, 1–14 10.1016/S0378-5173(00)00508-1 (doi:10.1016/S0378-5173(00)00508-1) [DOI] [PubMed] [Google Scholar]
- 18.Liaw J., Chang S. F., Hsiao F. C. 2001. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther. 8, 999–1004 10.1038/sj.gt.3301485 (doi:10.1038/sj.gt.3301485) [DOI] [PubMed] [Google Scholar]
- 19.Tong Y. C., Chang S. F., Liu C. Y., Kao W. W., Huang C. H., Liaw J. 2007. Eye drop delivery of nano-polymeric micelle formulated genes with cornea-specific promoters. J. Gene Med. 9, 956–966 10.1002/jgm.1093 (doi:10.1002/jgm.1093) [DOI] [PubMed] [Google Scholar]
- 20.Chiappetta D. A., Sosnik A. 2007. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 66, 303–317 10.1016/j.ejpb.2007.03.022 (doi:10.1016/j.ejpb.2007.03.022) [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Lopez J., Alvarez-Lorenzo C., Taboada P., Sosnik A., Sandez-Macho I., Concheiro A. 2008. Self-associative behavior and drug-solubilizing ability of poloxamine (tetronic) block copolymers. Langmuir 24, 10 688–10 697 10.1021/la8016563 (doi:10.1021/la8016563) [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Lorenzo C., Rey-Rico A., Sosnik A., Taboada P., Concheiro A. 2010. Poloxamine-based nanomaterials for drug delivery. Front. Biosci. 2, 424–440 [DOI] [PubMed] [Google Scholar]
- 23.Kabanov A. V., Batrakova E. V., Miller D. W. 2003. Pluronic® block copolymers as modulators of drug efflux transporter activity in the blood-brain barrier. Adv. Drug Deliv. Rev. 55, 151–164 10.1016/s0169-409x(02)00176-x (doi:10.1016/s0169-409x(02)00176-x) [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Lorenzo C., Rey-Rico A., Brea J., Loza M. I., Concheiro A., Sosnik A. 2010. Inhibition of P-glycoprotein pumps by PEO-PPO amphiphiles: branched versus linear derivatives. Nanomedicine UK 5, 1371–1383 10.2217/nnm.10.53 (doi:10.2217/nnm.10.53) [DOI] [PubMed] [Google Scholar]
- 25.Cuestas M. L., Sosnik A., Mathet V. L. 2011. Poloxamines display a multiple inhibitory activity of ATP-Binding Cassette (ABC) transporters in cancer cell lines. Mol. Pharmaceutics 8, 1152–1164 10.1021/mp2000132 (doi:10.1021/mp2000132) [DOI] [PubMed] [Google Scholar]
- 26.Dey S., Patel J., Anand B. S., Jain-Vakkalagadda B., Kaliki P., Pal D., Ganapathy V., Mitra A. K. 2003. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 44, 2909–2918 10.1167/iovs.02-1142 (doi:10.1167/iovs.02-1142) [DOI] [PubMed] [Google Scholar]
- 27.Chiappetta D. A., Facorro G., Rubin de Celis E., Sosnik A. 2011. Synergistic encapsulation of the anti-HIV agent efavirenz within mixed poloxamine/poloxamer polymeric micelles. Nanomedicine NBM 7, 624–637 10.1016/j.nano.2011.01.017 (doi:10.1016/j.nano.2011.01.017) [DOI] [PubMed] [Google Scholar]
- 28.Xiuli L., Jian X., Wanguo H., Dejun S. 2004. Effect of additives on the cloud points of two tri-block copolymers in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 237, 1–6 10.1016/j.colsurfa.2004.02.008 (doi:10.1016/j.colsurfa.2004.02.008) [DOI] [Google Scholar]
- 29.NICEATM-ICCVAM 2011. In vitro test methods for detecting ocular corrosives and severe irritants. See http://iccvam.niehs.nih.gov/methods/ocutox/ivocutox/ocu_brd_hetcam.htm (accessed June 2011)
- 30.Barreiro-Iglesias R., Alvarez-Lorenzo C., Concheiro A. 2003. Controlled release of estradiol solubilized in carbopol/surfactant aggregates. J. Control. Release 93, 319–330 10.1016/j.jconrel.2003.08.015 (doi:10.1016/j.jconrel.2003.08.015) [DOI] [PubMed] [Google Scholar]
- 31.Liu C., Chang K., Wang Y. 2010. A novel biodegradable amphiphilic diblock copolymers based on poly(lactic acid) and hyaluronic acid as biomaterials for drug delivery. J. Polymer Res. 17, 459–469 10.1007/s10965-009-9332-5 (doi:10.1007/s10965-009-9332-5) [DOI] [Google Scholar]
- 32.Parsaee S., Sarbolouki M. N., Parnianpour M. 2002. In vitro release of diclofenac diethylammonium from lipid-based formulations. Int. J. Pharm. 241, 185–190 10.1016/s0378-5173(02)00238-7 (doi:10.1016/s0378-5173(02)00238-7) [DOI] [PubMed] [Google Scholar]
- 33.Wei Z., Hao J., Yuan S., Li Y., Juan W., Sha X., Fang X. 2009. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int. J. Pharm. 376, 176–185 10.1016/j.ijpharm.2009.04.030 (doi:10.1016/j.ijpharm.2009.04.030) [DOI] [PubMed] [Google Scholar]
- 34.Li L., Tan Y. B. 2008. Preparation and properties of mixed micelles made of Pluronic polymer and PEG-PE. J. Colloid Interface Sci. 317, 326–331 10.1016/j.jcis.2007.09.053 (doi:10.1016/j.jcis.2007.09.053) [DOI] [PubMed] [Google Scholar]
- 35.Chiappetta D. A., Hocht C., Taira C., Sosnik A. 2010. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability. Nanomedicine UK 5, 11–23 10.2217/nnm.09.90 (doi:10.2217/nnm.09.90) [DOI] [PubMed] [Google Scholar]
- 36.Chiappetta D. A., Hocht C., Sosnik A. 2010. A highly concentrated and taste-improved aqueous formulation of efavirenz for a more appropriate pediatric management of the anti-HIV therapy. Curr. HIV Res. 8, 223–231 [DOI] [PubMed] [Google Scholar]
- 37.Parekh P., Singh K., Marangoni D. G., Bahadur P. 2011. Micellization and solubilization of a model hydrophobic drug nimesulide in aqueous salt solutions of Tetronic T904. Colloids Surf. B Biointerfaces 83, 69–77 10.1016/j.colsurfb.2010.10.046 (doi:10.1016/j.colsurfb.2010.10.046) [DOI] [PubMed] [Google Scholar]
- 38.Kadam Y., Singh K., Marangoni D. G., Ma J. H., Aswal V. K., Bahadur P. 2010. Thermodynamic of micelle formation of nonlinear block co-polymer Tetronic® T904 in aqueous salt solution. Colloids Surf. A Physicochem. Eng. Asp. 369, 121–127 10.1016/j.colsurfa.2010.08.010 (doi:10.1016/j.colsurfa.2010.08.010) [DOI] [Google Scholar]
- 39.Chiappetta D. A., Alvarez-Lorenzo C., Rey-Rico A., Taboada P., Concheiro A., Sosnik A. 2010. N-alkylation of poloxamines modulates micellar assembly and encapsulation and release of the antiretroviral efavirenz. Eur. J. Pharm. Biopharm. 76, 24–37 10.1016/j.ejpb.2010.05.007 (doi:10.1016/j.ejpb.2010.05.007) [DOI] [PubMed] [Google Scholar]
- 40.Clint J. H. 1975. Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 1 71, 1327–1334 10.1039/F19757101327 (doi:10.1039/F19757101327) [DOI] [Google Scholar]
- 41.Silva R. C. d., Loh W. 1998. Effect of additives on the cloud points of aqueous solutions of ethylene oxide-propylene oxide-ethylene oxide block copolymers. J. Colloid Interface Sci. 202, 385–390 10.1006/jcis.1998.5456 (doi:10.1006/jcis.1998.5456) [DOI] [Google Scholar]
- 42.Chakraborty S., Shukla D., Jain A., Mishra B., Singh S. 2009. Assessment of solubilization characteristics of different surfactants for carvedilol phosphate as a function of pH. J. Colloid Interface Sci. 335, 242–249 10.1016/j.jcis.2009.03.047 (doi:10.1016/j.jcis.2009.03.047) [DOI] [PubMed] [Google Scholar]
- 43.Alvarez-Lorenzo C., Gonzalez-Lopez J., Fernandez-Tarrio M., Sandez-Macho I., Concheiro A. 2007. Tetronic micellization, gelation and drug solubilization: influence of pH and ionic strength. Eur. J. Pharm. Biopharm. 66, 244–252 10.1016/j.ejpb.2006.10.010 (doi:10.1016/j.ejpb.2006.10.010) [DOI] [PubMed] [Google Scholar]
- 44.Alvarez-Lorenzo C., Concheiro A., Sosnik A. 2009. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles and gels in drug delivery: state-of-the-art and future perspectives. In Handbook of hydrogels: properties, preparation and applications (ed. Stein D. B.), pp. 449–484 Hauppauge, NY: Nova Publishers [Google Scholar]
- 45.Tonge S., Jones L., Goodall S., Tighe B. 2001. The ex vivo wettability of soft contact lenses. Curr. Eye Res. 23, 51–59 [DOI] [PubMed] [Google Scholar]
- 46.Valdes T. I., Kreutzer D., Moussy F. 2002. The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials. J. Biomed. Mater. Res. 62, 273–282 10.1002/jbm.10152 (doi:10.1002/jbm.10152) [DOI] [PubMed] [Google Scholar]
- 47.Cazedey E. C. L., Carvalho F. C., Fiorentino F. A. M., Gremião M. P. D., Salgado H. R. N. 2009. Corrositex®, BCOP and HET-CAM as alternative methods to animal experimentation. Braz. J. Pharm. Sci. 45, 759–766 10.1590/S1984-82502009000400021 (doi:10.1590/S1984-82502009000400021) [DOI] [Google Scholar]
- 48.Stehle R. G., Higuchi W. I. 1967. Diffusional model for transport rate studies across membranes. J. Pharm. Sci. 56, 1367–1368 10.1002/jps.2600561040 (doi:10.1002/jps.2600561040) [DOI] [PubMed] [Google Scholar]
- 49.Di Tommaso C., Torriglia A., Furrer P., Behar-Cohen F., Gurny R., Möller M. 2011. Ocular biocompatibility of novel cyclosporin a formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) micelle carriers. Int. J. Pham. 416, 515–524 10.1016/j.ijpharm.2011.01.004 (doi:10.1016/j.ijpharm.2011.01.004) [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro A., Veiga F., Santos D., Torres-Labandeira J. J., Concheiro A., Alvarez-Lorenzo C. 2011. Receptor-based biomimetic NVP/DMA contact lenses for loading/eluting carbonic anhydrase inhibitors. J. Membr. Sci. 383, 60–69 10.1016/j.memsci.2011.08.030 (doi:10.1016/j.memsci.2011.08.030) [DOI] [Google Scholar]