Abstract
In order to elucidate the main factor governing the capacity for apatite formation of titanium (Ti), Ti was exposed to HCl or NaOH solutions with different pH values ranging from approximately 0 to 14 and then heat-treated at 600°C. Apatite formed on the metal surface in a simulated body fluid, when Ti was exposed to solutions with a pH less than 1.1 or higher than 13.6, while no apatite formed upon exposure to solutions with an intermediate pH value. The apatite formation on Ti exposed to strongly acidic or alkaline solutions is attributed to the magnitude of the positive or negative surface charge, respectively, while the absence of apatite formation at an intermediate pH is attributed to its neutral surface charge. The positive or negative surface charge was produced by the effect of either the acidic or alkaline ions on Ti, respectively. It is predicted from the present results that the bone bonding of Ti depends upon the pH of the solution to which it is exposed, i.e. Ti forms a bone-like apatite on its surface in the living body and bonds to living bone through the apatite layer upon heat treatment after exposure to a strongly acidic or alkaline solution.
Keywords: titanium, NaOH or HCl treatments, apatite-forming ability, simulated body fluid, zeta potential, X-ray photoelectron spectroscopy
1. Introduction
Titanium (Ti) and its alloys are widely used in various types of implants in orthopaedic and dental fields, because of their good biocompatibility and high mechanical strength [1]. It is reported that they could have bonded to living bone after long-term implantation. However, they do not always bond to living bone, especially in a short period of time after implantation [2] and hence, their fixation in the living body is not always stable. Fast and reliable bonding to the bone is desirable for orthopaedic and dental implants. Various attempts have been made to induce bone bonding by techniques such as ion implantation [3–5], electrochemical [6–10] and hydrothermal treatment [11–15]. Certain chemical and heat treatments have also been attempted [16–21]. Improvement in bone bonding has been reportedly induced by these treatments. However, some reports have attributed the improvement to the surface roughness increased by the treatments, while others have pointed to the formation of specific crystalline phases such as anatase and rutile induced by the treatments. There is thus inadequate consistency in these interpretations, and the purpose of the present paper is to inquire into the main factor governing the bone-bonding properties of Ti.
We previously reported that Ti and its alloys spontaneously tightly bond to living bone when soaked in NaOH solution and then subjected to heat treatment [22–24]. These treatments have been applied to a porous Ti metal layer of an artificial hip joint, and the resulting bioactive hip joint has been used clinically in Japan since 2007 [25]. On the other hand, it was also shown that Ti spontaneously bonds to living bone when soaked in a H2SO4/HCl mixed acid solution and then subjected to heat treatment [26]. The bonding of the Ti subjected to NaOH and acid treatments to living bone has been attributed to surface apatite formation in the living body [24,26]. However, the dependence of this apatite formation on Ti metal upon the pH of the exposed solution has not been reported.
In the present study, Ti was exposed to simple aqueous solutions in which the pH was systematically changed from approximately 0 to 14 by HCl and NaOH solutions, and then subjected to a heat treatment. Surface apatite formation was examined in a simulated body fluid (SBF) using an ion concentration almost equal to that in human blood plasma [27], in comparison with those on Ti prior to heat treatment. The dependence of their apatite-forming ability on the pH of the solution is discussed in terms of surface roughness, the kind of the surface crystalline phase and the surface charges.
It has been shown that various kinds of bone-bonding bioactive ceramics have the capacity to form a surface apatite layer in SBF and to bond to living bone through this surface apatite layer in vivo [27]. The bone-bonding properties of Ti are discussed in terms of apatite-forming ability.
2. Material and methods
2.1. Preparation of the samples
Commercially pure Ti (Grade no. 2; Kobe Steel, Ltd, Japan) was cut into rectangular samples having the dimensions of 10 × 10 × 1 mm3, abraded with a no. 400 diamond plate, washed with acetone, 2-propanol and ultra pure water for 30 min each in an ultrasonic cleaner and then dried overnight in an oven at 40°C. Each specimen was soaked in 5 ml of HCl or NaOH solution at concentrations ranging from 0.5 mM to 5 M and water at 60°C in an oil bath, shaken at 120 strokes min−1 for 24 h, and then washed with flowed ultra-pure water for 30 s. The washing time was carefully selected as the shortest time when any precipitations from surplus NaOH solution could not be detected on the specimen treated with the NaOH solution. The pH values of the solutions are given in table 1. The specimens removed from the solutions were heated to 600°C at a rate of 5°C min−1 in an Fe–Cr electric furnace under an ambient atmosphere, maintained at this temperature for 1 h and then cooled naturally to room temperature in the furnace.
Table 1.
pH of exposed solution.
| solution | HCl |
water | NaOH |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 M | 1 M | 100 mM | 0.5 mM | 0.5 mM | 100 mM | 1 M | 5 M | ||
| pH | approx. 0 | 0.1 | 1.1 | 3.4 | 6–7 | 10.8 | 12.9 | 13.6 | approx. 14 |
2.2. Surface analysis of the treated Ti metals
The surface of the Ti specimens treated as described in §2.1 was analysed using thin-film X-ray diffraction (TF-XRD, RINT-2500, Rigaku Co., Japan). The X-ray source was CuKα, and the angle of the incident beam was set to 1° against the sample surface.
The same surface was coated with a Pt/Pd film and observed under field emission scanning electron microscopy (FE-SEM, Hitachi S-4300, Hitachi, Japan).
The depth profiles of various elements near the surface of the Ti specimens subjected to the acid and heat treatments were analysed using radio frequency (RF) glow discharge optical emission spectroscopy (GD-OES, GD-Profiler 2, Horiba Co., Japan) under Ar sputtering at an Ar pressure of 600 Pa. An RF electric field with a power of 35 W was applied at a regular interval of 20 ms.
Ti plates having the dimensions of 13 × 33 × 1 mm3 were prepared for measurement of the zeta potential, where the volume of the NaOH and HCl solutions and water for the surface treatments was increased to 20 ml. The Ti specimens soaked in the solutions and then heat-treated were electrically grounded to discharge any stray charges, and were immediately placed in the zeta potential and particle size analyser (ELS-Z1, Otsuka Electronics Co., Japan) using a glass cell for the plate sample. The zeta potential of the specimens was measured under an applied voltage of 40 V in 10 or 50 mM NaCl solution dispersing monitor particles of polystyrene latex particles (diameter = 500 nm) coated with hydroxyl propyl cellulose. When 40 V of the voltage was applied, the flow of electro-osmosis depending on the surface charge of the specimens was generated. The zeta potential was calculated from the distribution of the flow of electro-osmosis by monitoring the migration velocity of the monitor particles. Five samples were measured for each experimental condition, and the average value was used in the analysis.
2.3. Examination of the apatite formation in an simulated body fluid
The Ti specimens treated as described earlier were soaked in 30 ml of an acellular SBF at 36.5°C with ion concentrations (Na+ = 142.0, K+ = 5.0, Mg2+ = 1.5, Ca2+ = 2.5, Cl– = 147.8, HCO3– = 4.2, HPO42– = 1.0, and SO42– = 0.5 mM) nearly equal to those in human blood plasma. The SBF was prepared by dissolving reagent-grade NaCl, NaHCO3, KCl, K2HPO4 · 3H2O, MgCl2 · 6H2O, CaCl2 and Na2SO4 (Nacalai Tesque Inc., Japan) in ultra-pure water, and buffered at pH = 7.40 using tris (hydroxymethyl) aminomethane [(CH2OH)3CNH2] and 1 M HCl (Nacalai Tesque Inc.) [27].
After soaking in the SBF for 3 days, the surface was analysed for apatite formation with TF-XRD and FE-SEM using the methods described in §2.2.
The surfaces of the Ti specimens treated as described in §2.1 and soaked in SBF for various periods were analysed using X-ray photoelectron spectroscopy (XPS, ESCA-3300KM, Shimadzu Co., Japan), with MgKα radiation (λ = 9.8903 Å) as the X-ray source. The XPS take-off angle was set at 45°, which enabled the system to detect photoelectrons to a depth of 5–10 nm from the surface of the substrate. The binding energy of the measured spectra was calibrated by reference to the C1S peak of the surfactant CH2 groups on the substrate occurring at 284.6 eV.
3. Results
3.1. Surface structure of the treated Ti metal
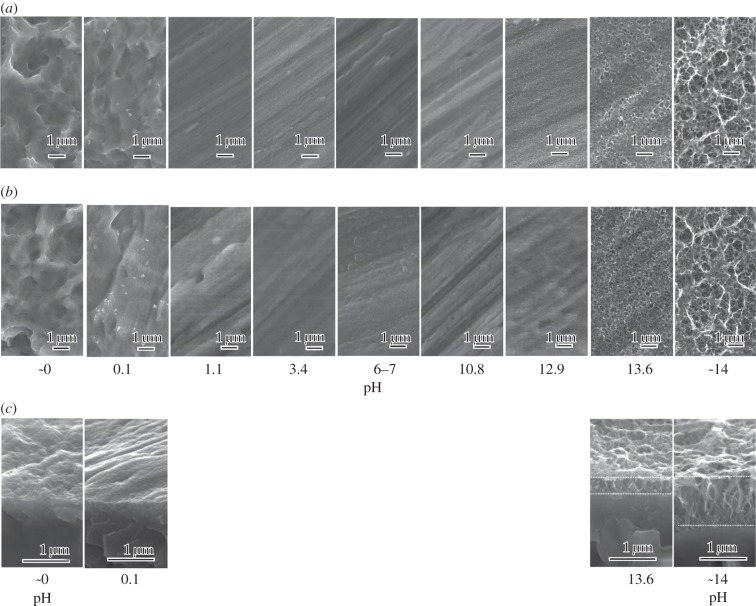
Figure 1 shows the FE-SEM micrographs of the surface of Ti specimens exposed to solutions with the different pHs, and also those that were subsequently heat-treated. Cross-sectional photographs of the Ti specimens exposed to solutions with the low or high pHs and subsequently heat-treated are also shown. It can be seen from figure 1 that a ripple-like micrometre scale roughness was formed on the Ti surface by exposure to solutions with a pH lower than 0.1, while a nanometre scale roughness consisting of a network of feather-like phases that were elongated perpendicularly to the surface was formed on the Ti surface by exposure to solutions with a pH higher than 13.6. The Ti surface morphology was not changed by exposure to solutions with intermediate pHs ranging from 1.1 to 12.9. None of these topographies were essentially changed by the subsequent heat treatment.
Figure 1.
(a) Before and (b) after heat treatment. FE-SEM photographs of surfaces of Ti as exposed to solutions with different pHs, and those subsequently subjected to heat treatment. (c) Cross-sectional photographs of Ti metals exposed to solutions with low and high pHs and subsequently heat-treated are given at the bottom.
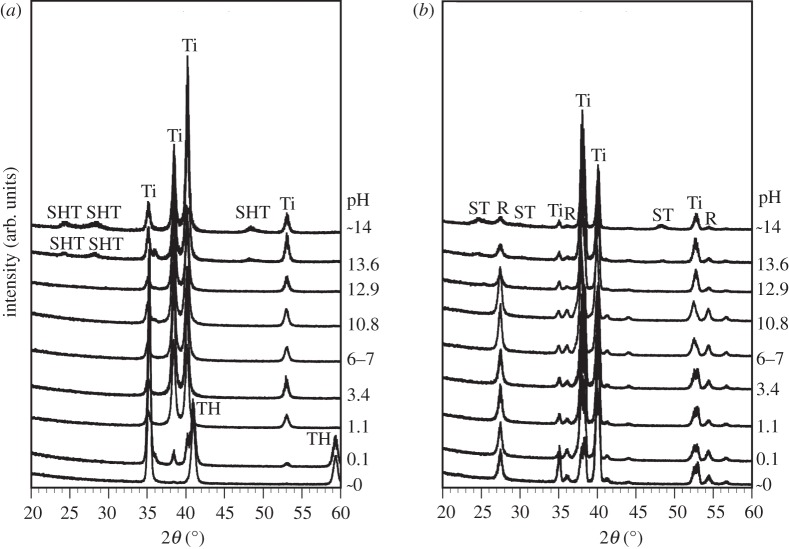
Figure 2 shows TF-XRD patterns of the surfaces of the Ti specimens exposed to solutions with the different pHs, and also those that were subsequently heat-treated. It can be seen from figure 2 that titanium hydride (TH; TiHx) [26] or sodium hydrogen titanate (SHT; NaxH2–xTi3O7, 0 < x < 2) [28] formed on the surface of Ti when treated with solutions of pH values lower than 0.1 or higher than 13.6, respectively. After the subsequent heat treatment, all the Ti specimens formed a titanium oxide of rutile phase irrespective of the pH of the solution. Only the pH values higher than 13.6 resulted in the formation of sodium titanate (Na2Ti6O13) in addition to rutile after the heat treatment.
Figure 2.
(a) Before and (b) after heat treatment. TF-XRD patterns of surfaces of Ti as exposed to solutions with different pHs, and those subsequently subjected to heat treatment. Ti, α Ti; TH, titanium hydride; SHT, sodium hydrogen titanate; ST, sodium titanate; R, rutile.
3.2. Zeta potential of the treated Ti metal
The zeta potential of a metal specimen with no electrically insulating oxide layer or one that was too thin cannot be measured by the present method, and hence the Ti specimens as exposed to the solutions could not be measured, because no or only a very thin oxide layer exists on their surfaces. This suggests that their zeta potentials are almost zero.
The zeta potentials of the Ti specimens subjected to the heat treatment after exposure to the solutions is shown in figure 3 as a function of the solution pH. Ti specimens exposed to solutions with a pH lower than 1.1 displayed a positive zeta potential higher than 5 mV, whereas those exposed to solutions with a pH higher than 13.6 displayed a negative zeta potential less than −10 mV. Those exposed to solutions with intermediate pH values that ranged from 3.4 to 12.9 had a zeta potential of approximately zero.
Figure 3.
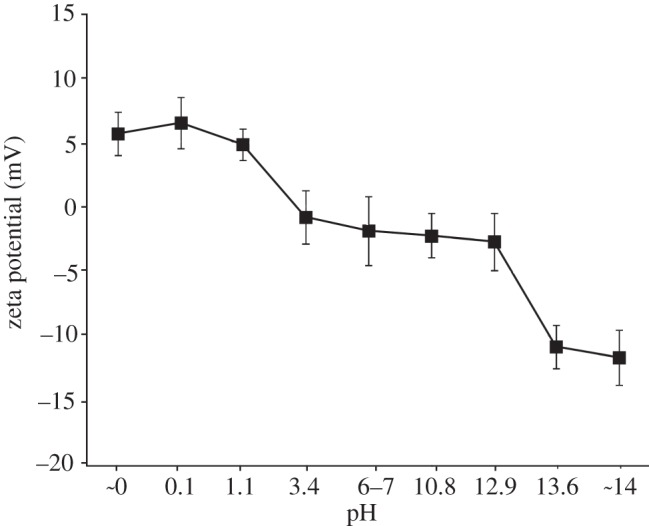
Zeta potentials of Ti as exposed to solutions with different pHs and subsequently subjected to heat treatment.
3.3. Distribution of elements near the surface
Figure 4 shows the depth profile of the GD-OES spectra of Ti specimens exposed to the solution with a pH ∼ 0 and also those that were subsequently subjected to heat treatment, as a function of sputtering time. Large amounts of H and O besides Ti were detected in the surface layer of the specimen exposed to the acid solution because of the formation of TH and titanium oxide by the acid treatment. It should be noted here that a small amount of Cl was also detected on its surface layer. The results show that H was remarkably reduced, while O was increased by the heat treatment because of transformation of the TH to titanium oxide. It should be noted here once again that a small amount of Cl was detected even after the heat treatment.
Figure 4.
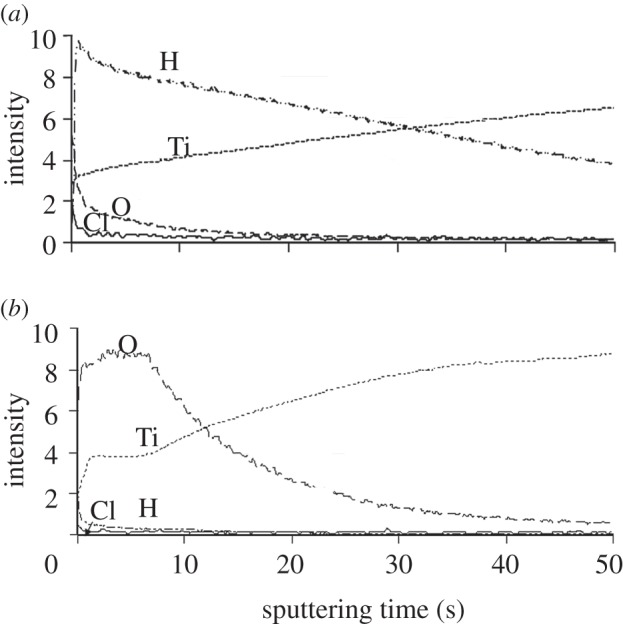
(a) Before and (b) after heat treatment. Depth profile of GD-OES spectra of surfaces of Ti as-exposed to the solutions with pH ∼ 0 and that subsequently subjected to heat treatment, as a function of sputtering time.
Depth profiles of elements near the surfaces of Ti specimens exposed to the NaOH solution with pH ∼ 14 and those that were subsequently subjected to heat treatment were previously examined by Auger electron spectroscopy and already published [29]. According to the result, Na and O penetrated into the Ti specimen up to a depth of about 1 μm as a result of the NaOH treatment and their concentrations gradually decreased with increasing depth. Only O penetrated into a deeper region, while Na did not show any change in its distribution by the subsequent heat treatment.
3.4. Apatite-forming ability of treated Ti metal in simulated body fluid
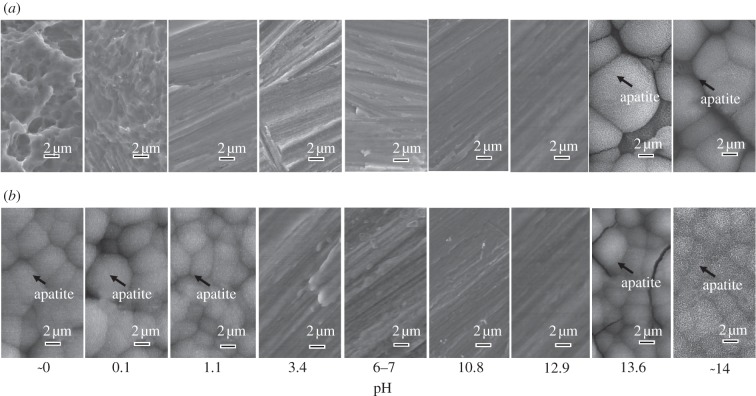
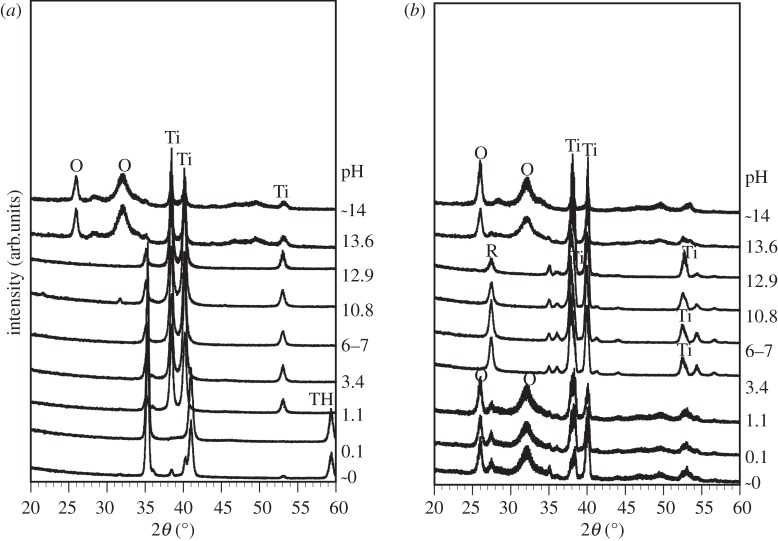
The FE-SEM micrographs in figure 5 show the surface of the Ti specimens soaked in SBF for 3 days after exposure to solutions with different pHs, and also those after the subsequent heat treatment. The TF-XRD patterns in figure 6 are of the surfaces of the Ti specimens soaked in SBF for 3 days after exposure to the solutions with the different pHs, and also those after the subsequent heat treatment. The spherical particles observed in the FE-SEM micrographs of the Ti surfaces were identified as crystalline apatite from the XRD patterns in figure 6. The Ti specimens as exposed to the solutions did not form the apatite on their surfaces except in the case of the solutions with pHs higher than 13.6, where only a relatively small amount of apatite was formed. In contrast, the Ti specimens after the heat treatment exhibited a large amount of apatite on their surfaces when they were exposed to solutions with a pH lower than 1.1 or higher than 13.6. Ti specimens exposed to solutions with an intermediate pH that ranged from 3.4 to 12.9 did not form any apatite, even after the heat treatment.
Figure 5.
(a) Before and (b) after heat treatment. FE-SEM photographs of surfaces of Ti soaked in SBF for 3 days after exposure to solutions with different pHs, and those after subsequent heat treatment.
Figure 6.
(a) Before and (b) after heat treatment. TF-XRD patterns of surfaces of Ti soaked in SBF for 3 days after exposure to solution with different pHs, and those after subsequent heat treatment, Ti, α Ti; TH, titanium hydride; R, rutile; O, apatite.
3.5. X-ray photoelectron spectroscopy spectra of the treated Ti metal
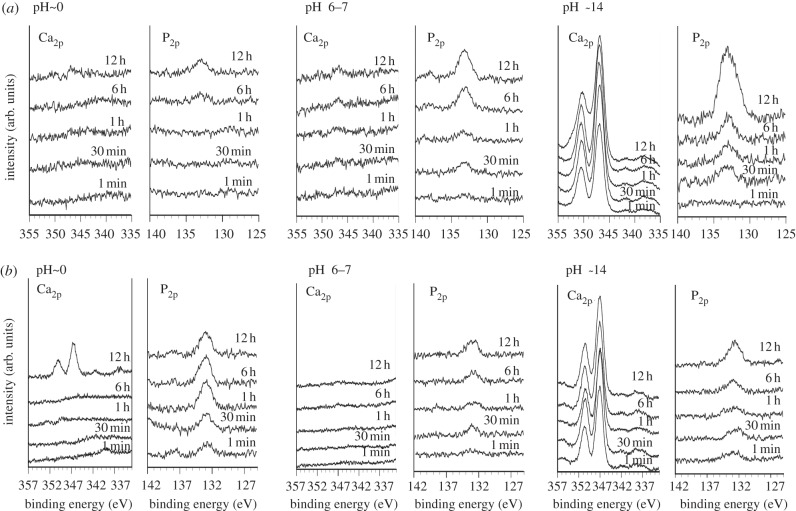
Figure 7 shows the Ca2P and P2P XPS spectra of the Ti surfaces exposed to solutions of pH values of approximately 0, 6–7 and 14 and those subsequently heat-treated as a function of soaking time in SBF. It can be seen that Ti specimens before heat treatment adsorb a small amount of the calcium and phosphate ions almost simultaneously, independent of pH, except in cases of a high pH, where it first selectively adsorbs the calcium ions, and then the phosphate ions.
Figure 7.
(a) XPS spectra of surfaces of Ti as exposed to solutions with pHs approximately 0, 6–7 and 14, and (b) those subsequent heat-treated as a function of soaking time in SBF.
In contrast, Ti specimens exposed to a low pH solution and subsequently heat-treated first selectively adsorb phosphate ions and then the calcium ions, whereas those exposed to a high pH and subsequently heat-treated do the opposite, i.e. first selectively adsorbing calcium ions and then phosphate ions. Ti specimens exposed to an intermediate pH adsorb a small amount of the calcium and phosphate ions almost simultaneously, even after the heat treatment.
4. Discussion
It is apparent from figures 5 and 6 that the Ti specimens did not form surface apatite in SBF when exposed to the solutions, except when it was exposed to a strongly alkaline solution, which resulted in a small amount of apatite formation, whereas those that were subsequently heat-treated exhibited a large amount of apatite after exposure to strongly acidic or alkaline solutions.
Micrometre or nanometre scale roughness was produced on Ti specimens when they were exposed to strongly acidic or alkaline solutions, and this was not changed by the subsequent heat treatment, as shown in figure 1. This indicates that the surface morphology is not responsible for the increased apatite formation induced by the heat treatment. It should be emphasized that even the highly porous surface formed by the NaOH treatment was not responsible for apatite formation, because the highly porous surface was apparently not changed by the subsequent heat treatment, whereas the apatite formation was remarkably increased by the heat treatment, as shown by higher density of the apatite for heat-treated specimen on FE-SEM picture in figure 5.
TH or SHT was precipitated on Ti specimens when they were exposed to strongly acidic or alkaline solutions, and the precipitate was transformed into rutile or sodium titanate accompanied by rutile upon subsequent heat treatment (figure 2). This suggests that the rutile and sodium titanate are responsible for the increased apatite formation induced by the heat treatment. However, rutile was also detected on heat-treated Ti specimens after exposure to solutions with intermediate pH values (figure 2), which did not form the apatite. This indicates that the composition and structure of the rutile phase is not responsible for the increase in apatite formation induced by the heat treatment.
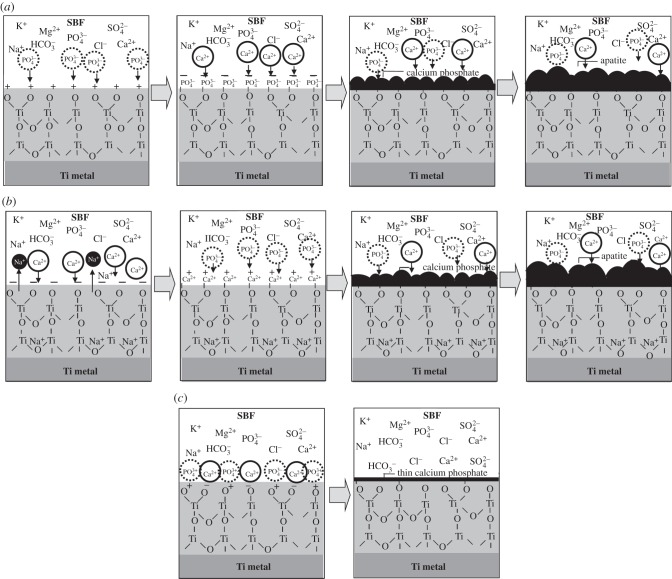
The Ti specimens exposed to the solutions displayed a zeta potential of approximately zero, independent of the pH of the exposed solution, whereas those that were subsequently heat-treated displayed a certain level of positive or negative zeta potential when they were exposed to a strongly acidic or alkaline solution, respectively. This indicates that the certain level of the positive or negative surface charges is responsible for the apatite formation induced by the heat treatment. It is suggested that the positively charged surface first selectively adsorbs the negatively charged phosphate ions on its surface. As the phosphate ions accumulate, the surface becomes negatively charged. As a result, the positively charged calcium ions are adsorbed on its surface so as to produce a calcium phosphate which is eventually transformed into apatite, as shown in figure 8a. On the other hand, the negatively charged surface is assumed to first selectively adsorb positively charged calcium ions and then the negatively charged phosphate ions so as to also form apatite, as shown in figure 8b.
Figure 8.
Schematic of ion adsorption on (a) positively charged, (b) negatively charged and (c) neutrally charged Ti metal.
It is shown by the XPS spectra in figure 7 that the former type of phosphate and calcium ion adsorption occurred on heat-treated Ti specimens after exposure to strongly acidic solutions, while the latter type of sequential adsorption occurred on heat-treated Ti specimens after the strongly alkaline solutions.
The Ti specimen that was heat-treated after exposure to solutions with intermediate pH values exhibited a zeta potential of almost zero (figure 3). This means that the positively and negatively charged sites are balanced and hence the surface is neutrally charged [30]. It is assumed that each Ti site is able to simultaneously adsorb negatively charged phosphate ions and positively charged calcium ions, respectively, as shown in figure 8c. As a result, a thin calcium phosphate layer is formed on their surfaces and their charges are soon neutralized, and hence the calcium phosphate layer does not grow into the thick apatite layer in a short period of time. It is also evident from the XPS spectra in figure 7 that this type of simultaneous adsorption of the small amount of the phosphate and calcium ions occurred on Ti specimens that were heat-treated after exposure to solutions with intermediate pH value. However, the calcium phosphate layer was so thin that it was not apparently observed under FE-SEM with the resolution of several nanometres in figure 5, and precipitation of apatite on the specimens was not detected by TF-XRD patterns in figure 6. The apatite formation on heat-treated Ti specimens after strong acid or alkali solutions is thus attributed to their positive or negative surface charges, respectively.
The reason why Ti specimens that are heat-treated after exposure to the strong acid solutions are charged positively may be due to the titanium oxide adsorbed with the Cl− ions on their surfaces. It is assumed that the Cl− ions were adsorbed on the TH on the surface of the Ti specimens when they were exposed to the strong acid solutions, and they remained on the titanium oxide that was formed on the Ti specimens by the subsequent heat treatment. The adsorption of the Cl− ions on the TH on the Ti specimens exposed to the acid solutions and the adsorption of those on the titanium oxide on Ti specimens that were subsequently heat-treated were demonstrated by the GD-OES spectra shown in figure 4. These adsorbed Cl− ions may become dissociated via exchange with OH− ions in SBF so as to give rise to an acidic environment. It has been reported by Textor et al. [30] that titanium oxide is positively charged by forming larger numbers of Ti-OH2+ groups in acidic solutions. A recent report has shown that the positive charge and apatite formation on the Ti metal can be induced by not only Cl−, but also other acid radicals such as NO3− and SO42− [31].
The reason why Ti specimens that are heat-treated after exposure to strongly alkaline solutions are charged negatively may be due to the sodium titanate on their surfaces. The sodium titanate releases its Na+ ions via exchange with the H3O+ ions in SBF to form Ti-OH groups on its surface [32–35] by the following reaction
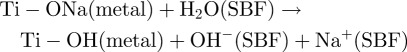 |
This increases the pH of the surrounding SBF because of the consumption of H3O+ ions. It has been reported by Textor et al. [30] that titanium oxide is negatively charged by forming Ti-O− groups in alkaline solution.
In the case of the Ti specimens prior to heat treatment, they became covered with a thin titanium oxide layer when they were exposed to the solutions with intermediate pH values, similar to the untreated Ti specimen. This titanium oxide layer has an almost neutral surface charge (figure 3) and hence does not induce the apatite formation. They did form TH on their surfaces when they were exposed to the strongly acidic solutions. This phase is electrically conductive [36]. Therefore, their surfaces are hardly charged even though they adsorbed Cl− ions, and hence did not induce the apatite formation.
Ti specimens formed an SHT on their surfaces upon exposure to strongly alkaline solutions. The SHT also releases its Na+ ions via exchange with H3O+ ions in SBF to form negatively charged Ti-OH groups [37]. However, SHT is electrically conductive, and hence its negative surface charge is so small that it cannot be measured by the present method. Therefore, Ti specimens exposed to the strongly alkaline solution induced apatite formation, but its magnitude was smaller than that of the Ti specimen that was subsequently heat-treated, as shown in figure 5. It is apparent from these findings that the Ti specimen that is heat-treated after exposure to a strongly acidic or alkaline solution can induce surface apatite formation in SBF, because of its large positive or negative surface charges.
It has been shown for various kinds of ceramics, including glasses, glass-ceramics and sintered crystalline ceramics of various compositions, that materials that have the capacity to form a surface apatite layer in the living body are able to bond to living bone through the apatite layer [27], and are useful as long as they contain neither cytotoxic nor antigenic components, and furthermore, their apatite formation can be reproduced even in an acellular SBF having ion concentrations almost equal to those of human blood plasma [27]. Bohner et al. [38] and Pan et al. [39] recently pointed out that certain resorbable ceramics bond to living bone without any formation of a surface apatite layer. These exceptional cases were also pointed out in the paper described earlier [27]. This means that the rule described earlier should be applied with caution in the case of resorbable materials. However, Ti is not a resorbable material, and contains neither cytotoxic nor antigenic components. It has already been shown that Ti and various kinds of Ti-based alloys (such as Ti–6Al–4V, Ti–15Mo–5Zr–3Al and Ti–15Zr–4Nb–4Ta) that are able to form apatite on their surfaces in SBF, bond to living bone through the apatite layer formed on their surfaces in the living body [23,24,26,40–47].
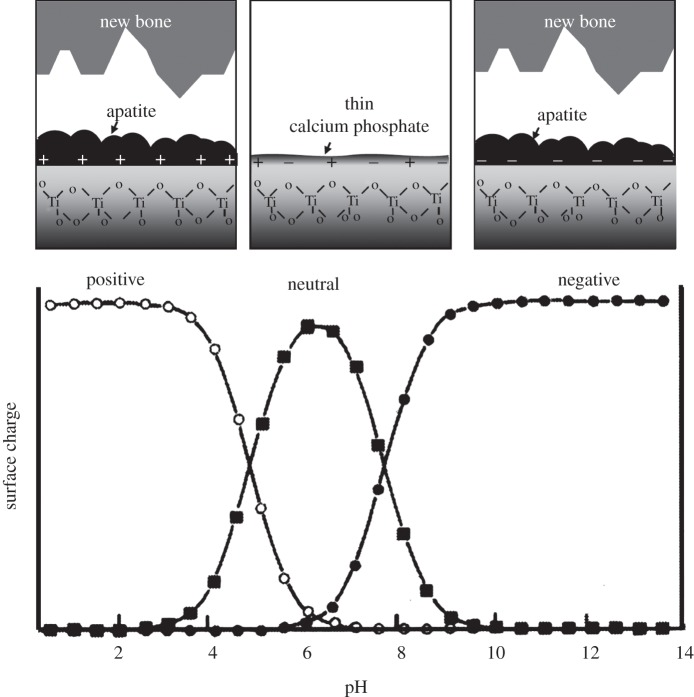
In view of these findings, it is predicted from the present results that Ti forms a bone-like apatite surface layer in the living body and bonds to living bone through the apatite layer upon exposure to a strongly acidic or alkaline solution and then heat-treated, because its surface is then either positively or negatively charged in the living body. In contrast with this, Ti exposed to solutions with intermediate pH values and subsequently subjected to the heat treatment forms only a thin amorphous calcium phosphate layer in the living body, and hence possesses a good compatibility with the bone but does not bond to bone, because the surface is almost neutrally charged, as shown in figure 9. The Ti not subjected to heat treatment after the solution treatment displayed little or no surface apatite formation in the living body, and hence do not bond to living bone, because their surfaces are almost entirely neutrally charged.
Figure 9.
Apatite formation and bone-bonding of Ti as a function of pH of exposed solution. Lower part of this figure was reproduced from Textor et al. [30] after modification.
5. Conclusion
Ti specimens that were heat-treated after exposure to strong acid or alkali solutions showed remarkable apatite formation on their surfaces in SBF, while those heat-treated after exposure to solutions with intermediate pHs did not show any such apatite formation.
The remarkable apatite formation of Ti exposed to the strongly acidic and alkaline solutions is attributed to the magnitude of their positive and negative surface charges, respectively. The positive and negative surface charges are produced by the dissociated chloride ions and the released sodium ions, respectively. The lack of apatite formation on the Ti exposed to intermediate pH solutions is attributed to their neutral surface charges. Ti that were not subjected to heat treatment after the solutions showed no or only a little apatite formation on their surfaces in SBF because of their neutral surface charge.
It is predicted from the present results that the bone-bonding property of Ti strongly depends upon the pH of the solution used, i.e. Ti forms bone-like apatite on its surface in the living body and bonds to living bone through the apatite layer, if it is heat-treated after exposure to a strongly acidic or alkaline solution.
References
- 1.Brunette D. M., Tengvall P., Textor M., Thomsen P. 2001. Titanium in medicine. Berlin, Germany: Springer [Google Scholar]
- 2.Yan W. Q., Nakamura T., Kobayashi M., Kim H. M., Miyaji F., Kokubo T. 1997. Bonding of chemically treated titanium implants to bone. J. Biomed. Mater. Res. 37, 267–275 10.1002/(SICI)1097-4636(199711)37:2,267::AID-JBM17.3.0.CO;2-B (doi:10.1002/(SICI)1097-4636(199711)37:2,267::AID-JBM17.3.0.CO;2-B) [DOI] [PubMed] [Google Scholar]
- 3.Hanawa T., Kamimura Y., Yamamoto S., Kohgo T., Amemiya A., Ukai M., Murakami K., Asaoka K. 1997. Early bone formation around calcium-ion-implanted titanium inserted into rat tibia. J. Biomed. Mater. Res. 36, 131–136 (doi:10.1002/(SICI)1097-4636(199707)36:1<131::AID-JBM16>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 4.Armitage D. A., Mihoc R., Tate T. J., McPhail D. S., Chater R., Hobkirk J. A., Shinawi L., Jones F. H. 2007. The oxidation of calcium implanted titanium in water: a depth profiling study. Appl. Surf. Sci. 253, 4085–4093 10.1016/j.apsusc.2006.09.006 (doi:10.1016/j.apsusc.2006.09.006) [DOI] [Google Scholar]
- 5.Nayab H., Jones F. H., Olsen I. 2007. Effects of calcium ion-implantation of titanium on bone cell function in vitro. J. Biomed. Mater. Res. 83A, 296–302 10.1002/jbm.a.31218 (doi:10.1002/jbm.a.31218) [DOI] [PubMed] [Google Scholar]
- 6.Sul Y. T. 2003. The significance of the surface properties of oxidized titanium to the bone response: special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 24, 3893–3907 10.1016/S0142-9612(03)00261-8 (doi:10.1016/S0142-9612(03)00261-8) [DOI] [PubMed] [Google Scholar]
- 7.Song W. H., Ryu H. S., Hong S. H. 2005. Apatite induction on Ca-containing titania formed by micro-arc oxidation. J. Am. Ceram. Soc. 88, 2642–2644 10.1111/j.1551-2916.2005.00476.x (doi:10.1111/j.1551-2916.2005.00476.x) [DOI] [Google Scholar]
- 8.Frojd V., Franke-Stenport V., Meirelles L., Wennerberg A. 2008. Increased bone contact to a calcium-incorporated oxidized commercially pure titanium implant: an in-vivo study in rabbits. Int. J. Oral Maxillofac. Surg. 37, 561–566 10.1016/j.ijom.2008.01.020 (doi:10.1016/j.ijom.2008.01.020) [DOI] [PubMed] [Google Scholar]
- 9.Wu J., Liu Z. M., Zhao X. H., Gao Y., Hu J., Gao B. 2010. Improved biological performance of microarc-oxidized low-modulus Ti-24Nb-4Zr-7.9Sn alloy. J. Biomed. Mater. Res. 92B, 298–306 10.1002/jbm.b.31515 (doi:10.1002/jbm.b.31515) [DOI] [PubMed] [Google Scholar]
- 10.Whiteside P., Matykina E., Gough J. F., Skeldon P., Thompson G. E. 2010. In vitro evaluation of cell proliferation and collagen synthesis on titanium following plasma electrolytic oxidation. J. Biomed. Mater. Res. 94A, 38–46 10.1002/jbm.a.32664 (doi:10.1002/jbm.a.32664) [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa M., Zhang L., Udoh K., Matsuya S., Ishikawa K. 2005. Effects of hydrothermal treatment with CaCl2 solution on surface property and cell response of titanium implants. J. Mater. Sci., Mater. Med. 16, 985–991 10.1007/s10856-005-4753-0 (doi:10.1007/s10856-005-4753-0) [DOI] [PubMed] [Google Scholar]
- 12.Park J. W., Park K. B., Suh J. Y. 2007. Effects of calcium ion incorporation on bone healing of Ti6Al4V alloy implants in rabbit tibiae. Biomaterials 28, 3306–3313 10.1016/j.biomaterials.2007.04.007 (doi:10.1016/j.biomaterials.2007.04.007) [DOI] [PubMed] [Google Scholar]
- 13.Ueda M., Ikeda M., Ogawa M. 2009. Chemical–hydrothermal combined surface modification of titanium for improvement of osteointegration. J. Mater. Sci. Eng. C 29, 994–1000 10.1016/j.msec.2008.09.002 (doi:10.1016/j.msec.2008.09.002) [DOI] [Google Scholar]
- 14.Chen X. B., Li Y. C., Plessis J. D., Hodgson P. D., Wen C. 2009. Influence of calcium ion deposition on apatite-inducing ability of porous titanium for biomedical applications. Acta Biomater. 5, 1808–1820 10.1016/j.actbio.2009.01.015 (doi:10.1016/j.actbio.2009.01.015) [DOI] [PubMed] [Google Scholar]
- 15.Park J. W., Kim Y. J., Jang J. H., Kwon T. G., Bae Y. C., Suh J. Y. 2010. Effects of phosphoric acid treatment of titanium surfaces on surface properties, osteoblast response and removal of torque forces. Acta Biomater. 6, 1661–1670 10.1016/j.actbio.2009.10.011 (doi:10.1016/j.actbio.2009.10.011) [DOI] [PubMed] [Google Scholar]
- 16.Wang X. X., Hayakawa S., Tsuru K., Osaka A. 2002. Bioactive titania gel layers formed by chemical treatment of Ti substrate with a H2O2/HCl solution. Biomaterials 23, 1353–1357 10.1016/S0142-9612(01)00254-X (doi:10.1016/S0142-9612(01)00254-X) [DOI] [PubMed] [Google Scholar]
- 17.Wang X. X., Yan W., Hayakawa S., Tsuru K., Osaka A. 2003. Apatite deposition on thermally and anodically oxidized titanium surfaces in a simulated body fluid. Biomaterials 24, 4631–4637 10.1016/S0142-9612(03)00357-0 (doi:10.1016/S0142-9612(03)00357-0) [DOI] [PubMed] [Google Scholar]
- 18.Wu J. M., Hayakawa S., Tsuru K., Osaka A. 2004. Low temperature preparation of anatase and rutile layers on titanium substrates and their ability to induce in vitro apatite deposition. J. Am. Ceram. Soc. 87, 1635–1642 10.1111/j.1551-2916.2004.01635.x (doi:10.1111/j.1551-2916.2004.01635.x) [DOI] [Google Scholar]
- 19.Lu X., Wang Y., Yang X., Zhang Q., Zhao Z., Weng L. T., Leng Y. 2008. Spectroscopic analysis of titanium surface functional groups under various surface modification and their behaviors in vitro and in vivo. J. Biomed. Mater. Res. 84A, 523–534 10.1002/jbm.a.31471 (doi:10.1002/jbm.a.31471) [DOI] [PubMed] [Google Scholar]
- 20.Sugino A., Ohtsuki C., Tsuru K., Hayakawa S., Nakano T., Okazaki Y., Osaka A. 2009. Effect of spatial design and thermal oxidation on apatite formation on Ti–15Zr–4Ta–4Nb alloy. Acta Biomater. 5, 298–304 10.1016/j.actbio.2008.07.014. (doi:10.1016/j.actbio.2008.07.014.) [DOI] [PubMed] [Google Scholar]
- 21.Karthega M., Rajendran N. 2010. Hydrogen peroxide treatment on Ti–6Al–4V alloy: a promising surface modification technique for orthopaedic application. Appl. Surf. Sci. 256, 2176–2183 10.1016/j.apsusc.2009.09.069 (doi:10.1016/j.apsusc.2009.09.069) [DOI] [Google Scholar]
- 22.Kokubo T., Miyaji F., Kim H. M., Nakamura T. 1996. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J. Am. Ceram. Soc. 79, 1127–1129 10.1111/j.1151-2916.1996.tb08561.x (doi:10.1111/j.1151-2916.1996.tb08561.x) [DOI] [Google Scholar]
- 23.Kim H. M., Miyaji F., Kokubo T., Nakamura T. 1996. Preparation of bioactive Ti and its alloy via simple chemical surface treatment. J. Biomed. Mater. Res. 32, 409–417 10.1002/(SICI)1097-4636(199611)32:3%3C409::AID-JBM14%3E3.0.CO;2-B (doi:10.1002/(SICI)1097-4636(199611)32:3<409:: AID-JBM14>3.0.CO;2-B) [DOI] [PubMed] [Google Scholar]
- 24.Nishiguchi S., Fujibayashi S., Kim H. M., Kokubo T., Nakamura T. 2003. Biology of alkali- and heat-treated titanium implants. J. Biomed. Mater. Res. 67A, 26–35 10.1002/jbm.a.10540 (doi:10.1002/jbm.a.10540) [DOI] [PubMed] [Google Scholar]
- 25.Kawanabe K., Ise K., Goto K., Akiyama H., Nakamura T., Kaneuji A., Sugimori T., Matsumoto T. 2009. A new cementless total hip arthoplasty with bioactive titanium porous-coating by alkaline and heat treatment: average 4.8-year results. J. Biomed. Mater. Res. 90B, 476–481 10.1002/jbm.b.31309 (doi:10.1002/jbm.b.31309) [DOI] [PubMed] [Google Scholar]
- 26.Kokubo T., Pattanayak D. K., Yamaguchi S., Takadama H., Matsushita T., Kawai T., Takemoto M., Fujibayashi S., Nakamura T. 2010. Positively charged bioactive Ti metal prepared by simple chemical and heat treatments. J. R. Soc. Interface 7, S503–S513 10.1098/rsif.2010.0129.focus (doi:10.1098/rsif.2010.0129.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokubo T., Takadama H. 2006. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27, 2907–2915 10.1016/j.biomaterials.2006.01.017 (doi:10.1016/j.biomaterials.2006.01.017) [DOI] [PubMed] [Google Scholar]
- 28.Sun X., Li Y. 2003. Synthesis and characterization of ion-exchangeable titanate nanotubes. Chem. Eur. J. 9, 2229–2238 10.1002/chem.200204394 (doi:10.1002/chem.200204394) [DOI] [PubMed] [Google Scholar]
- 29.Kim H.-M., Miyaji F., Kokubo T., Nishiguchi S., Nakamura T. 1999. Graded surface structure of bioactive titanium prepared by chemical treatment. J. Biomed. Mater. Res. 45, 100–107 (doi:10.1002/(SICI)1097-4636(199905)45:2<100::AID-JBM4>3.0.CO;2-0) [DOI] [PubMed] [Google Scholar]
- 30.Textor M., Sittig C., Frauchiger V., Tosatti S., Brunette D. M. 2001. Properties and biological significance of natural oxide films on titanium and its alloys. In Titanium in medicine (eds Brunette D. M., Tengvall P., Textor M., Thomsen P.), pp. 171–230 Berlin, Germany: Springer [Google Scholar]
- 31.Pattanayak D. K., Yamaguchi S., Matsushita T., Kokubo T. 2011. Nanostructured positively charged bioactive TiO2 layer formed on Ti metal by NaOH, acid and heat treatments. J. Mater. Sci: Mater. Med. 22, 1803–1812 10.1007/s10856-011-4372-x (doi:10.1007/s10856-011-4372-x) [DOI] [PubMed] [Google Scholar]
- 32.Kawai T., Kizuki T., Takadama T., Matsushita T., Unuma H., Nakamura T., Kokubo T. 2010. Apatite formation on surface titanate layer with different Na content on Ti metal. J. Ceram. Soc. Japan 118, 19–24 [Google Scholar]
- 33.Takadama H., Kim H.-M., Kokubo T., Nakamura T. 2001. An X-ray photoelectron spectroscopy study of the process of apatite formation on bioactive titanium metal. J. Biomed. Mater. Res. 55, 185–193 (doi:10.1002/1097-4636(200105)55:2<185::AID-JBM1005>3.0.CO;2-P) [DOI] [PubMed] [Google Scholar]
- 34.Takadama H., Kim H.-M., Kokubo T., Nakamura T. 2001. TEM-EDX study of mechanism of bonelike apatite formation on bioactive titanium metal in simulated body fluid. J. Biomed. Mater. Res. 57, 441–448 10.1002/1097-4636(20011205)57:3%3C441::AID-JBM1187%3E3.0.CO;2-B (doi:10.1002/1097-4636(20011205)57:3<441::AID-JBM1187>3.0.CO;2-B) [DOI] [PubMed] [Google Scholar]
- 35.Kim H. M., Himeno T., Kawashita M., Lee J. H., Kokubo T., Nakamura T. 2003. Surface potential change in bioactive titanium metal during the process of apatite formation in simulated body fluid. J. Biomed. Mater. Res. A 67, 1305–1309 10.1002/jbm.a.20039 (doi:10.1002/jbm.a.20039) [DOI] [PubMed] [Google Scholar]
- 36.Ito M., Setoyama D., Matsunaga J., Muta H., Kurosaki K., Masayoshi U., Yamanaka S. 2006. Electrical and thermal properties of titanium hydrides. J. Alloys Compd. 420, 25–28 10.1016/j.jallcom.2005.10.032 (doi:10.1016/j.jallcom.2005.10.032) [DOI] [Google Scholar]
- 37.Kim H.-M., Miyaji F., Kokubo T., Nakamura T. 1997. Apatite-forming ability of alkali-treated Ti metal in body environment. J. Ceram. Soc. Japan 105, 111–116 [Google Scholar]
- 38.Bohner M., Lemahitre J. 2009. Can bioactivity be tested in vitro with SBF solution? Biomaterials 30, 2175–2179 10.1016/j.biomaterials.2009.01.008 (doi:10.1016/j.biomaterials.2009.01.008) [DOI] [PubMed] [Google Scholar]
- 39.Pan H., Zhao X., Darvell B. W., Lu W. W. 2010. Apatite-formation ability: predictor of ‘bioactivity’? Acta Biomater. 6, 4181–4188 10.1016/j.actbio.2010.05.013 (doi:10.1016/j.actbio.2010.05.013) [DOI] [PubMed] [Google Scholar]
- 40.Nishiguchi S., Kato H., Fujita H., Kim H. M., Miyaji F., Kokubo T., Nakamura T. 1999. Enhancement of bone-bonding strengths of titanium alloy implants by alkali and heat treatments. J. Biomed. Mater. Res. (Appl Biomater.) 48, 689–696 10.1002/(SICI)1097-4636(1999)48:5%3C689::AID-JBM13%3E3.0.CO;2-C (doi:10.1002/(SICI)1097-4636(1999)48:5<689::AID-JBM13>3.0.CO;2-C) [DOI] [PubMed] [Google Scholar]
- 41.Kim H. M., Takadama H., Miyaji F., Kokubo T., Nishiguchi S., Nakamura T. 2000. Formation of bioactive functionally graded structure on Ti–6Al–4V alloy by chemical surface treatment. J. Mater. Sci., Mater. Med. 11, 555–559 10.1023/A:1008924102096 (doi:10.1023/A:1008924102096) [DOI] [PubMed] [Google Scholar]
- 42.Kim H. M., Takadama H., Kokubo T., Nishiguchi S., Nakamura T. 2000. Formation of a bioactive graded surface structure on Ti–15Mo–5Zr–3Al alloy by chemical treatments. Biomaterials 21, 353–358 10.1016/S0142-9612(99)00190-8 (doi:10.1016/S0142-9612(99)00190-8) [DOI] [PubMed] [Google Scholar]
- 43.Takemoto M., Fujibayashi S., Neo M., So K., Akiyama N., Matsushita T., Kokubo T., Nakamura T. 2007. A porous bioactive titanium implant for spinal interbody fusion: an experimental study using a canine model. J. Neurosurg. Spine 7, 435–443 10.3171/SPI-07/10/435 (doi:10.3171/SPI-07/10/435) [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi S., Takadama H., Matsushita T., Nakamura T., Kokubo T. 2010. Apatite-forming ability of Ti–15Zr–4Nb–4Ta alloy induced by calcium solution treatment. J. Mater. Sci., Mater. Med. 21, 439–444 10.1007/s10856-009-3904-0 (doi:10.1007/s10856-009-3904-0) [DOI] [PubMed] [Google Scholar]
- 45.Kizuki T., Takadama H., Matsushita T., Nakamura T., Kokubo T. 2010. Preparation of bioactive Ti metal surface enriched with calcium ions by chemical treatment. Acta Biomater. 6, 2836–2842 10.1016/j.actbio.2010.01.007 (doi:10.1016/j.actbio.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 46.Fukuda A., et al. 2011. Bone bonding bioactivity of Ti metal and Ti–Zr–Nb–Ta alloys with Ca ions incorporated on their surfaces by simple chemical and heat treatment. Acta Biomater. 7, 1379–1386 10.1016/j.actbio.2010.09.026 (doi:10.1016/j.actbio.2010.09.026) [DOI] [PubMed] [Google Scholar]
- 47.Pattanayak D. K., Fukuda A., Matsushita T., Takemoto M., Fujibayashi S., Sasaki K., Nishida N., Nakamura T., Kokubo T. 2011. Bioactive Ti metal analogous to human cancellous bone: fabrication by selective laser melting and chemical treatments. Acta Biomater. 7, 1398–1406 10.1016/j.actbio.2010.09.034 (doi:10.1016/j.actbio.2010.09.034) [DOI] [PubMed] [Google Scholar]