Abstract
Easy-to-use dipstick tests for lead have been developed by immobilizing nanoparticle–DNAzyme conjugates on lateral flow devices and their application for detecting lead in paints is demonstrated.
Designing easy-to-use biosensors for trace metal ions in the environment is of considerable importance1 as these metal ions are large in number, small in quantity and high in toxicity. Metal-specific DNAzymes, functional DNA molecules that can catalyze a reaction in the presence of a particular metal ion, have emerged as a new class of metal-ion sensors1e,2 because DNAzymes with desired metal specificity and affinity can be obtained by a combinatorial biology technique called in vitro selection.3 When combined with gold nanoparticles (AuNPs), the DNAzymes have been transformed into highly sensitive and selective colorimetric biosensors, producing color changes between red (dispersed AuNPs) to blue (aggregated AuNPs) in response to a target recognized by DNA.2c,4 Using this method, we have demonstrated colorimetric biosensors for metal ions such as Pb2+, UO2 2+ and Cu2+.2b,k,5
While these colorimetric biosensors have taken an important step towards real-time sensing as the signal is detectable by the naked eye, without the need for expensive instrumentation, they still require laboratory type operations, such as precise transfer and mixing of multiple solutions. In addition, although the sensitivity is high when the absorbance is recorded using a UV-Vis spectrophotometer, it is often difficult to distinguish the red color of dispersed nanoparticles against a blue background from the aggregates, particularly at low metal-ion concentrations. Furthermore, AuNPs are not very stable in the solution state; they are vulnerable to aggregation under a variety of conditions thereby making it difficult to store the sensors for a long period of time.
Lateral flow devices are an ideal platform for making dipstick type tests to further improve the performance of DNAzyme–AuNP colorimetric sensors. In addition to eliminating precise solution transfer and allowing separation of AuNPs to make it easier to distinguish colors, the reagents can be prepared in a dry or nearly dry state, making the device stable at ambient conditions for a long period of time. The home pregnancy test is the most commonly used application of lateral flow devices that use antibodies for detection. Being more stable to denaturation, DNA is an attactive molecule to replace antibodies in the dipstick tests. Despite the promise, DNA-based dipstick tests are not common. Glynou et al. reported a lateral flow device for the detection of DNA.6 To expand on the range of analytes detected, we previously reported dipstick tests for the detection of adenosine and cocaine using AuNP conjugated to aptamers.7 A paper based bioassay using aptamers and the protein enzyme DNAase I which also involved the disassembly of nanoparticle aggregates that were dried onto paper substrates was reported by Yingfu Li and coworkers.4f Even though our previously reported methodology can be applied to almost any target for which aptamers can be obtained, because aptamers with high affinity for metal ions have been difficult to select, this methodology has not been applied to dipstick tests for metal ions. To extend the applicability of this test for metal ions, metal-specific DNAzymes are an excellent choice. However, it is not trivial to adopt the aptamer-based dipstick methodology by simply replacing aptamers with DNAzymes because DNAzymes undergo not only binding as aptamers do, but also catalytic activity and product release, making the design more complicated. Herein, we report a method to convert DNAzyme–AuNPs into dipstick tests for metal ions, specifically for Pb2+. In the process, we showed that the cross-link based method used in the previous aptamer–AuNP system4f,7 did not work for the DNAzyme–AuNP system. Instead, we succeeded in developing a non-cross-linked DNAzyme–AuNP system for detection. Furthermore, we have demonstrated the dipstick tests to be ideal for detection of lead in household paints, in accordance with the EPA defined threshold of 1 mg cm−2 Pb2+ for paint to be classified as a lead-based paint.8
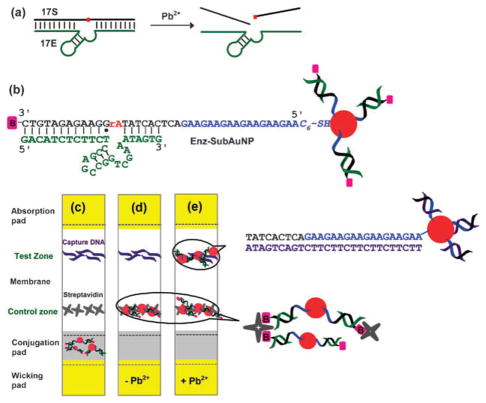
We chose the 8–17 DNAzyme to construct the dipstick tests for Pb2+ because of its very high activity as shown by a fast cleavage rate (estimated kobs ~50 min−1 at pH 7.0).9 Fig. 1a shows its reaction scheme. In the presence of Pb2+, the enzyme strand (called 17E) catalyzes the cleavage of the substrate (called 17S) at the single ribo-adenosine base (shown in red). Based on this reaction scheme, the 8–17 DNAzyme has been previously converted into fluorescent,2a,d colorimetric2c,5a,b,d and electrochemical sensors2i for Pb2+. Unlike aptamer-based colorimetric tests, the presence of target does not cause immediate disassembly of the aggregates in the DNAzyme-based colorimetric tests.4f,7 Disassembly in the case of DNAzymes has been shown to require heating2c or the use of invasive DNA strands5b to release the cleaved product trapped in the nanoparticle aggregates. Either of these methods will lead to added complexity and they are not feasible for lateral flow devices. Therefore, we decided to use an alternative approach that does not involve formation of nanoparticle aggregates and thus the detection is not based on a change in the optical properties of AuNPs.
Fig. 1.
(a) The 8–17 DNAzyme reaction. In the presence of Pb2+, the 17E enzyme catalyzes the cleavage of the substrate, 17S at the single ribo-linkage (shown in red). (b) Modified 8–17 construct conjugated to AuNPs (called Enz-SubAuNP) used for the dipstick tests. (c) Assembled lateral flow device. (d) Negative control. In the absence of Pb2+, AuNP-uncleaved substrate is captured at the control zone via streptavidin–biotin interaction, producing a single red line. (e) Positive test. Substrate is cleaved in the presence of Pb2+ and the AuNP-cleaved product migrates beyond the control zone to be captured at the test zone by hybridization to complementary DNA. Two red lines are produced.
In our scheme, the 8–17 DNAzyme was modified to form the construct shown in Fig. 1b. The 17S substrate was modified on the 3′ end to have a biotin moiety. On the 5′ side, 18 additional bases (AAG)6 were added to act as a site for DNA hybridization required for the capture of cleaved product. In addition, the 5′ end was functionalized with a thiol group in order to conjugate the substrate to 13 nm AuNPs. When the original 8–17 DNAzyme construct with symmetric 9 base pairs on either substrate-binding arm was tested, this construct failed to display a positive signal as designed (see ESI, S2†). To facilitate the release of the cleavage product without compromising the binding of the substrate, three bases were deleted from the 3′ end of the enzyme and two bases were added to the 5′ end of the 17E enzyme. The shortened arm facilitated the release of the cleaved product after reaction, while the overall stability of the construct before cleavage was maintained due to the extra base-pairing on the opposite arm. The enzyme–substrate complex was prepared in a buffer containing 25 mM Tris-HCl (pH 8.0), 100 mM NaCl, and 8% sucrose by hydridizing the enzyme to the substrate conjugated to AuNPs and this was referred to as Enz-SubAuNP. Sucrose was added to the buffer in order to keep the DNA hybridized and facilitate rehydration of the complex.
The lateral flow device was constructed using a Millipore Assembly kit by placing of four overlapping pads on a backing (see ESI, S1†). Streptavidin and capture DNA were applied on the capture zone and test zone of the membrane, respectively and the complex, Enz-SubAuNP was spotted on the conjugation pad and allowed to dry for 8 h (Fig. 1c). In order to perform the test, the dipstick was dipped in a flow buffer containing 25 mM Tris (pH 8.0) and 30 mM NaCl which rehydrated the Enz-SubAuNP complex. In the absence of Pb2+, the substrate would remain uncleaved and Enz-SubAuNP would migrate on the membrane till it reached the control zone, where the biotin-containing Enz-SubAuNP could be captured by streptavidin, thus producing a single red line at the control zone (Fig. 1d). In the presence of Pb2+, the substrate would be cleaved, and the cleaved product would migrate past the control zone to be captured at the test zone by a 27 base long DNA sequence complementary to the cleaved substrate piece (called capture DNA), producing a red line at the test zone. Since the cleavage reaction may not be 100% complete, the positive tests would normally result in two red lines, one being the cleaved product and the other being the uncleaved enzyme-substrate (Fig. 1e).
To determine the sensitivity of the dipstick test, the lateral flow devices were dipped in a flow buffer containing varying amounts of Pb2+. As expected, a single red line was observed at the control zone in the absence of Pb2+. In contrast, the presence of Pb2+ resulted in a second red line at the test zone, and its intensity increased with increasing Pb2+ concentration (Fig. 2a). This test can be qualitative or semi-quantitative because a color chart can be used to estimate the Pb2+ concentration, like a pH paper. The detection limit for this test was determined to be ~5 μM. This detection limit is higher than ~0.1 μM reported for the DNAzyme–AuNP sensor in solution.2c This is not surprising, as when the same DNAzyme was immobilized on a gold surface, the reaction was slowed down by diffusion limitation of Pb2+ to the DNAzyme active site on the surface, resulting in long reaction time (up to 1 h).2d Since the reaction on the lateral flow device takes place within 10 min, it is difficult to complete such a slow reaction on the surface.
Fig. 2.
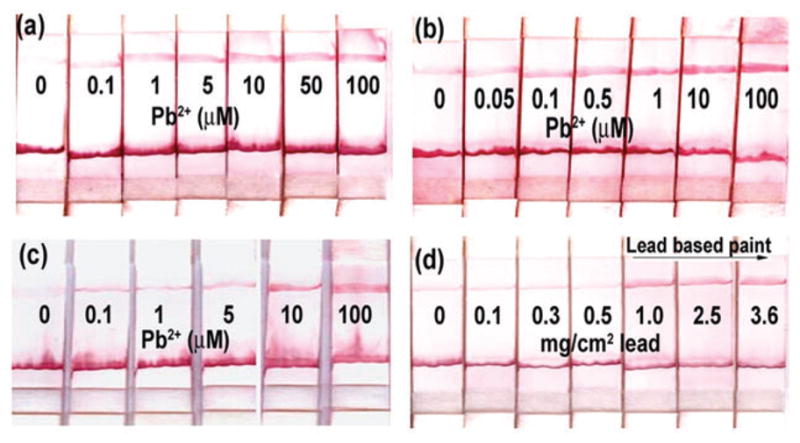
Results of dipstick test for lead: (a) performance when the Enz-SubAuNP was pre-immobilized on the conjugate pad and the Pb2+ reaction occurred on the surface of the device; (b) performance when Enz-SubAuNP was allowed to react with Pb2+ in solution and then placed on the conjugate pad; (c) performance when 5 μM EDTA was added to the system to shift the dynamic range; (d) detection of lead in paint around the US federal threshold for leaded paints (1 mg cm−2).
To further improve the sensitivity of the system, we decided to perform the Pb2+-induced cleavage reaction in solution and use the lateral flow device as a medium to visualize the results. To the Enz-SubAuNP complex in buffer, Pb2+ was added and the reaction was allowed to proceed for 15 min. This mixture was then placed on the conjugate pad of a lateral flow device and the device was dipped in the flow buffer (Fig. 2b). A clear red line at the test zone can be seen at 0.5 μM Pb2+, and thus the sensitivity is ~10 times better than the system shown in Fig. 2a. The sensitivity of this lateral flow device shown in Fig. 2b is also good in comparison with our previously reported colorimetric lead sensor that is based on the disassembly of AuNPs and produces a blue to red color change with Pb2+.2c Although the detection limit for the solution-based method is 0.1 μM when the absorbance is measured using a UV-Vis instrument, it is difficult to visualize the red color below 5 μM Pb2+, because of the large blue background from the aggregates. Thus, this lateral flow device provides a~10-fold improvement in sensitivity for visual detection over the previously reported colorimetric sensor.2c Furthermore, performing the reaction in solution only requires one additional step of placing the reacted DNAzyme construct on the conjugation pad, which can be carried out using a dropper. In addition to the improved sensitivity, the selectivity of the test for Pb2+ over other divalent metal ions at 10 μM concentration is maintained, although it is lower than that of the previously reported 17E construct (see ESI, S3†).2c
In order to test the efficacy of the dipstick test for practical applications, we investigated the use of the lateral flow device for detecting lead in paints. The US Department of Housing and Development (HUD) classifies paint to be lead-based if it contains more than 1 mg cm−2 of lead and an ideal test should provide a positive response above the cut-off and negative response below it.8 Paint samples were spiked with known amounts of lead salt and painted on a solid surface, over which another layer of paint was applied to simulate multiple layers of paints seen in old houses (see ESI, S4a†). The lead was extracted from the paint by suspending the paint samples in 10% acetic acid for 3 h. Since our sensor has a low detection limit, we used EDTA, which is a good chelator for Pb2+ to tune the dynamic range of this sensor. Metal ions chelated to EDTA are not available for interaction with the DNAzyme. In addition to tuning the dynamic range, EDTA also eliminates any non-specific cleavage due to trace metals present in the buffer that may occur during annealing or storage, thus reducing the likelihood of any false positives. The test demonstrated in Fig. 2b was repeated with 5 μM EDTA in the buffer, which resulted in the detection limit shifting to 10 μM Pb2+, and the faint red line seen at the test zone in the absence of Pb2+ was completely eliminated (Fig. 2c). For the lead-paint tests, a calculated amount of EDTA (50 μM, see ESI, S4b†) was added to the DNAzyme construct such that it could chelate Pb2+ extracted from those samples that have lower lead content than the federally defined threshold for lead-based paint. Fig. 2d depicts the results from dipstick tests performed with paint samples with varying amounts of Pb2+. A red line at the test zone is observed only in samples containing 1 mg cm−2 lead or higher, whereas paint samples with lower Pb2+ content produce a line at the control zone only, due to the absence of free Pb2+ in solution.
In conclusion, we have used the 8–17 DNAzyme and AuNPs to construct an easy-to-use dipstick test for Pb2+ with a detection limit of ~0.5 μM without the use of any instrumentation to visualize the results. The dipstick test shows promising results for the detection of Pb2+ extracted from paints. Such a simple dipstick test will find wide use in household and other environmental applications.
Supplementary Material
Acknowledgments
We wish to thank the US Department of Energy (DE-FG02-08ER64568), National Institute of Health (ES016865), Department of House and Urban Development (ILLHT0112-06) and the National Science Foundation (Grant no. CTS-0120978 and DMI-0328162) for financial support.
Footnotes
Electronic supplementary information (ESI) available: Experimental section; optimization of DNAzyme construct; specificity data; details for detection of lead in paint. See DOI: 10.1039/b917772h
Notes and references
- 1.(a) Jiang P, Guo Z. Coord Chem Rev. 2004;248:205–229. [Google Scholar]; (b) Chen P, Greenberg B, Taghavi S, Romano C, van der Lelie D, He C. Angew Chem, Int Ed. 2005;44:2715–2719. doi: 10.1002/anie.200462443. [DOI] [PubMed] [Google Scholar]; (c) Domaille DW, Que EL, Chang CJ. Nat Chem Biol. 2008;4:168–175. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]; (d) Nolan EM, Lippard SJ. Chem Rev. 2008;108:3443–3480. doi: 10.1021/cr068000q. [DOI] [PubMed] [Google Scholar]; (e) Mazumdar D, Liu J, Lu Y. In: Nanotechnology Applications for Clean Water. Diallo M, Duncan J, Savage N, Street A, Sustich R, editors. William Andrews; Norwich, NY: 2009. pp. 427–446. [Google Scholar]
- 2.(a) Li J, Lu Y. J Am Chem Soc. 2000;122:10466–10467. [Google Scholar]; (b) Lu Y. Chem–Eur J. 2002;8:4588–4596. doi: 10.1002/1521-3765(20021018)8:20<4588::AID-CHEM4588>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]; (c) Liu J, Lu Y. J Am Chem Soc. 2003;125:6642–6643. doi: 10.1021/ja034775u. [DOI] [PubMed] [Google Scholar]; (d) Swearingen CB, Wernette DP, Cropek DM, Lu Y, Sweedler JV, Bohn PW. Anal Chem. 2005;77:442–448. doi: 10.1021/ac0401016. [DOI] [PubMed] [Google Scholar]; (e) Lu Y, Liu J. Curr Opin Biotechnol. 2006;17:580–588. doi: 10.1016/j.copbio.2006.10.004. [DOI] [PubMed] [Google Scholar]; (f) Shen Y, Mackey G, Rupcich N, Gloster D, Chiuman W, Li Y, Brennan JD. Anal Chem. 2007;79:3494–3503. doi: 10.1021/ac070235u. [DOI] [PubMed] [Google Scholar]; (g) Liu J, Brown AK, Meng X, Cropek DM, Istok JD, Watson DB, Lu Y. Proc Natl Acad Sci U S A. 2007;104:2056–2061. doi: 10.1073/pnas.0607875104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Liu J, Lu Y. J Am Chem Soc. 2007;129:9838–9839. doi: 10.1021/ja0717358. [DOI] [PubMed] [Google Scholar]; (i) Xiao Y, Rowe AA, Plaxco KW. J Am Chem Soc. 2007;129:262–263. doi: 10.1021/ja067278x. [DOI] [PubMed] [Google Scholar]; (j) Hollenstein M, Hipolito C, Lam C, Dietrich D, Perrin DM. Angew Chem, Int Ed. 2008;47:4346–4350. doi: 10.1002/anie.200800960. [DOI] [PubMed] [Google Scholar]; (k) Liu J, Cao Z, Lu Y. Chem Rev. 2009;109:1948–1998. doi: 10.1021/cr030183i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Wang H, Kim Y, Liu H, Zhu Z, Bamrungsap S, Tan W. J Am Chem Soc. 2009;131:8221–8226. doi: 10.1021/ja901132y. [DOI] [PubMed] [Google Scholar]
- 3.Breaker RR, Joyce GF. Chem Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 4.(a) Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]; (b) Stojanovic MN, Landry DW. J Am Chem Soc. 2002;124:9678–9679. doi: 10.1021/ja0259483. [DOI] [PubMed] [Google Scholar]; (c) Niazov T, Pavlov V, Xiao Y, Gill R, Willner I. Nano Lett. 2004;4:1683. [Google Scholar]; (d) Huang CC, Huang YF, Cao Z, Tan W, Chang HT. Anal Chem. 2005;77:5735–5741. doi: 10.1021/ac050957q. [DOI] [PubMed] [Google Scholar]; (e) Liu J, Lu Y. Angew Chem, Int Ed. 2006;45:90–94. [Google Scholar]; (f) Zhao W, Ali MM, Aguirre SD, Brook MA, Li Y. Anal Chem. 2008;80:8431–8437. doi: 10.1021/ac801008q. [DOI] [PubMed] [Google Scholar]; (g) Li J, Yao J, Zhong W. Chem Commun. 2009:4962–4964. doi: 10.1039/b910251e. [DOI] [PubMed] [Google Scholar]
- 5.(a) Liu J, Lu Y. Chem Mater. 2004;16:3231–3238. [Google Scholar]; (b) Liu J, Lu Y. J Am Chem Soc. 2005;127:12677–12683. doi: 10.1021/ja053567u. [DOI] [PubMed] [Google Scholar]; (c) Liu J, Lu Y. J Am Chem Soc. 2007:4872–4874. doi: 10.1039/b712421j. [DOI] [PubMed] [Google Scholar]; (d) Wang Z, Lee JH, Lu Y. Adv Mater. 2008;20:3263–3267. [Google Scholar]; (e) Lee JH, Wang Z, Liu J, Lu Y. J Am Chem Soc. 2008;130:14217–14226. doi: 10.1021/ja803607z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glynou K, Ioannou PC, Christopoulos TK, Syriopoulou V. Anal Chem. 2003;75:4155–4160. doi: 10.1021/ac034256+. [DOI] [PubMed] [Google Scholar]
- 7.(a) Liu J, Mazumdar D, Lu Y. Angew Chem, Int Ed. 2006;45:7955–7959. doi: 10.1002/anie.200603106. [DOI] [PubMed] [Google Scholar]; (b) Liu J, Lu Y. Nat Protoc. 2006;1:246–252. doi: 10.1038/nprot.2006.38. [DOI] [PubMed] [Google Scholar]
- 8.Public Law 102-550; Residential Lead-Based Paint Hazard Reduction Act of the housing and Community Development Act of 1992. Pittsburgh: 1992. [Google Scholar]
- 9.Brown AK, Li J, Pavot CMB, Lu Y. Biochemistry. 2003;42:7152–7161. doi: 10.1021/bi027332w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.