
Introduction
While the evolution of computed tomography imaging in the last 2 decades has been driven almost exclusively by improvements in the instrumentation and processing algorithms, there have been comparatively modest advances in contrast agent technology. A notable change in the last decade has been the development of blood pool contrast agents based on nanoparticle technology. While not yet ready for clinical use, the stable and uniform opacification provided by these agents in normal vasculature and controlled extravasation in compromised vasculature enables novel techniques for imaging and diagnosis of pathologies. This manuscript presents preclinical examples demonstrating cardiovascular pathologies and tumor characterization by high-resolution computed tomography imaging.
Introduction
High-resolution computed tomography (CT) imaging has experienced a rapid evolution in the last 10 years, driven primarily by the development of multi-row detector spiral scanning and cone-beam methods.1 Where the state-of-the-art about a decade ago was a 4-row detector, contemporary machines today boast 128 detector rows or more with rotation speeds on the order of 0.25 seconds as well as dual energy technologies, allowing for multi-element decomposition.2–4 Flat-panel digital detector systems have become the standard in angiography, and rotation of the source-detector pair for computed-tomographic reconstructions in C-arm systems has entered the clinical arena.5 With this rapid evolution in technology, methods enabling soft tissue and blood pool contrast have remained practically unchanged. The development of nonionic contrast agents in the 1970s remains the most significant advancement in contrast agents for CT imaging.6 7 Nonionic iodinated molecules exemplified by iohexol and iodixanol have become the mainstay of CT contrast agents in spite of their well-recognized limitations. First, iodine has a relatively low absorption coefficient for soft X-rays, necessitating a delivery of large volumes of contrast agent in order to elicit a reasonable signal.8 While CT is relatively forgiving due to the ability of the tomographic method to discriminate line-of-sight background, planar angiography is much more susceptible and often necessitates tens of milliliters per injection, with 20 to 50 injections not uncommonly used in many procedures.9 A consequence of this large concentration of iodinated molecules in the contrast agent formulation is high osmolality, leading to local irritation, burning sensation, and, in extreme cases, membrane rupture and hemolysis at the injection site.10 11 Second, and most surprisingly, there has been very little attention paid to the pharmacokinetics of contrast agents. Indeed, the initial direction of scanners — towards rapid scanning to overcome the very rapid clearance of injected contrast agents —has had a “self-fulfilling” effect, whereby contrast agents have always been designed to have rapid clearance. This rapid clearance occurs invariably via the renal route, and the high osmolality (and viscosity) of the agents leads to acute renal toxcity.12 Indeed, as much as 5% to 10% of the general population, and 25% to 40% of the renally susceptible population, suffers from contrast-induced nephropathy (CIN) after a contrast-CT study.13 14 Ironically, it is this susceptible population that most needs the CT study in the first place. Third, the rapid clearance kinetics (t1/2 ~ 5 minutes) necessitates a bolus injection and careful timing of the scan to correctly trace the bolus at the moment of entry into the anatomy of interest. This can be challenging, particularly for left-heart-based imaging, since the intravenous bolus undergoes significant dissipation and dispersion in the pulmonary vasculature prior to collection in, and ejection from, the left ventricle. Thus, at most imaging sites, a “bolus tracking” scan is implemented, which continuously scans a sentinel section immediately upstream of the anatomical region of interest, triggering the scan upon arrival of contrast in the sentinel.15 However, the bolus tracking scan results in continuous exposure to X-rays and dramatically increases the total X-ray dose in such procedures. Indeed, in the pediatric population, where sensitivity to X-ray dose is particularly high and scan doses have been progressively reduced, the bolus tracking contribution to the total dose can be as high as 30% to 40%.16 In spite of all these limitations, however, CT imaging has experienced nothing but continuous growth over the last decade and remains the most widespread imaging technique after ultrasound.
The shortcomings of conventional contrast agent kinetics have also been turned into powerful strengths by the clever use of delayed-phase imaging. Taking advantage of the rapid renal clearance of contrast agents, arteriography has become the main forte of contrast-enhanced CT. A few venography techniques, such as the lower-extremity runoff, are commonly practiced. By the same token, significant challenges remain in renal vasculature mapping and hepatic imaging, where the majority of the blood supply is of venous origin.17 18 Delayed-phase imaging and perfusion kinetics have also been developed to characterize tumors using the well-known Patlak method.19
However, it has always been clear that better control over the pharmacokinetics, coupled with a longer circulation time for contrast agents, would offer significant benefits. First, it would eliminate the need for high-speed scans because there would no longer be concerns about “missing” the bolus. Second, the delayed clearance would reduce acute renal load and probably reduce the incidence of CIN. Third, it would enable universal venography. One also imagines that with longer blood residence times, new delayed-phase imaging techniques would emerge. At short time scales, in just hours, one could enforce a constant input function and therefore increase the precision of dynamic estimations. At long time scales, on the order of days, one envisions new dynamics hitherto unexplored because input functions have usually decayed to zero. All of these potential advantages have spurred significant interest in the development of long-circulating (“blood pool”) agents.
The first foray into the development of nanoparticle blood pool agents happened in the 1980s.20 In a parallel development in the drug delivery field, the liposome, a bilayer bounded vesicle, was designed and built as a carrier for chemotherapeutic, antibiotic, and antifungal drugs.21–23 In the contrast agent arena, however, poor loading efficiency of the iodinated active molecule and rapid hepatic sequestration led to very limited success.24 25 A clinical trial of iodinated liposomes was terminated due to adverse events.26 In the late 1990s, an iodinated triglyceride backbone was used to form triglyceride particles with long-circulating properties and enjoyed significant use in preclinical imaging.27 In the late 2000s, researchers developed an emulsion carrying iodinated molecules in its hydrophobic internal phase.28 While not strictly a long-circulating blood pool agent, this material does have a longer circulation time than conventional contrast agents. However, it is primarily intended to target macrophages and is being developed for clinical use in tracking pathologies that accumulate macrophages,29 such as atherosclerotic plaques, certain tumors, and sites of inflammation and infection.
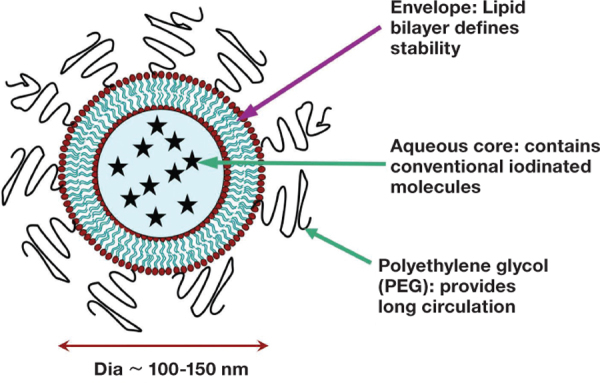
A true blood pool agent (NCTX) has been designed and extensively tested by the authors of this paper. NCTX is a PEGylated liposomal particle containing clinically used, nonionic iodinated contrast agent (Figure 1). In 2002, the first in vivo experiments with this agent demonstrated cardiac imaging in a rabbit model using a 4-slice CT scanner.30 Since that landmark experiment, the agent has been successfully used to image the mouse vasculature in practically every anatomical region. Imaging of pulmonary emboli using liposomal contrast agent has been demonstrated in rabbits and pigs. Imaging of coronary artery stenosis using the liposomal blood pool contrast agent has been demonstrated in a sheep model. Complete mapping of the hepatic vasculature, including the arterial, venous, and portal circulation, has been demonstrated in small and large animal models.31 32 Delayed-phase imaging (72 to 120 hours) has been used to characterize tumor vascular permeability.33 Preliminary studies in mice have demonstrated that the tumor uptake of liposomal contrast agents can facilitate differentiation of malignant and benign lung nodules. Stratification of breast tumors has allowed us to identify those tumors that are treatable by PEGylated liposomal doxorubicin and those that are not likely to respond.33 34 For the first time ever, the existence of extratumoral blood vessels that exhibit vascular permeability usually only attributed to intratumoral neovasculature has been demonstrated.32 Additionally, variants of this agent that target macrophages and highlight atherosclerotic plaques have also been recently demonstrated. The remainder of this paper, therefore, focuses on these capabilities of the liposomal blood pool agent. The processes used for the production of this agent are exhaustively documented in previous publications and are not reproduced here.31–33 We focus instead on the highlights of the imaging studies.
Figure 1.
Illustration of a liposomal blood pool contrast agent.
Whole-Body Vascular Imaging
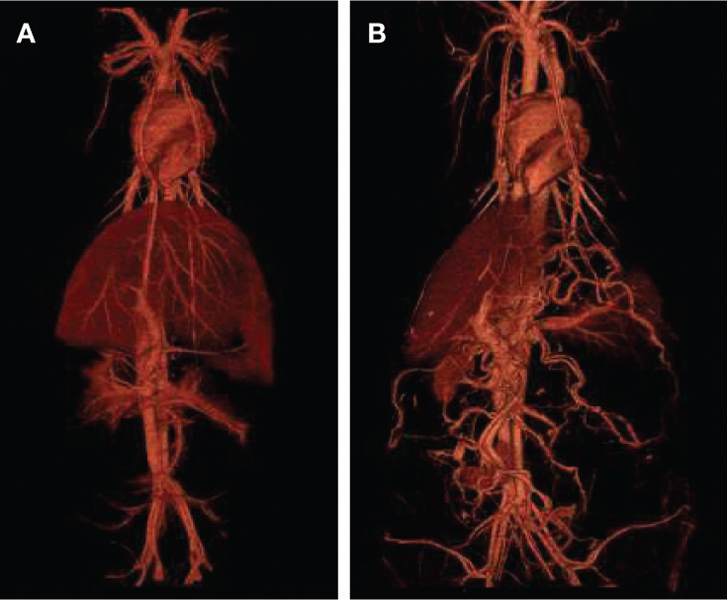
The pharmacokinetics and biodistribution of liposomal contrast agents have been studied in mice.31 32 Uniform and stable blood attenuation is obtained immediately after systemic administration of the liposomal contrast agent. The blood pool attenuation remains relatively uniform for several hours post administration, with attenuation decay gradually occurring over a period of several days. CT angiography studies performed in small and large animals demonstrated excellent visualization of the entire blood circulatory system using a single dose of liposomal contrast agent (Figure 2).
Figure 2.
3D volume-rendered images demonstrating whole-body vasculature in a pig (A) and sheep (B) model obtained after administration of liposomal blood pool contrast agent.
Cardiovascular Imaging in Large Animals
Unlike humans, cardiovascular CT imaging in small animals remains a major challenge.35 Due to higher heart rates (300 to 600 beats per minute) and respiration rates (80 to 120 per minute) in rodents, cardiorespiratory-gated scans typically take 8 to 10 minutes per cardiac phase cycle (~120 minutes for 12 cardiac phases). Stable and uniform opacification is required for this entire period, before gated imaging is feasible. The liposomal agent has enabled such studies. The uniform opacification of the cardiac chambers also facilitates determination of cardiac function parameters, thus enabling facile cardiac phenotyping in rodent models.
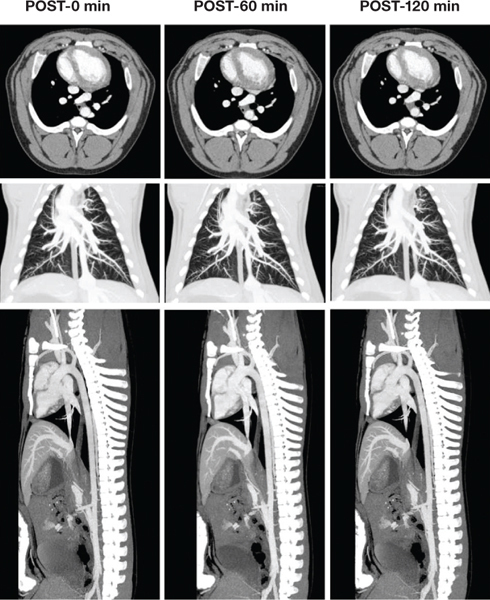
Cardiopulmonary vascular imaging has also been demonstrated in large animal models. Excellent visualization of the coronary arteries was demonstrated in a sheep model (Figure 3). Simultaneous imaging of pulmonary vasculature, heart, and descending aorta has also been demonstrated using a single injection of liposomal contrast agent (Figure 4). The availability of such an agent could facilitate the total diagnosis of acute chest pain, including the three critical differentials: myocardial infarction, pulmonary embolism, and aortic dissection, the aptly named “triple rule-out.”
Figure 3.
Cardiac CT angiography in a sheep demonstrating visualization of the coronary arteries at various time points (in minutes) after administration of liposomal contrast agent. LCA: left coronary artery; LAD: left anterior descending artery; LCX: left circumflex coronary artery.
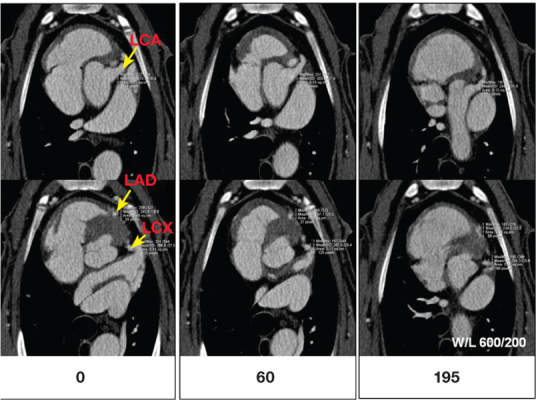
Figure 4.
Simultaneous visualization of pulmonary vasculature, heart, and descending aorta in a sheep model. Top row: axial images demonstrating uniform and stable attenuation in cardiac chambers. Middle row: coronal thick slab maximum intensity projection (MIP) images demonstrating the pulmonary vasculature. Bottom row: sagittal thick slab MIP images demonstrating the descending aorta. Images were acquired at various time points after a single injection of liposomal blood pool contrast agent.
Imaging of Pulmonary Embolism
Imaging of pulmonary emboli (PE) using liposomal contrast agent has been demonstrated in a rabbit36 and a pig model. Autologous blood clots, administered directly into the pulmonary artery, were confirmed using conventional contrast-enhanced CT scan. After washout of conventional agent, the liposomal blood pool contrast agent was administered and imaging was performed to evaluate clot visibility. A majority of clots detected on conventional PE scan were also demonstrated in images acquired with the liposomal contrast agent. Both segmental and subsegmental clots were demonstrated in images acquired using the liposomal contrast agent. Longitudinal imaging demonstrated visualization of PE for several hours. In the rabbit model, the liposomal contrast agent also enabled therapeutic tracking of clots after administration of recombinant tissue plasminogen activator (rtPA). Thus, a single dose of liposomal contrast agent enabled an “image and treat approach” (i.e., visualize pulmonary emboli and assess efficacy of treatment). The personalized imaging approach described in this study could have implications in the management of patients with acute stroke.
Similar to the rabbit study, longitudinal follow-up of pulmonary emboli after administration of the liposomal contrast agent has been demonstrated in a pig model (Figure 5). Uniform blood attenuation was obtained in both the arterial and venous phase, including the peripheral vasculature. In the clinic, this could facilitate workups of patients with venous thromboembolic (VTE) disease, since a single injection of blood pool contrast agent would facilitate simultaneous diagnosis of pulmonary embolism and deep vein thrombosis.
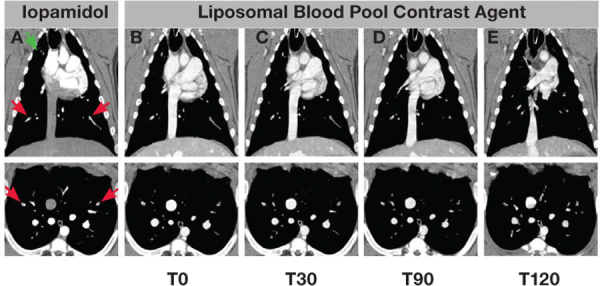
Figure 5.
Imaging of pulmonary embolism in a pig model. Representative coronal (top row) and axial images (bottom row) demonstrating visualization of bilateral lobe PE (red arrows) and right upper lung clot (green arrow) in conventional scan (column A) and at various time points after a single injection of liposomal contrast agent. Images using the liposomal contrast agent were acquired immediately after administration (T0, column B), after 30 minutes (T30, column C), after 90 minutes (T90, column D), and after 120 minutes (T120, column E). Note that the right upper lung clot moves out of plane by T30.
Cancer Imaging
The nanoscale size of liposomal carriers provides unique ways to detect and characterize solid tumors. Unlike conventional contrast agents that undergo rapid wash-in and wash-out between the vascular compartment and the tumor interstitial space, the transport of liposomal contrast agent within tumor tissue is primarily governed by convection.37 The extravasation of liposomal nanoparticles from the “leaky” tumor vascular compartment into the interstitial space occurs very slowly, typically on the time frame of hours-to-days instead of the seconds-to-minutes known for conventional contrast agent. These nanoparticles extravasate and accumulate in tumor tissues via the “enhanced permeation and retention” (EPR) effect.38 In fact, the efficacy of several nanoparticle-based chemotherapeutics, including Doxil® (PEGylated liposomal doxorubicin), is dependent on the EPR effect.39
Liposomal contrast agents can enable evaluation of solid tumors using two approaches (Figure 6). Early-phase imaging, defined as imaging within a few hours after administration of the contrast agent, enables visualization and characterization of tumor vasculature. During early-phase imaging, the liposomal contrast agent primarily resides within the vascular compartment, thus facilitating assessment of tumor perfusion. Delayed-phase imaging, defined as imaging at least 24 hours after administration of the contrast agent, enables visualization of tumor tissue due to signal enhancement from accumulation of liposomes within the tumor interstitial space. The utility of liposomal contrast agent for CT imaging and functional interrogation of solid tumors has been demonstrated in a mouse model of triple-negative breast cancer.32 Early-phase imaging enabled visualization of not only intratumoral vessels, but also tumor vessel co-option. Delayed-phase imaging demonstrated visualization of intratumoral regions with highly permeable vasculature (Figure 6). More interestingly, visualization of permeable vessels beyond tumor margins was also demonstrated (Figure 7).
Figure 6.
Dynamic imaging of tumors using liposomal contrast agent. Early-phase imaging (Post-0 hours) enables visualization of tumor vasculature and therefore assesses tumor perfusion. Delayed-phase imaging (post-24 hours and beyond) enables visualization of tumor due to heterogeneous signal enhancement from accumulation of liposomal nanoparticles within the tumor interstitial space.32
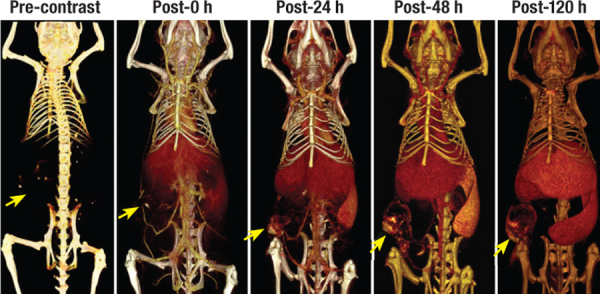
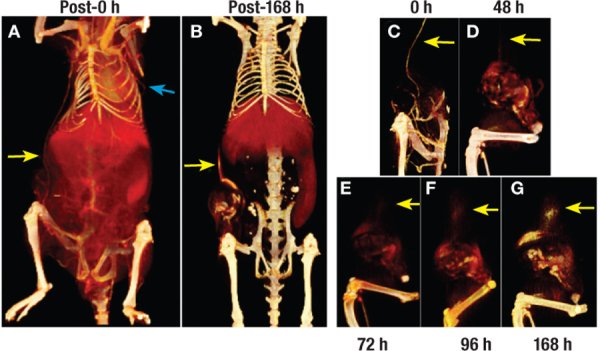
Figure 7.
Functional imaging of tumor vasculature. Visualization of co-opted (yellow arrow) and intratumoral vasculature in a mouse model of breast cancer. Longitudinal imaging enabled functional evaluation of both intratumoral and extratumoral blood vessels. The highly permeable nature of co-opted tumor blood vessel located outside the tumor margins resulted in diffuse signal enhancement due to extravasation of liposomal contrast agent into the perivascular space (yellow arrow).32
Dynamic imaging of liposomal contrast agent uptake in tumor tissue has also been used to assess tumor malignancy.40 In a rat model of mammary adenocarcinoma, it was shown that the rate of tumor uptake of liposomal contrast agent, defined as tumor vascular permeability, correlated with the tumor growth rate; rapidly growing tumors, an indicator of tumor malignancy, demonstrated higher uptake of liposomal contrast agent compared to slow growing tumors. In the same tumor model, quantitative measurement of liposomal-based tumor vascular permeability correlated with the expression levels of angiogenic biomarkers, namely vascular endothelial growth factor (VEGF) and VEGF receptor-2.40 The study demonstrated the benefit of liposomal contrast agent as an imaging surrogate for molecular profiling of angiogenic biomarkers within individual tumors.
Monitoring Chemotherapy
The use of liposomal contrast agents for prognostication and monitoring the efficacy of nano-chemotherapeutics has been demonstrated in preclinical studies. When injected intravenously into rats bearing a syngeneic mammary adenocarcinoma, liposomal contrast agent helped to predict the outcome of subsequent chemotherapy using a nanoparticle chemotherapeutic, PEGylated liposomal doxorubicin (PLD).33 The tumor uptake rate of liposomal contrast agent, as measured by the X-ray image using a clinical mammography system, correlated with the response of tumors upon treatment with PLD. Thus, in individual rats bearing tumors of identical size and morphology, those specific tumors that demonstrated high liposomal contrast agent uptake were most susceptible to treatment by PLD. If validated in the clinic, the liposomal contrast agent could be used as a prognosticator of liposomal chemotherapy outcome, providing obvious clinical value for personalizing therapy.
Conclusion
Blood pool iodinated agents represent a novel class of CT contrast agents. The preclinical applications described here provide a glimpse of their potential use in cardiovascular imaging. Clinical translation of these agents could not only improve current imaging but also provide a new paradigm for detection and management of vascular diseases.
Conflict of Interest Disclosures: All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and the following were reported: Drs. Annapragada and Hoffman are founders and significant shareholders of Marval Biosciences Inc., and Dr. Ghaghada is a significant shareholder of Marval Biosciences Inc.
Funding/Support: Drs. Annapragada’s and Hoffman’s work was partially funded by an unrestricted grant from Marval Biosciences Inc. and a grant from the National Institute of Biomedical Imaging and Bioengineering (2R44EB04700) awarded to Marval Biosciences Inc. (PI: R. Lebovitz). Dr. Karathanasis has received funding support from the American Cancer Society (IRG-91-022-18) and pilot funding from the Case Comprehensive Cancer Center (P30 CA043703).
References
- 1.Pan X, Siewerdsen J, La Riviere PJ, Kalender WA. Anniversary paper. Development of X-ray computed tomography: the role of medical physics and AAPM from the 1970s to present. Med Phys. 2008 Aug;35(8):3728–39.. doi: 10.1118/1.2952653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersilka M, Bruder H, Krauss B, Stierstorfer K, Flohr TG. Technical principles of dual source CT. Eur J Radiol. 2008 Dec;68(3):362–8.. doi: 10.1016/j.ejrad.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, Cheung AC, Bartling SH, Lisauskas J, Grasruck M, Leidecker C, et al. Flat-panel volume CT: fundamental principles, technology, and applications. Radiographics. 2008 Nov-Dec;28(7):2009–22.. doi: 10.1148/rg.287085004. [DOI] [PubMed] [Google Scholar]
- 4.Hsiao EM, Rybicki FJ, Steigner M. CT coronary angiography: 256-slice and 320-detector row scanners. Curr Cardiol Rep. 2010 Jan;12(1):68–75.. doi: 10.1007/s11886-009-0075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eide KR, Ødegård A, Myhre HO, Lydersen S, Hatlinghus S, Haraldseth O. DynaCT during EVAR — a comparison with multidetector CT. Eur J Vasc Endovasc Surg. 2009 Jan;37(1):23–30.. doi: 10.1016/j.ejvs.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Almén T. New media. Experience from 10 years of development of water-soluble nonionic contrast media. Invest Radiol. 1980 Nov-Dec;15(6 Suppl):S283–8.. doi: 10.1097/00004424-198011001-00062. [DOI] [PubMed] [Google Scholar]
- 7.Almén T. Development of nonionic contrast media. Invest Radiol. 1985 Jan-Feb;20(1 Suppl):S2–9.. doi: 10.1097/00004424-198501002-00003. [DOI] [PubMed] [Google Scholar]
- 8.National Institute of Standards and Technology [Internet]. Gaithersburg MD: U.S. Dept. of Commerce; c1996-2011. X-Ray Mass Attenuation Coefficients; 2009 Sep 17 [cited 2011 Nov 2]. Available from: http://physics.nist.gov/PhysRefData/XrayMassCoef/tab3.html. [Google Scholar]
- 9.Marenzi G, Assanelli E, Campodonico J, Lauri G, Marana I, De Metrio M, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009 Feb 3;150(3):170–7.. doi: 10.7326/0003-4819-150-3-200902030-00006. [DOI] [PubMed] [Google Scholar]
- 10.Wang CL, Cohan RH, Ellis JH, Adusumilli S, Dunnick NR. Frequency management, and outcome of extravasation of nonionic iodinated contrast medium in 69,657 intravenous injections. Radiology. 2007 Apr;243(1):80–7.. doi: 10.1148/radiol.2431060554. [DOI] [PubMed] [Google Scholar]
- 11.Namasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: an update. Curr Probl Diagn Radiol. 2006 Jul-Aug;35(4):164–9.. doi: 10.1067/j.cpradiol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Rudnick MR, Goldfarb S. Pathogenesis of contrast-induced nephropathy: experimental and clinical observations with an emphasis on the role of osmolality. Rev Cardiovasc Med. 2003;4 Suppl 5:S28–33.. [PubMed] [Google Scholar]
- 13.Tumlin J, Stacul F, Adam A, Becker CR, Davidson C, Lameire N, et al. Pathophysiology of contrast-induced nephropathy. Am J Cardiol. 2006 Sep 18;98(6A):14K–20K.. doi: 10.1016/j.amjcard.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010 Jan;5(1):4–9.. doi: 10.2215/CJN.05200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallett RL, Fleischmann D. Tools of the trade for CTA: MDCT scanners and contrast medium injection protocols. Tech Vasc Interv Radiol. 2006 Dec;9(4):134–42.. doi: 10.1053/j.tvir.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 16. Krishamurthy, Rajesh (Texas Children’s Hospital Houston, TX). Conversation with: Ananth Annapragada (The Singleton Department of Pediatric Radiology, Texas Children’s Hospital, Houston, Texas). 2011 Jul 8. [Google Scholar]
- 17.Saad WE, Saad N. Computer tomography for venous thromboembolic disease. Radiol Clin North Am. 2007 May;45(3):423–45.. doi: 10.1016/j.rcl.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005 Mar;234(3):661–73.. doi: 10.1148/radiol.2343031362. [DOI] [PubMed] [Google Scholar]
- 19.Miles K. Functional Computed Tomography. In: Padhani AR Choyke P, editors. New techniques in oncological imaging. New York, NY: Taylor & Francis; 2006. pp. 245-272. [Google Scholar]
- 20.Seltzer SE, Davis MA, Adams DF, Shulkin PM, Landis WJ, Havron A. Liposomes carrying diatrizoate. Characterization of biophysical properties and imaging applications. Invest Radiol. 1984 Mar-Apr;19(2):142–51.. doi: 10.1097/00004424-198403000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Gregoriadis G. Liposomes in therapeutic and preventive medicine: the development of the drug-carrier concept. Ann N Y Acad Sci. 1978;308:343–70.. doi: 10.1111/j.1749-6632.1978.tb22034.x. [DOI] [PubMed] [Google Scholar]
- 22.Lasic DD, Papahadjopoulos D. Liposomes revisited. Science. 1995 Mar 3;267(5202):1275–6.. doi: 10.1126/science.7871422. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Berestein G. Liposomes as carriers of antimicrobial agents. Antimicrob Agents Chemother. 1987 May;31(5):675–8.. doi: 10.1128/aac.31.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seltzer SE, Blau M, Herman LW, Hooshmand RL, Herman LA, Adams DF, et al. Contrast material-carrying liposomes: biodistribution, clearance, and imaging characteristics. Radiology. 1995 Mar;194(3):775–81.. doi: 10.1148/radiology.194.3.7862978. [DOI] [PubMed] [Google Scholar]
- 25.Desser TS, Rubin DL, Muller H, McIntire GL, Bacon ER, Toner JL. Blood pool and liver enhancement in CT with liposomal lodixanol: comparison with lohexol. Acad Radiol. 1999 Mar;6(3):176–83.. doi: 10.1016/S1076-6332(99)80404-8. [DOI] [PubMed] [Google Scholar]
- 26.Leander P, Höglund P, Børseth A, Kloster Y, Berg A. A new liposomal liver-specific contrast agent for CT: first human phase-I clinical trial assessing efficacy and safety. Eur Radiol. 2001;11(4):698–704.. doi: 10.1007/s003300000712. [DOI] [PubMed] [Google Scholar]
- 27.Lee FT, Jr, Chosy SG, Naidu SG, Goldfarb S, Weichert JP, Bakan DA, et al. CT depiction of experimental liver tumors: contrast enhancement with hepatocyte-selective iodinated triglyceride versus conventional techniques. Radiology. 1997 May;203(2):465–70.. doi: 10.1148/radiology.203.2.9114106. [DOI] [PubMed] [Google Scholar]
- 28.Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007 May;13(5):636–41.. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 29.Van Herck JL, De Meyer GR, Martinet W, Salgado RA, Shivalkar B, De Mondt R, et al. Multi-slice computed tomography with N1177 identifies ruptured atherosclerotic plaques in rabbits. Basic Res Cardiol. 2010 Jan;105(1):51–9.. doi: 10.1007/s00395-009-0052-0. [DOI] [PubMed] [Google Scholar]
- 30.Kao CY, Hoffman EA, Beck KC, Bellamkonda RV, Annapragada AV. Long-residence-time nanoscale liposomal iohexol for X-ray-based blood pool imaging. Acad Radiol. 2003 May;10(5):475–83.. doi: 10.1016/s1076-6332(03)80055-7. [DOI] [PubMed] [Google Scholar]
- 31.Mukundan S, Jr, Ghaghada KB, Badea CT, Kao CY, Hedlund LW, Provenzale JM, et al. A liposomal nanoscale contrast agent for preclinical CT in mice. AJR Am J Roentgenol. 2006 Feb;186(2):300–7.. doi: 10.2214/AJR.05.0523. [DOI] [PubMed] [Google Scholar]
- 32.Ghaghada KB, Badea CT, Karumbaiah L, Fettig N, Bellamkonda RV, Johnson GA, et al. Evaluation of tumor microenvironment in an animal model using a nanoparticle contrast agent in computed tomography imaging. Academic Radiology. 2011;18(1):20–30.. doi: 10.1016/j.acra.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karathanasis E, Suryanarayanan S, Balusu SR, McNeeley K, Sechopoulos I, Karellas A, et al. Imaging nanoprobe for prediction of outcome of nanoparticle chemotherapy by using mammography. Radiology. 2009(a);250(2):398–406.. doi: 10.1148/radiol.2502080801. [DOI] [PubMed] [Google Scholar]
- 34.Karathanasis E, Chan L, Balusu SR, D’Orsi CJ, Annapragada AV, Sechopoulos I, et al. Multifunctional nanocarriers for mammographic quantification of tumor dosing and prognosis of breast cancer therapy. Biomaterials. 2008 Dec;29(36):4815–22.. doi: 10.1016/j.biomaterials.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Badea CT, Fubara B, Hedlund LW, Johnson GA. 4-D micro-CT of the mouse heart. Mol Imaging. 2005 Apr-Jun;4(2):110–6.. doi: 10.1162/15353500200504187. [DOI] [PubMed] [Google Scholar]
- 36.Burke SJ, Annapragada A, Hoffman EA, Chen E, Ghaghada KB, Sieren J, et al. Imaging of pulmonary embolism and t-PA therapy effects using MDCT and liposomal iohexol blood pool agent preliminary results in a rabbit model. Acad Radiol. 2007 Mar;14(3):355–62.. doi: 10.1016/j.acra.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain RK. Transport of molecules particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63.. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 38.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Controlled Release. 2000;65(1-2):271–84.. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 39.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–36.. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 40.Karathanasis E, Chan L, Karumbaiah L, McNeeley K, D’Orsi CJ, Annapragada AV, et al. Tumor vascular permeability to a nanoprobe correlates to tumor-specific expression levels of angiogenic markers. PLoS One. 2009 Jun 9;4(6):e5843.. doi: 10.1371/journal.pone.0005843. [DOI] [PMC free article] [PubMed] [Google Scholar]


