
Introduction
The common goal in using liposomes for diagnostics and therapy is to deliver a pharmaceutic to the injured area. Liposomes are spherical, self-closed structures formed by one or several concentric lipid bilayers with an aqueous phase inside and between the lipid bilayers. Their ability to entrap different water-soluble compounds within the inner aqueous phase and lipophilic agents between liposomal bilayers has made them useful for delivery of different kinds of drugs and for carrying diagnostic agents in all imaging modalities — gamma-scintigraphy, magnetic resonance imaging (MRI), computed tomography (CT) imaging, and sonography. Liposomal modification with polyethylene glycol (PEG) increases their field of usage by enhancing circulation time and attachment of antibodies or different targeting moieties to their surface to target specific affected areas. Such modified liposomes and immunoliposomes can be considered for intravascular drug delivery, using cells and noncellular components (such as endothelial cells, subendothelial structures, and blood components) as the targeted sites for diagnosing and treating the most important cardiac pathologies, including myocardial infarction, coronary thrombosis, and atherosclerosis (Figure 1).
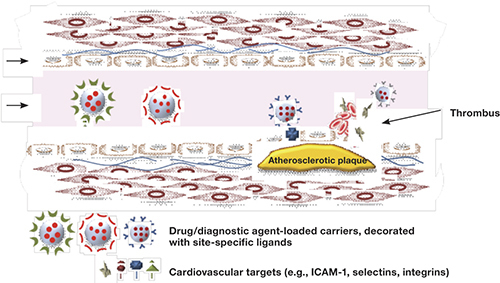
Figure 1.
Diagram of an arterial section with atherosclerotic plaque and thrombus, showing possible interaction with vascular target antigens attached to nanocarriers that are loaded with therapeutic or site-specific diagnostic ligands.
Accumulation of Liposomes and Immunoliposomes in the Ischemic Heart
Occlusion of coronary arteries by thrombi results in myocardial infarction (MI). Timely reperfusion is required to protect the myocardium from more permanent damage. During the ischemic phase and following reperfusion, extensive myocardial cell death occurs within the ischemic zone. 1 Cardiac myocyte death during coronary occlusion occurs mostly by apoptosis 2 or necrosis. 3 4
The ability of plain, unmodified liposomes to accumulate in regions of experimental MI was described long ago when 99mTc-DTPA was used as an MRI contrast agent. 5 However, to get a clear picture, the imaging agent needed to be delivered in relatively high quantity to the area of investigation. This problem was solved when immunoliposomes were used for the first time to visualize MI. 6 To enhance binding to a damaged area, liposomes with covalently attached antibodies to canine cardiac myosin were labeled with 111InCl3 and used to “actively” target infarcted myocardium in a dog experimental MI model. After temporary occlusion of the left coronary artery, animals were injected with antimyosin-labeled liposomes. Infarct imaging performed with a gamma camera showed the accumulation of immunoliposomes in the infarcted zone.
However, rapid sequestering of liposomes or immunoliposomes by the reticuloendothelial system in the infarct zone, with its limited blood supply, and opsonization of liposomes by plasma proteins lowers the ability of liposomes to reach the damaged organ. One of the ways to increase liposome circulation time is by coating them with polyethylene glycol (PEG). This decreases the rate of liposome opsonization and their recognition by liver cells, which then significantly increases nanoparticle lifetime in the blood. 7 The long-circulating PEG-coated liposomes can be made to target an antigen by incorporating antibodies onto the liposome’s surface. In an in vivo study, PEG-liposomes radiolabeled with 111In and with attached anti-myosin antibodies were used to target experimental MI in dogs and rabbits and provided good gamma-immunoscintigraphic images of the infarct within several hours of intravenous administration. 8
Myocardial infarcts were also visualized by sonography when gas-filled liposomes were used for infarct imaging in rabbits. 9 Use of echogenic liposomes for diagnosing cardiovascular pathology will be discussed below.
Protection of Infarcted Myocardium with Liposomal Formulations
“Plug and Seal” Mechanism
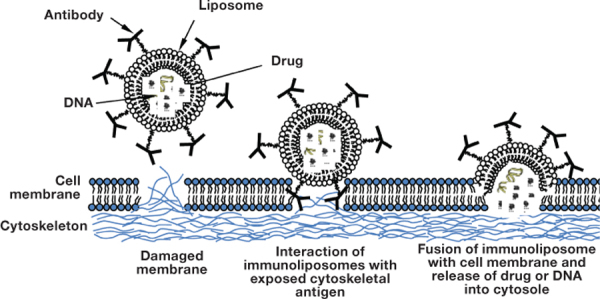
The presence of membrane lesions in ischemic tissue, in the form of microscopic holes, permits washout of intracellular macromolecules into the circulation. Intracellular proteins, including the components of the cytoskeleton (myosin, vimentin), can become exposed through these holes to the surroundings. Antibodies against intracellular cytoskeletal antigens could be used to reveal cell membrane lesions and, if coupled to liposomes, could deliver phospholipid vesicles to an affected cell’s surface. This suggestion was evaluated with antibody-directed liposomes to cytoskeletal antigens in an attempt to prevent or reduce hypoxia-induced release of intracellular contents and cell death by sealing (plugging) the membrane lesions. Antimyosin antibody was used to indicate the region of necrotic myocyte hypoxia-induced damage. Prevention of necrotic cell death by plugging and sealing was demonstrated in H9C2 rat embryonic cardiocytes under hypoxia conditions using myosin as the anchor antigen on the cell surface and the antimyosin antibody as the targeting device attached to the liposomes (Figure 2). 10 An important step was later made by Khaw’s group when it was shown in a Langendorff isolated rat heart model that treatment with cytoskeketal antigen-specific immunoliposomes can preserve cell and organ viability and function. 11
Figure 2.
Representation of the targeted delivery process, using antimyosin-labeled immunoliposomes loaded with drug or DNA.
Antimyosin immunoliposomes were also used in an in vivo rabbit model of experimental left ventricular MI. Mean myocardial infarct size in rabbit hearts treated with immunoliposomes was five times smaller than in those treated with nonspecific control liposomes or vehicle. 12 Immunoliposome targeting of areas of inflammation after an acute MI could also provide the means by which proangiogenic compounds can be selectively delivered to an infarcted region. The adhesion of a drug carrier composed of immunoliposomes coated with an antibody to P-selectin was quantified in a rat model of MI following left coronary artery ligation. 13 After treatment with anti-P-selectin-conjugated liposomes containing vascular endothelial growth factor (VEGF), noticeable improvements in cardiac function and post-infarct vasculature areas quantified in the model were accompanied by a 21% increase in the number of anatomical vessels and a 74% increase in the number of perfused vessels in the infarcted region of treated animals. 14
Liposomal Drug Delivery
The depletion of high-energy phosphates during ischemia or hypoxia is the fundamental cause of cell damage. Intravenous infusion of strongly charged adenosine triphosphate (ATP) as an additional energy supply is insufficient because of its molecular charge and short half-life in circulation. To protect ATP from degradation, Xu et al. tested different liposomal formulations in vitro and in vivo. 15 Biodistribution studies demonstrated significant accumulation of ATP liposomes in ischemia-damaged canine myocardium. 15
Later, Verma et al. demonstrated that optimized and targeted liposomes loaded with ATP can produce cardioprotective effects ex vivo in the Langendorff isolated rat heart model. 16 ATP-liposomes (ATP-L) injected 1 minute before starting a period of global ischemia provided significant protection to the ischemic myocardium after 30 minutes of reperfusion. At the end of reperfusion, the left ventricular end diastolic pressure was significantly reduced with ATP-L (61%) compared to the Krebs-Henseleit (KH) buffer. 16 When myosin-specific monoclonal antibodies were also attached to the liposomes, the left ventricular developed pressure significantly recovered to above 80% of the baseline compared to 25% in the KH buffer group. 17 This protective effect also depended on the amount of the antibody attached to the liposomal surface. The ATP-L were used for in vivo study in rabbits with an induced MI. Liposomes were administered by intracoronary infusion followed by 30 minutes of occlusion and 3 hours of reperfusion. The final irreversible damage in ATP-L-treated animals was approximately 30% of the total area at risk as compared with about 60% in the group treated with empty liposomes and roughly 70% in the KH buffer-treated group. 18
Coenzyme Q10 (CoQ10) has been reported as a potential agent for treatment and prevention of ischemia-reperfusion injury, hypertension, hyperlipidemia, coronary artery disease, and heart failure. 19 The possibility for reducing the experimental myocardial infarct size by intracoronary infusion of CoQ10 liposomes was again evaluated with the rabbit model of acute experimental infarction. 20 CoQ10-loaded empty liposomes or control buffer were administered by intracoronary infusion followed by 30 minutes of occlusion and 3 hours of reperfusion. The final irreversible damage in CoQ10-liposome-treated animals was only 30% of the total area at risk compared to the control. Thus, the ischemic heart was effectively protected by enhanced intracellular delivery of liposomal CoQ10.
Large-scale clinical trials have suggested the potential value of adenosine as another cardioprotective therapy for patients with acute MI, 21 but this agent has an extremely short half-life and causes hypotension and bradycardia because of its vasodilatory and negative chronotropic effects. 22 An experimental infarction study in rats, however, showed that an infusion with PEGylated liposomes augmented the cardioprotective effects of adenosine against ischemia/reperfusion injury and reduced its unfavorable hemodynamic effects. 23
Liposomal Gene Delivery
The treatment of myocardial ischemia using gene therapy is a rather novel but promising approach. When coupled to liposomes, antimyosin antibodies can deliver phospholipid vesicles to the affected cell surface and not only plug them directly into membrane holes but bring DNA entrapped in liposomes to the cytosol of damaged H9C1 embryonic cardiomyocytes in vitro. 24 All cell viability tests demonstrated a highly improved survival of hypoxic cells in the presence of immunoliposomes (up to 95% survival after 24 hours of hypoxia). Under hypoxic conditions, immunoliposomes successfully delivered entrapped plasmid to the H9C2 cells.
Gene delivery to targeted cells may be increased not only with specific antimyosin antibody but by combining specificity with enhanced intracellular penetration. 25 Double-targeted delivery systems were first used in vitro when liposome-plasmid DNA complexes modified with cell-penetrating transactivating transcriptional activator peptide (TATp) and/or monoclonal antimyosin antibody were tested with normoxic and hypoxic H9C2 cardiomyocytes. Transfection under both conditions was enhanced by the presence of TATp and was further enhanced by the additional modification with antibodies. In the in vivo rat study with experimental MI, an increased accumulation of immunoliposomes modified with TATp and an enhanced transfection of cardiomyocytes in the ischemic zone was clearly demonstrated. 25
Liposomes for Diagnosis of Thrombosis and Monitoring Thrombolytic Therapy
Visualization of thrombi and thrombolytic therapy are now mostly based on targeted delivery of contrast agents and thrombolytic drugs, such as the enzymes urokinase, streptokinase, and tissue plasminogen activator (tPA). The most important disadvantage of these pharmaceutics is their short plasma half-life. To solve this problem, liposomal formulations of thrombolytic enzymes were developed; 99mTc-radiolabeled fibrinolytic urokinase or streptokinase 26 27 was loaded onto liposomes and demonstrated ample enzymatic capacity and a slow release profile. Tracking the biodistribution behavior of these preparations by γ-imaging showed an increased thrombus uptake of the liposomal enzymes compared to that of the free streptokinase and an improved imaging quality of thrombi.
One of the additional approaches in this area is based on the concept of acoustically reflective (echogenic) liposomes (ELIP) that can be targeted to promote site-specific acoustic enhancement. 28 29 Liposomes conjugated with antifibrinogen antibodies via a thioether linkage acquired the ability to attach to fibrin-coated surfaces and thrombi in cell culture and in blood-flow models. 30 A recent study in an in vivo rabbit aorta clot model showed that ultrasound enhances thrombolysis when combined with a thrombolytic and a contrast agent. 31 Thrombi were created in denuded abdominal aorta, and tPA-loaded echogenic liposomes or empty liposomes were injected. Doppler ultrasound treatment resulted in earlier and more complete recanalization rates with tPA-loaded ELIP.
Thrombolytic Therapy with Polymeric Nanocarriers and Immunoliposomes
Several types of nanocarriers for delivery of thrombolytic drugs have been used to decrease ischemic damage after thrombus formation. One of the first thrombolytic agents, heparin, is degraded to inactive oligomer fragments when ingested orally, while systemic administration is complicated by the need for continuous infusion and the potential for uncontrolled hemorrhage. In 1988, Kim et al. showed that liposomal heparin administered intravenously into rats stayed in plasma longer than untreated heparin. 32 The prolonged biological activity was apparently due to a gradual release of heparin from the liposomes entrapped in the reticuloendothelial system and not due to prolonged circulation in the blood. Inhalable liposomal heparin was later studied in rat models of pulmonary embolism and deep vein thrombosis. 33 A once-every-other-day inhaled dose of PEGylated liposomes loaded with low-molecular-weight heparin showed similar efficacy in reducing thrombus weight as with a once-daily dose of subcutaneously administered drug. Liposomal prostaglandin E1 administered intravenously before thrombolytic therapy resulted in a significant shortening of thrombolysis time, improvement in coronary patency and blood flow during reperfusion, and a reduction in infarct size. 34
For treatment of MI, a liposome-encapsulated thrombolytic agent (t-PA) was compared with free t-PA in a rabbit jugular vein thrombosis model. 35 Injection of liposomal t-PA had significantly better thrombolytic efficiency than equimolar doses of free t-PA. On the other hand, liposome encapsulation of t-PA did not affect the systemic activation of alpha 2-antiplasmin and plasminogen. For this model, improved thrombolytic efficacy of t-PA is achieved by liposome encapsulation.
Echogenic liposomes were used to further develop the targeted delivery of t-PA and to investigate the effect of ultrasound exposure on thrombolytic efficacy. Following a 50% t-PA entrapment into ELIP, ex vivo porcine clots treated with tPA-loaded echogenic liposomes were lysed with an effect similar to treatment with free t-PA. 36 T-PA can also be delivered as a PEGylated anionic gelatin complex 37 or in microbubbles with the Arg-Gly-Asp-Ser (RGDS) tetrapeptide as a targeting agent. 38 In both cases, after IV injection in a rabbit model, t-PA was released from the nano-sized delivery complex when exposed to ultrasound.
To test whether liposomal entrapment would enhance the streptokinase effect, a fibrinolytic formulation with a different mode of action and inactivation (liposomal streptokinase) was compared with free enzyme in an experimental rabbit model of thrombolysis. 39 40 The result showed that both liposomes and polymer microcapsules reduced time to reperfusion and residual clot mass and improved return of blood flow compared to identical dosages of free streptokinase in the thrombosed rabbit carotid artery. The fibrinogen-mimetic cyclic arginine-glycine-aspartate (RGD) peptide, which has an affinity for activated platelets, may be useful for the targeted delivery of thrombolytic agents. Viadya et al. developed RGD-peptide-conjugated liposomes loaded with streptokinase. 41 This in vitro drug release study showed that nearly 40% of the entrapped drug was released in 12 hours in phosphate buffered saline (pH 7.4). However, on incubation with activated platelets, about 90% of the drug was released within 45 minutes. Urokinase, another member of the thrombolytic cascade, can also be delivered by thrombus-targeted immunoliposomes incorporating a D-dimer monoclonal antibody to liposomes, and it increases the thrombolytic efficiency compared to free drug in a rabbit pulmonary thromboembolism model. 42
Targeting of Atherosclerotic Lesions for Tomographic Imaging and Treatment
Localization and visualization of atheromas has usually been performed with various antibodies labeled with radioactive γ-emitting isotopes lllIn- and 99mTc or heavy metals bound to the antibody via chemical incorporation of a chelating group. 43 44 Later liposomal formulations were designed for MRI of atherosclerotic lesions. E-selectin-expressing human umbilical vein endothelial cells (HUVEC) were incubated with PEGylated paramagnetic fluorescently labeled liposomes carrying anti-E-selectin monoclonal antibody as a targeting ligand. In this study, both MRI and fluorescence microscopy revealed specific association of the liposomal MRI contrast agent with stimulated HUVEC, 45 suggesting that this newly developed MRI system may serve as a useful diagnostic tool to investigate these pathological processes in vivo.
For early detection of atherosclerotic plaques by computed tomographic (CT) imaging, Danila et al. investigated the use of immunoliposomes loaded with the CT contrast agent. 46 Anti-ICAM-1 monoclonal antibodies covalently attached to PEGylated liposomes loaded with iohexol bound specifically to activated human coronary artery endothelial cells in cell culture. Thus, iohexol-filled immunoliposomes have potential use in CT angiography for noninvasive detection of atherosclerotic plaques that are prone to rupture.
The ICAM-1 antibodies were also used to develop acoustically reflective liposomes that can be conjugated for site-specific acoustic enhancement. 47 In Yucatan mini pigs with experimental atherosclerosis, conjugated liposomes retained their acoustically reflective properties and provided ultrasonic image enhancement of their targeted structures. Liposomes conjugated to antifibrinogen attached to thrombi and to fibrous portions of the atheroma, whereas liposomes conjugated to anti-ICAM-1 attached to early atheromas. Ultrasound-enhanced delivery of Rhodamine-labeled echogenic liposomes (Rh-ELIP) within the arterial wall to detect atheroma was observed in an ex vivo mouse model. 48 Subendothelial penetration of Rh-ELIP was present in all ultrasound-treated aortae and was absent in those not exposed to ultrasound.
Almer et al. recently investigated a promising new candidate for improved visualization of atherosclerotic plaques. 49 Globular domain of adiponectin (gAd) was coupled to liposomes and evaluated for its potency to characterize in critical scenarios within early and advanced atherosclerotic plaque lesions using an atherosclerotic mouse model. 49 Ex vivo imaging performed by confocal laser scanning microscopy showed a strong fluorescent signal at the surface of atherosclerotic plaques treated with gAd-coupled nanoparticles.
Imaging of atherosclerotic plaque can also be performed using paramagnetic gadolinium liposomes enriched with phosphatidylserine (PS) for targeting macrophages mimicking apoptosis. 50 In vivo performance of Gd-PS-liposomes was evaluated in the ApoE(-/-) mouse model with an MRI system, which revealed rapid and significant image enhancement of the aortic wall after injection. Gd-PS-enriched liposomes enhanced atherosclerotic plaque and colocalized with macrophages in experimental atherosclerosis. Gadolinium-labeled liposomes were used as multifunctional nanocarriers for simultaneous monitoring and delivery of an anti-inflammatory drug to treat atherosclerosis. A liposomal formulation of glucocorticoids (L-PLP) was developed and applied intravenously in a rabbit model of atherosclerosis. 51 MRI was used to monitor the delivery of L-PLP into atherosclerotic plaques. Significant anti-inflammatory effects were observed as early as 2 days and lasted up to at least 7 days after administration of a single dose of L-PLP. This study was a two-pronged strategy for efficient treatment of atherosclerotic plaque and the application of noninvasive and clinically approved imaging techniques to monitor delivery and therapeutic responses.
Miscellaneous Drug Delivery with Liposomes in the Cardiovascular System
The unique properties of liposomes, such as their biodegradability, low toxicity, high carrying capacity, and ease of preparation, make them an excellent candidate for drug encapsulation that can be given with reduced pain upon injection. Vesicles formulated with phospholipids and PEG-HS, an excipient chosen to modulate the bilayer properties, were selected as the drug delivery system for istaroxime, 52 which represents a promising and safe treatment of both acute and chronic heart failure.
The ability of liposomes not only to release the encapsulated drug, but also to capture compounds from the bloodstream, can be used to treat cardiovascular intoxication. Long-circulating liposomes with a transmembrane pH gradient were developed, and in vivo scavenging properties were demonstrated by examining the drug’s pharmacokinetics as a scavenging nanocarrier for diltiazem intoxication. 53
Pulmonary complications are common in cardiovascular pathology. In these cases, liposomal formulations have also proved to be useful for therapy and diagnosis. Plain liposomes have been shown to protect vasoactive intestinal peptide (VIP) from rapid proteolytic degradation in rats. 54 This peptide exhibits a very short period of activity in the lung, but the liposomal VIP remains intact after an inhalative administration, which is preferable in lung pathology. To enhance therapeutic or diagnostic effects of liposomal formulations, different targeting moieties were used for liposome modification. In the early 1990s, it was shown that PEGylated liposomes with attached monoclonal antibodies to pulmonary endothelial cells have prolonged circulation time and increased binding in lungs. 55 56 Later, different molecular determinants on the surface of pulmonary endothelial cells were used for targeted delivery of liposomal nanocarriers. 57 58
Liposomes can be used not only as an independent drug delivery system but also in combination with polymer-coated stents. Stents coated with polymeric material containing dispersed or encapsulated drugs have been developed and used as a noninvasive method to deal with stent-related complications. The possibility of coating polymer-covered stents with heparin-encapsulating liposomes was investigated for improvement of their haemocompatibility. 59 Encapsulated heparin retained its biological functionality, and release time depended on the lipid composition and method of liposome preparation. The same strategy used to develop the latest generation of drug-eluting stents was applied to show the possibility for gene delivery. To reduce coronary restenosis, Brito et al. 60 developed a gene-eluting stent with liposome/DNA complexes immobilized on the stainless steel surface. In vivo studies in an iliac artery restenosis model in rabbits showed that green fluorescent protein expression in arterial tissues developed after 24 hours of implantation.
Future Applications
One of the important and promising directions of drug delivery systems for cardiovascular therapy and imaging has been the attempt to combine molecular imaging for detection of inflammation, apoptosis, extracellular matrix, and angiogenesis with drug delivery of a therapeutic agent. 61 Using a broad spectrum of targeting moieties such as vascular and intracellular adhesion molecules, selectins, macrophages and their scavenger receptors, and integrins, new nanocarriers may be developed for noninvasive treatment of patients with cardiovascular diseases to reduce the morbidity and mortality rates.
Conflict of Interest Disclosure: All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: The authors have no funding disclosures.
Contributor Information
Tatyana S. Levchenko, The Center for Pharmaceutical Biotechnology and Nanomedicine, Northeastern University, Boston, Massachusetts
William C. Hartner, The Center for Pharmaceutical Biotechnology and Nanomedicine, Northeastern University, Boston, Massachusetts
Vladimir P. Torchilin, The Center for Pharmaceutical Biotechnology and Nanomedicine, Northeastern University, Boston, Massachusetts
References
- 1.Freude B, Masters TN, Robicsek F, Fokin A, Kostin S, Zimmermann R, et al. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol. 2000 Feb;32(2):197–208. doi: 10.1006/jmcc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994 Oct;94(4):1621–8. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005 Mar 31;434(7033):652–8. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 4.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960 Jul;70:68–78. [PubMed] [Google Scholar]
- 5.Caride VJ, Zaret BL. Liposome accumulation in regions of experimental myocardial infarction. Science. 1977 Nov 18;198(4318):735–8. doi: 10.1126/science.910155. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin VP, Khaw BA, Smirnov VN, Haber E. Preservation of antimyosin antibody activity after covalent coupling to liposomes. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1114–9. doi: 10.1016/0006-291x(79)92123-5. [DOI] [PubMed] [Google Scholar]
- 7.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990 Jul 30;268(1):235–7. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 8.Torchilin VP, Klibanov AL, Huang L, O’Donnell S, Nossiff ND, Khaw BA. Targeted accumulation of polyethylene glycol-coated immunoliposomes in infarcted rabbit myocardium. FASEB J. 1992 Jun;6(9):2716–9. doi: 10.1096/fasebj.6.9.1612296. [DOI] [PubMed] [Google Scholar]
- 9.Unger E, Shen DK, Fritz T, Lund P, Wu GL, Kulik B, et al. Gas-filled liposomes as echocardiographic contrast agents in rabbits with myocardial infarcts. Invest Radiol. 1993 Dec;28(12):1155–9. doi: 10.1097/00004424-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Khaw BA, Torchilin VP, Vural I, Narula J. Plug and seal: prevention of hypoxic cardiocyte death by sealing membrane lesions with antimyosin-liposomes. Nat Med. 1995 Nov;1(11):1195–8. doi: 10.1038/nm1195-1195. [DOI] [PubMed] [Google Scholar]
- 11.Khaw BA, Khudairi T. Dose-response to cytoskeletal-antigen specific immunoliposome therapy for preservation of myocardial viability and function in langendorff instrumented rat hearts. J Liposome Res. 2007;17(2):63–77. doi: 10.1080/08982100701375035. [DOI] [PubMed] [Google Scholar]
- 12.Khaw BA, DaSilva J, Hartner WC. Cytoskeletal-antigen specific immunoliposome-targeted in vivo preservation of myocardial viability. Journal of Controlled Release. 2007 Nov 16;120(1-2):35–40. doi: 10.1016/j.jconrel.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Scott RC, Wang B, Nallamothu R, Pattillo CB, Perez-Liz G, Issekutz A, et al. Targeted delivery of antibody conjugated liposomal drug carriers to rat myocardial infarction. Biotechnology and Bioengineering. 2007 Mar 1;96(4):795–802. doi: 10.1002/bit.21233. [DOI] [PubMed] [Google Scholar]
- 14.Scott RC, Rosano JM, Ivanov Z, Wang B, Chong P, Issekutz, AC, et al. Targeting VEGF-encapsulated immunoliposomes to MI heart improves vascularity and cardiac function. FASEB J. 2009 Oct;23(10):3361–7. doi: 10.1096/fj.08-127373. [DOI] [PubMed] [Google Scholar]
- 15.Xu GX, Xie XH, Liu FY, Zang DL, Zheng DS, Huang DJ, et al. Adenosine triphosphate liposomes: encapsulation and distribution studies. Pharm Res. 1990 May;7(5):553–7. doi: 10.1023/a:1015837321087. [DOI] [PubMed] [Google Scholar]
- 16.Verma DD, Levchenko TS, Bernstein EA, Torchilin VP. ATP-loaded liposomes effectively protect mechanical functions of the myocardium from global ischemia in an isolated rat heart model. J Control Release. 2005 Nov 28;108(2-3):460–71. doi: 10.1016/j.jconrel.2005.08.029. Epub 2005 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma DD, Levchenko TS, Bernstein EA, Mongayt D, Torchilin VP. ATP-loaded immunoliposomes specific for cardiac myosin provide improved protection of the mechanical functions of myocardium from global ischemia in an isolated rat heart model. J Drug Target. 2006 Jun;14(5):273–80. doi: 10.1080/10611860600763103. [DOI] [PubMed] [Google Scholar]
- 18.Verma DD, Hartner WC, Levchenko TS, Bernstein EA, Torchilin VP. ATP-loaded liposomes effectively protect the myocardium in rabbits with an acute experimental myocardial infarction. Pharmaceutical Research. 2005 Dec;22(12):2115–20. doi: 10.1007/s11095-005-8354-x. [DOI] [PubMed] [Google Scholar]
- 19.Sarter B. Coenzyme Q10 and cardiovascular disease: a review. J Cardiovasc Nurs. 2002 Jul;16(4):9–20. doi: 10.1097/00005082-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Verma DD, Hartner WC, Thakkar V, Levchenko TS, Torchilin VP. Protective effect of coenzyme Q10-loaded liposomes on the myocardium in rabbits with an acute experimental myocardial infarction. Pharm Res. 2007 Nov;24(11):2131–7. doi: 10.1007/s11095-007-9334-0. [DOI] [PubMed] [Google Scholar]
- 21.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol. 2005 Jun 7;45(11):1775–80. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Forman MB, Stone GW, Jackson EK. Role of adenosine as adjunctive therapy in acute myocardial infarction. Cardiovasc Drug Rev. 2006 Summer;24(2):116–47. doi: 10.1111/j.1527-3466.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 23.Takahama H, Minamino T, Asanuma H, Fujita M, Asai T, Wakeno M, et al. Prolonged targeting of ischemic/reperfused myocardium by liposomal adenosine augments cardioprotection in rats. J Am Coll Cardiol. 2009 Feb;53(8):709–17. doi: 10.1016/j.jacc.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Khaw BA, Narula J, Vural I, Torchilin VP. Cytoskeleton-specific immunoliposomes: sealing of hypoxic cells and intracellular delivery of DNA Int J Pharm. 1998 Mar;162(1-2):71–6. [Google Scholar]
- 25.Ko YT, Hartner WC, Kale A, Torchilin VP. Gene delivery into ischemic myocardium by double-targeted lipoplexes with anti-myosin antibody and TAT peptide. Gene Ther. 2009 Jan;16(1):52–9. doi: 10.1038/gt.2008.135. [DOI] [PubMed] [Google Scholar]
- 26.Erdogan S, Ozer AY, Bilgili H. In vivo behaviour of vesicular urokinase. Int J Pharm. 2005 May 13;295(1-2):1–6. doi: 10.1016/j.ijpharm.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Erdogan S, Ozer AY, Volkan B, Caner B, Bilgili H. Thrombus localization by using streptokinase containing vesicular systems. Drug Deliv. 2006 Jul-Aug;13(4):303–9. doi: 10.1080/10717540600559544. [DOI] [PubMed] [Google Scholar]
- 28.Alkan-Onyuksel H, Demos SM, Lanza GM, Vonesh MJ, Klegerman ME, Kane BJ, et al. Development of inherently echogenic liposomes as an ultrasonic contrast agent. J Pharm Sci. 1996 May;85(5):486–90. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton A, Huang SL, Warnick D, Stein A, Rabbat M, Madhav T, et al. Left ventricular thrombus enhancement after intravenous injection of echogenic immunoliposomes: studies in a new experimental model. Circulation. 2002 Jun 11;105(23):2772–8. doi: 10.1161/01.cir.0000017500.61563.80. [DOI] [PubMed] [Google Scholar]
- 30.Demos SM, Onyuksel H, Gilbert J, Roth SI, Kane B, Jungblut P, et al. In vitro targeting of antibody-conjugated echogenic liposomes for site-specific ultrasonic image enhancement. J Pharm Sci. 1997 Feb;86(2):167–71. doi: 10.1021/js9603515. [DOI] [PubMed] [Google Scholar]
- 31.Laing ST, Moody M, Smulevitz B, Kim H, Kee P, Huang S, et al. Ultrasound-enhanced thrombolytic effect of tissue plasminogen activator-loaded echogenic liposomes in an in vivo rabbit aorta thrombus model — brief report. Arterioscler Thromb Vasc Biol. 2011 Jun;31(6):1357–9. doi: 10.1161/ATVBAHA.111.225938. Epub 2011 Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim TD, Kambayashi J, Sakon M, Tsujinaka T, Ohshiro T, Mori T. Metabolism of liposome-encapsulated heparin. Thromb Res. 1989 Nov 1;56(3):369–76. doi: 10.1016/0049-3848(89)90249-1. [DOI] [PubMed] [Google Scholar]
- 33.Bai S, Gupta V, Ahsan F. Cationic liposomes as carriers for aerosolized formulations of an anionic drug: safety and efficacy study. Eur J Pharm Sci. 2009 Sep 10;38(2):165–71. doi: 10.1016/j.ejps.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feld S, Li G, Amirian J, Felli P, Vaughn WK, Accad M, et al. Enhanced thrombolysis, reduced coronary reocclusion and limitation of infarct size with liposomal prostaglandin E1 in a canine thrombolysis model. J Am Coll Cardiol. 1994 Nov 1;24(5):1382–90. doi: 10.1016/0735-1097(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 35.Heeremans JL, Prevost R, Bekkers ME, Los P, Emeis JJ, Kluft C, et al. Thrombolytic treatment with tissue-type plasminogen activator (t-PA) containing liposomes in rabbits: a comparison with free t-PA. Thromb Haemost. 1995 Mar;73(3):488–94. [PubMed] [Google Scholar]
- 36.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119(6):777–84.. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uesugi Y, Kawata H, Jo J, Saito Y, Tabata Y. An ultrasound-responsive nano delivery system of tissue-type plasminogen activator for thrombolytic therapy. J Control Release. 2010 Oct 15;147(2):269–77. doi: 10.1016/j.jconrel.2010.07.127. [DOI] [PubMed] [Google Scholar]
- 38.Hua X, Liu P, Gao YH, Tan KB, Zhou LN, Liu Z, et al. Construction of thrombus-targeted microbubbles carrying tissue plasminogen activator and their in vitro thrombolysis efficacy: a primary research. J Thromb Thrombolysis. 2010 Jul;30(1):29–35. doi: 10.1007/s11239-010-0450-z. [DOI] [PubMed] [Google Scholar]
- 39.Leach JK, O’Rear EA, Patterson E, Miao Y, Johnson AE. Accelerated thrombolysis in a rabbit model of carotid artery thrombosis with liposome-encapsulated and microencapsulated streptokinase. Thromb Haemost. 2003 Jul;90(1):64–70. [PubMed] [Google Scholar]
- 40.Leach JK, Patterson E, O’Rear EA. Improving thrombolysis with encapsulated plasminogen activators and clinical relevance to myocardial infarction and stroke. Clin Hemorheol Microcirc. 2004;30(3-4):225–8.. [PubMed] [Google Scholar]
- 41.Vaidya B, Nayak MK, Dash D, Agrawal GP, Vyas SP. Development and characterization of site specific target sensitive liposomes for the delivery of thrombolytic agents. Int J Pharm. 2011 Jan 17;403(1-2):254–61. doi: 10.1016/j.ijpharm.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Lü CP, Yang H, Wang J, Dong XL. [Thrombolysis of rabbit’s pulmonary embolism with thrombus-targeted urokinase immune liposome]. Zhonghua Xin Xue Guan Bing Za Zhi. 2009 Nov;37(11):1035–8. [PubMed] [Google Scholar]
- 43.Tekabe Y, Einstein AJ, Johnson LL, Khaw BA. Targeting very small model lesions pretargeted with bispecific antibody with 99mTc-labeled high-specific radioactivity polymers. Nucl Med Commun. 2010 Apr;31(4):320–7. doi: 10.1097/MNM.0b013e32833576e8. [DOI] [PubMed] [Google Scholar]
- 44.Narula J, Petrov A, Pak KY, Ditlow C, Chen F, Khaw BA. Noninvasive detection of atherosclerotic lesions by 99mTc-based immunoscintigraphic targeting of proliferating smooth muscle cells. Chest. 1997 Jun;111(6):1684–90. doi: 10.1378/chest.111.6.1684. [DOI] [PubMed] [Google Scholar]
- 45.Mulder WJ, Strijkers GJ, Griffioen AW, Van Bloois L, Molema G, Storm G, et al. A liposomal system for contrast-enhanced magnetic resonance imaging of molecular targets. Bioconjug Chem. 2004 Jul-Aug;15(4):799–806. doi: 10.1021/bc049949r. [DOI] [PubMed] [Google Scholar]
- 46.Danila D, Partha R, Elrod DB, Lackey M, Casscells SW, Conyers JL. Antibody-labeled liposomes for CT imaging of atherosclerotic plaques: in vitro investigation of an anti-ICAM antibody-labeled liposome containing iohexol for molecular imaging of atherosclerotic plaques via computed tomography. Tex Heart Inst J. 2009;36(5):393–403.. [PMC free article] [PubMed] [Google Scholar]
- 47.Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagarai A, Greene R, et al. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol. 1999 Mar;33(3):867–75. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
- 48.Hitchcock KE, Caudell DN, Sutton JT, Klegerman ME, Vela D, Pyne-Geithman GJ, et al. Ultrasound-enhanced delivery of targeted echogenic liposomes in a novel ex vivo mouse aorta model. J Control Release. 2010 Jun 15;144(3):288–95. doi: 10.1016/j.jconrel.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almer G, Wernig K, Saba-Lepek M, Haj-Yahya S, Rattenberger J, Wagner J, et al. Adiponectin-coated nanoparticles for enhanced imaging of atherosclerotic plaques. Int J Nanomedicine. 2011;6:1279–90. doi: 10.2147/IJN.S18739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maiseyeu A, Mihai G, Kampfrath T, Simonetti OP, Sen CK, Roy S, et al. Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J Lipid Res. 2009 Nov;50(11):2157–63. doi: 10.1194/jlr.M800405-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lobatto ME, Fayad ZA, Silvera S, Vucic E, Calcagno C, Mani V, et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm. 2010 Dec 6;7(6):2020–9. doi: 10.1021/mp100309y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luciani P, Fevre M, Leroux JC. Development and physico-chemical characterization of a liposomal formulation of istaroxime. Eur J Pharm Biopharm. 2011 Oct;79(2):285–93. doi: 10.1016/j.ejpb.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Bertrand N, Bouvet C, Moreau P, Leroux JC. Transmembrane pH-gradient liposomes to treat cardiovascular drug intoxication. ACS Nano. 2010 Dec 28;4(12):7552–8. doi: 10.1021/nn101924a. [DOI] [PubMed] [Google Scholar]
- 54.Stark B, Andreae F, Mosgoeller W, Edetsberger M, Gaubitzer E, Koehler G, et al. Liposomal vasoactive intestinal peptide for lung application: protection from proteolytic degradation. Eur J Pharm Biopharm. 2008 Sep;70(1):153–64. doi: 10.1016/j.ejpb.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Maruyama K, Kennel SJ, Huang L. Lipid composition is important for highly efficient target binding and retention of immunoliposomes. Proc Natl Acad Sci U S A. 1990 Aug; 87(15):5744–8. doi: 10.1073/pnas.87.15.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bloemen PG, Henricks PA, van Bloois L, van den Tweel MC, Bloem AC, Nijkamp FP, et al. Adhesion molecules: a new target for immunoliposome-mediated drug delivery. FEBS Lett. 1995 Jan 3;357(2):140–4. doi: 10.1016/0014-5793(94)01350-a. [DOI] [PubMed] [Google Scholar]
- 57.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009 Jan; 335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv. 2005 Sep;2(5):909–26. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 59.Koromila G, Michanetzis GP, Missirlis YF, Antimisiaris SG. Heparin incorporating liposomes as a delivery system of heparin from PET-covered metallic stents: effect on haemocompatibility. Biomaterials. 2006 Apr;27(12):2525–33. doi: 10.1016/j.biomaterials.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 60.Brito LA, Chandrasekhar S, Little SR, Amiji MM. In vitro and in vivo studies of local arterial gene delivery and transfection using lipopolyplexes-embedded stents. J Biomed Mater Res A. 2010 Apr;93(1):325–36. doi: 10.1002/jbm.a.32488. [DOI] [PubMed] [Google Scholar]
- 61.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic Nanomedicines. Acc Chem Res. 2011 May;44(10):1029–38. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]


