Abstract
Cardiovascular diseases are widely prevalent in western societies, and their associated costs number in the billions of dollars and affect millions of patients each year. Nanovectors targeted to tissues involved in cardiovascular diseases offer great opportunities to improve cardiovascular treatment through their imaging and drug delivery capabilities. Vascular-targeted imaging particles may permit the early identification of atherosclerosis, discriminate between stable and vulnerable atherosclerotic plaques, or guide surgeons as they work on fragile vasculature. Tailored therapeutic nanoparticles may provide safer, more efficient and effective intervention through localization and release of encapsulated therapeutics. Nanovector design involves numerous considerations such as fabrication material, particle size, and surface-modification with ligands for targeting and increasing blood circulation times. Complex blood rheology may affect the efficiency with which dissimilarsized particles target ligand receptors associated with disease. Additionally, the intended use of a nanovector is a critical factor in its design as some materials with poor drug-loading qualities or release kinetics may be suitable for imaging purposes only. Overall, vectors targeted to the vasculature will need to be efficient in avoiding blood clearance, honing to the target location, and binding at the desired site.
Keywords: vascular targeting, nanovectors, atherosclerosis, stroke, atheroma, molecular imaging, contrast agent, phospholipids, liposomes, micelles

Cardiovascular Disease and Nanovectors
Cardiovascular diseases (CVDs) encompass a wide variety of disorders affecting the blood vessels and heart. These conditions include angina, arrhythmias, atherosclerosis, cardiomyopathy, stroke, hypertension, myocarditis, and pericarditis. Nearly $0.5 trillion dollars per annum is spent on treating cardiovascular diseases. Coronary artery disease (CAD), stemming from atherosclerosis, is the leading cause of death from myocardial infarction in the western world. In the United States, CAD results in about one-third of total deaths.1 Many of these patients succumb to thrombi that form rapidly and occlude vessels completely after rupture of atherosclerotic plaques. In many cases, plaques that rupture are nonstenotic (most cause less than 50% luminal narrowing) and evade detection by traditional imaging methods (i.e., X-ray angiography, intravascular ultrasound).2–6 Consequently, treatment of CAD does not occur until the blood flow has been severely compromised, and it usually involves surgical intervention. Such an invasive procedure is by nature undesirable, does not address the underlying cause of the myocardial infarction, and thus fails to prevent reoccurrence. Statin treatment has been effective at reducing acute coronary complications due to atherosclerosis; nonetheless, acute complications continue to occur in more than half of the patients, and aggressive statin treatment has been associated with serious side effects.7 8 Development of effective noninvasive imaging methods for early detection and consequent therapy that can treat the underlying causes of CAD and other cardiovascular diseases remain a major focus of cardiovascular research. Nanovectors offer potential for improving current treatment options through more complete imaging information and delivery of drugs specifically targeted to tissues affected by disease.
There are numerous biochemical processes associated with the pathogenesis and destabilization of plaques that precede anatomical and physiological changes. By targeting the presence or activity of proteins associated with these biological processes, clinicians can identify nonstenotic vulnerable plaques before rupture and treat the underlying cause of plaque destabilization. The ability to detect these proteins with diagnostic imaging techniques has stimulated the development of targeted nanovectors containing contrast-enhancing agents. This form of imaging, known as molecular imaging, has been used to detect angiogenesis in early stage atherosclerosis and the activity of matrix metalloproteinases, a protease involved in plaque remodeling and destabilization.9–11 Upon diagnosing the stage and determining the extent of disease, nanovectors can transport therapeutics specifically to the diseased tissue, thus localizing treatment and reducing adverse side effects associated with systemic administration.12
To be successful, targeted drug delivery and/or imaging systems must reach their intended destination in functional form. This requires navigation through the blood stream to the target, including the ability to avoid the body’s clearance processes, to find the vessel wall from blood flow, and then to bind to the desired target. Synthesizing nanovectors that avoid immune clearance and thus possess increased circulation time is challenging since particles are typically quickly removed from the bloodstream. Approaches such as PEGylation and varying the size, shape, and composition of nanovectors may be explored to achieve this goal.
Cardiovascular Targets
Recent technologies have focused on discovering appropriate molecules to target for CVDs once the particle approaches the vessel wall. The vascular endothelium that lines blood vessels and creates a natural barrier separating blood from surrounding tissue is considered an attractive target for both drug delivery and imaging due to its proximity to intravenously administered therapy. Additionally, the unique markers expressed by endothelial cells during the progression of CAD offer an opportunity for the design of molecular imaging probes and targeted nanovectors for localized treatments. Proinflammatory markers such as selectins, VCAM-1, and ICAM-1 expressed during chronic inflammation, which is prominent in most CVDs, serve as prime targets for targeted nanovectors.13 Another means of directing nanovectors to CAD is to target fibrin clots formed at the site of atherosclerosis when blood comes into contact with exposed tissue within the plaque.14 While nanovectors may be targeted to biomarkers expressed by the endothelium, the endothelial cells themselves may not be the intended target of therapeutic action. For example, cells such as monocytes, T cells, and foam cells that are recruited into atherosclerotic plaques or the underlying tissue have served as targets.15 When the final destination of imaging and drug carriers is not the vascular endothelium but rather the underlying tissue/organ, particle internalization and/or transcytosis of the nanovector must be considered.16 17 Another possible approach for treating atherosclerosis could rely on targeting neovascularization of the vasa vasorum (network of small arteries in the vascular wall) that is strongly correlated with plaque growth and rupture.18
Particle Type
Particle material and fabrication technique are important design parameters that affect the performance of nanovectors. Several types of carriers have been proposed for use in the treatment and imaging of cardiovascular diseases including soluble carriers, viral carriers, lipid-based carriers, nano/microbubbles, polymeric, and inorganic-based nanocarriers (Figure 1).
Figure 1.
Schematic of (A) soluble, (B) polymer-based, and (C) lipid-based nanovectors.
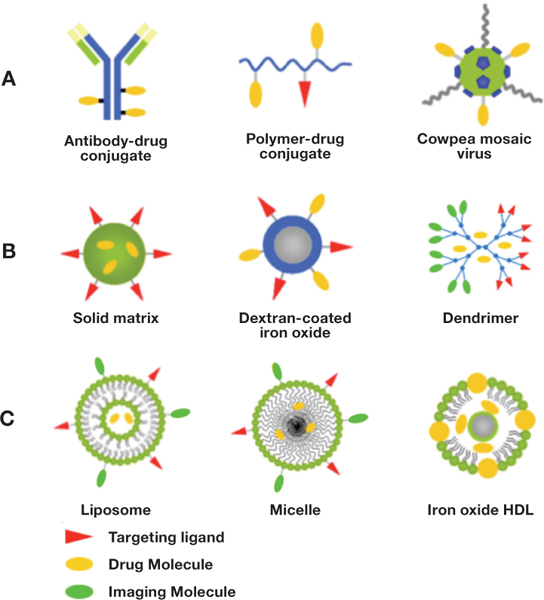
Soluble carriers include modified plasma proteins such as albumin, antibodies, and soluble biopolymers such as dextran and chitosan, and the design is such that the active agent is covalently linked to the carrier. For example, albumin has been conjugated to gadolinium for use as an MRI contrast agent.19 These types of carriers are attractive due to their solubility in plasma and thus their ability to leave the bloodstream and pass into targeted cells. However, soluble carriers remain plagued with limited load-carrying capacity, lack of protection for the bounded therapeutics, and covalent linkage chemistry that may alter drug efficacy. As such, soluble carriers can have limitations for drug delivery but still be beneficial for imaging application and gene delivery.
Viral nanoparticles are advantageous due to their natural ability to avoid detection and clearance from the bloodstream, their innate targeting capability, and their ability to enter cells. For example, the cowpea mosaic virus (CPMV) may be used for vascular imaging and drug delivery due to its specific binding to vimentin, which is presented on endothelial cells during angiogenesis and also present during neovascularization of the vasa vasorum in atherosclerosis.20 21 Additionally, imaging of CPMV has shown their localization to inflamed endothelium.22 PEGylation of CPMV may permit the use of these viruses for other targets.23 The drawbacks to such nanoparticles are potential toxicity in humans and the requirement for them to be engineered and grown in bioreactors, which adds to the complexity of their design.
Several lipid-based nanovectors such as liposomes, micelles, and lipoproteins have been proposed for targeted drug delivery and imaging. Liposomes, phospholipid-based nanovesicles, are easy to fabricate and possess low toxicity and large versatility. Liposomes can be loaded with hydrophilic, hydrophobic, and lipophilic drugs for therapeutic applications. For diagnostic applications, contrast-generating material can be incorporated in the outer shell or entrapped within the core. For example, a group at Washington University School of Medicine has engineered a targeted paramagnetic nanoemulsion for molecular imaging of atherosclerotic plaques with MRI.9 24 The nanoemulsion consists of a liquid perfluorocarbon core coated with a lipid shell that contains gadolinium. Unfortunately, issues of gadolinium toxicity have been raised that may limit the utility of the paramagnetic nanoemulsion. Alternatively, perfluorocarbon nanoemulsions have been used for ultrasound-based molecular imaging of atherosclerosis.25 However, the nanoemulsions are weak scatterers due to their size and the incompressibility of the liquid core, and thus a large number of nanoemulsions are required in order to produce a detectable change in image contrast. Ideally, ultrasound contrast agents will contain gas, which is more compressible than liquid and thus is more echogenic. Liposomes containing liquid and gas have been engineered for ultrasound-based molecular imaging of several components of atheromas, including fibrin and adhesion molecules.26 27 In addition to imaging, ultrasound can be used to trigger the release of entrapped thrombolytic agents from echogenic liposomes.28 29 Thus, echogenic liposomes can serve as a nanovector platform for targeted image-guided drug delivery and treatment of thrombi.30
Micelles are limited to the entrapment of hydrophobic drugs, but with their smaller size, they may be able to enter tissue from the bloodstream. They may include multifunctional complexes with polymers and attachment of targeting ligands/contrast agents for imaging.14 Lipoproteins are also limited to hydrophobic drugs, and their loading and release is not as tunable as with other materials. Synthetic high-density lipoproteins (HDL) may be decorated with contrast agents such as gadolinium and used to target HDL receptors such as on macrophages.31 Alternatively, synthetic HDL can be combined with inorganics such as iron oxide to make iron oxide core HDL nanoparticles that utilize the natural HDL trafficking pathway with magnetic resonance contrast enhancement provided by iron oxide.32
Polymeric nanoparticles are widely proposed as vectors for targeted drug delivery due to their variety of materials, sizes, and shapes. Typical formulations include solid matrix, polymersomes, and dendrimers, and available biodegradable polymers include poly(lactide), poly(glycolide), their copolymer poly(lactide-co-glycolide), poly(caprolactone), and poly(ethylene glycol). Solid matrix particles come in a variety of shapes and sizes and may be decorated with a variety of targeting ligands. One disadvantage to solid matrix particles made from biodegradable polymers such as poly(lactide), poly(glycolide), and their copolymers is the acidic degradation environment that may degrade or damage certain loaded therapeutics, particularly proteins.33 Means to mitigate this acidity concern include the incorporation of trehalose or poorly soluble bases alongside the encapsulated drug, as this has been shown to increase the stability of encapsulated proteins.34 35 Polymersomes are made from amphipathic polymers and are similar to lipid-based liposomes in their membrane flexibility while maintaining better structural integrity and allowing for greater PEGylation. Dendrimers are very small, highly branched polymers that allow for the attachment of targeting ligands, imaging markers, and therapeutics; thus they can be useful for theranostic applications — the merging of therapeutics and diagnostics in a single-carrier system.19 36 However, their use in high concentrations can be toxic (depending on their surface characteristics), and their loading capacity is often low. Moreover, covalent bonding of therapeutics to dendrimer surface is frequently required when physical entrapment is not feasible, which potentially diminishes their efficacy as drug carriers.37 Thus, very much like soluble carriers, dendrimers may be best suited for gene delivery and imaging applications.38 Indeed, Gadomer-17, a polylysine dendrimer complexed with 24 Gd-DOTA (gadolinium-tetraazacyclododecane tetraacetic acid), has been explored for use as an MRI contrast agent and shows promise of in vivo efficacy with minimal toxicity.39
Particles made from inorganic materials such as gold, silver, silicon, iron oxides, and carbon have been explored for drug delivery. One concern in the design of such particles is the loading and release profiles of therapeutics, requiring tuning of pore sizes to achieve desired release. Iron oxide and polymer-coated iron oxide particles have been explored for MRI imaging of cardiovascular systems due to their paramagnetic properties.40 41 Iron oxide particles can be used as a contrast agent for both magnetic resonance and X-ray imaging modalities, opening the possibility of overlaying images from dual sources and thus allowing more detailed analysis of affected tissues.
Particle Size
Physical characteristics of drug or imaging carriers, including size and shape, will determine how these particles localize to the blood vessel wall in flow. Spheres in the nanometer to micrometer range made from many types of materials have been broadly explored as injectable drug carriers and imaging agents due to their ease of fabrication. Nanospheres are attractive for intravenous injection routes as they are more likely to clear the microcirculation, particularly in the lungs, since the smallest human capillaries are on the order of 5 microns. This constraint imposed by the capillaries eliminates larger spherical particles made from rigid materials due to the risk of vascular occlusion. Additionally, nanoparticles are less likely to be internalized by macrophages than microspheres possessing diameters from 2 to 3 μm.42 This is possibly due to the fact that the opsonization rate with serum proteins decreases with particle size.43
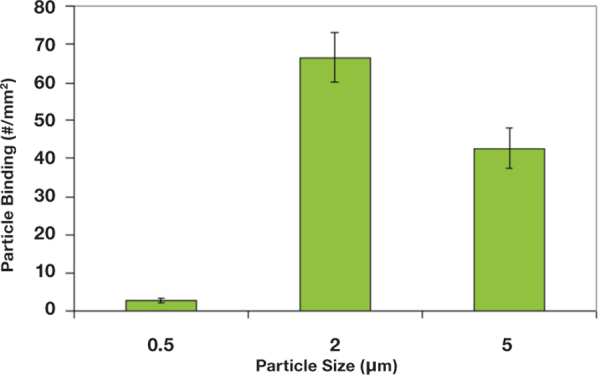
It has been recently reported, however, that microspheres with diameters ranging from 2 to 5 microns display significantly higher localization and binding to inflamed endothelial cell monolayers from bulk human blood flow than nanospheres with diameters from 100 to 500 nm as shown in Figure 2.44 This may be due to the impact of particle size on their interactions with red blood cells (RBCs). Larger particles (>2 μm in diameter) are preferentially excluded from the middle of blood flow and pushed to the wall, but nanospheres are likely small enough that they comfortably fit in the pocket between RBCs.45 It is likely that smaller nanoparticles, particularly in the tens-of-nanometers size range, are able to partition into plasma and show improved localization to the wall in bulk blood flow. However, the small size limits their utility for drug delivery due to a low capacity for carrying drugs.46 47
Figure 2.
Adhesion of nano/microspheres to activated endothelium from blood flow in a parallel plate flow chamber with a step channel. Blood flow is pulsatile between 120s-1 for 4 seconds and 1200s-1 for 2s over 5 minutes. Channel height at the entrance=127 μm and channel height at the main channel (after step)=508 μm.
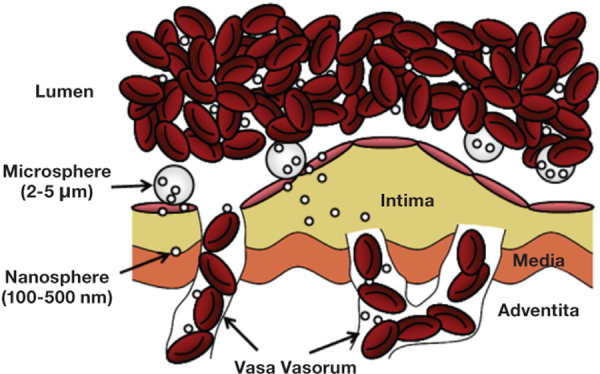
Efficiency of transport to the blood vessel wall where the particles may then adhere is more important for targeted drug delivery. High efficiencies of binding at the desired target allow for smaller concentrations of injected particles, lowering potentially harmful particle interactions with blood components and decreasing the risk of occlusion in arteries. For drug delivery, this translates to a lower systemic therapeutic amount, decreasing cost as well as deleterious side effects from potent drugs. For imaging, this amounts to better contrast and sensitivity per injection amount, which is important for imaging modalities that have relatively low detection sensitivity. For example, MRI has low detection sensitivity (i.e., 10-3 to 10-5 moles/L) compared to positron emission tomography (10-11 to 10-12 moles/L). Increasing the density of targeting moieties on the surface of paramagnetic nanovectors may increase the number that bind to the intended target, thus providing more material for contrast enhancement. However, increasing ligand density will have minimal effect if nanovector delivery to the wall is limiting.44 Alternatively, the concentration of paramagnetic material loaded onto a single nanovector can be increased, thus increasing the effect of each nanovector on the MR signal. Localization of a nanovector may also be increased by attaching the nanovector to micron-sized carriers that are highly efficient in traveling to the vessel wall. For example, Ananta et al. loaded nanoscale gadolinium-based contrast agents into porous silicon microparticles and showed an enhancement in contrast due to their geometrical confinement.48 For drug delivery, microcarriers would bind to the endothelial wall and release their nanocarrier load at the vessel wall, where they may transmigrate through the endothelium (Figure 3). This would require the design of microcarriers to release their load over a suitable time frame, perhaps involving fast-degrading polymers as a shell to release nanocarriers fairly quickly. For CVDs such as atherosclerosis that inflict larger arteries, the effective delivery of nanoparticles without a microcarrier system may be possible via the vasa vasorum that feed the wall of these arteries. As previously mentioned, associated inflammation and angiogenesis in these vessels may provide an avenue for targeting. However, only circumstantial evidence currently exists in the literature for nanoparticles localizing to the vasa vasorum.49 Certainly, nanoparticles may not be able to enter the vasa vasorum if they originate from the lumen of the coronary artery.50
Figure 3.
Schematics of microcarriers binding and releasing encapsulated nanovectors from blood flow at the endothelium.
Conclusion
Overall, there are advantages and disadvantages to differently sized particles for treatment and imaging of cardiovascular diseases. Nanoparticles are attractive as they offer low risk of vessel occlusion and avoidance of phagocytosis by macrophages, but they seem to lack efficiency in finding and binding the vessel wall from blood flow. These tradeoffs indicate an apparent need for further modification of particles by deviating from spherical shape or using micron-sized spherical carriers loaded with nanosphere cargo. Additionally, in vivo animal models may be insufficient to identify the best risk imaging and treatment carriers in humans; differences in vessel size and blood flow patterns (shear stress, red blood cell size, etc.) between animals necessitate trials in humans. Development of more effective methods of imaging for detection and consequent treatment that can address the fundamental causes of cardiovascular diseases and can identify those at greatest risk offer potential improvements in the treatment and outcomes of these diseases.
Conflict of Interest Disclosure: All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: Dr. Eniola-Adefeso acknowledges funding support from the American Heart Association (SDG 0735043N and Innovator 10IRG3490015) and the National Science Foundation (Brige EEC-0824182 and Career CBET-1054352). Dr. Heslinga acknowledges funding support from the American Heart Association (10PRE2840008).
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics — 2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215.. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose JA, Winters SL, Arora RR, Haft JI, Goldstein J, Rentrop KP, et al. Coronary angiographic morphology in myocardial infarction: a link between the pathogenesis of unstable angina and myocardial infarction. J Am Coll Cardiol. 1985;6(6):1233–8.. doi: 10.1016/s0735-1097(85)80207-2. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988 Jul;12(1):56–62.. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 4.Hackett D, Davies G, Maseri A. Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur Heart J. 1988 Dec;9(12):1317–23.. doi: 10.1093/oxfordjournals.eurheartj.a062449. [DOI] [PubMed] [Google Scholar]
- 5.Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992 Mar 15;69(8):729–32.. doi: 10.1016/0002-9149(92)90495-k. [DOI] [PubMed] [Google Scholar]
- 6.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995 Aug 1;92(3):657–71.. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002 Nov;8(11):1257–62.. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 8.Wierzbicki AS. Lipid-altering agents: the future. Int J Clin Pract. 2004 Nov;58(11):1063–72.. doi: 10.1111/j.1742-1241.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- 9.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003 Nov 4;108(18):2270–4.. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005 Apr 12;111(14):1800–5.. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amirbekian V, Aguinaldo JG, Amirbekian S, Hyafil F, Vucic E, Sirol M, et al. Atherosclerosis and matrix metalloproteinases: experimental molecular MR imaging in vivo. Radiology. 2009 May;251(2):429–38.. doi: 10.1148/radiol.2511080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006 Sep;26(9):2103–9.. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 13.Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004 May-Jun;13(3):125–38.. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- 14.Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M, et al. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9815–9.. doi: 10.1073/pnas.0903369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leuschner F, Nahrendorf M. Molecular imaging of coronary atherosclerosis and myocardial infarction: considerations for the bench and perspectives for the clinic. Circ Res. 2011 Mar 4;108(5):593–606.. doi: 10.1161/CIRCRESAHA.110.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery (review). Mol Membr Biol. 2010 Aug;27(4-6):190–205.. doi: 10.3109/09687688.2010.499548. [DOI] [PubMed] [Google Scholar]
- 17.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009 Jan;335(1):283–300.. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langheinrich AC, Sedding DG, Kampschulte M, Moritz R, Wilhelm J, Haberbosch WG, et al. 3-Deazaadenosine inhibits vasa vasorum neovascularization in aortas of ApoE(-/-)/LDL(-/-) double knockout mice. Atherosclerosis. 2009 Jan;202(1):103–10.. doi: 10.1016/j.atherosclerosis.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Nwe K, Milenic D, Bryant LH, Regino CA, Brechbiel MW. Preparation characterization and in vivo assessment of Gd-albumin and Gd-dendrimer conjugates as intravascular contrast-enhancing agents for MRI. J Inorg Biochem. 2011 May;105(5):722–7.. doi: 10.1016/j.jinorgbio.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koudelka KJ, Destito G, Plummer EM, Trauger SA, Siuzdak G, Manchester M. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog. 2009 May;5(5):e1000417.. doi: 10.1371/journal.ppat.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones MK, Itani RM, Wang H, Tomikawa M, Sarfeh IJ, Szabo S, et al. Activation of VEGF and Ras genes in gastric mucosa during angiogenic response to ethanol injury. Am J Physiol. 1999 Jun;276(6 Pt 1):G1345–55.. doi: 10.1152/ajpgi.1999.276.6.G1345. [DOI] [PubMed] [Google Scholar]
- 22.Shriver LP, Koudelka KJ, Manchester M. Viral nanoparticles associate with regions of inflammation and blood brain barrier disruption during CNS infection. J Neuroimmunol. 2009 Jun 25;211(1-2):66–72.. doi: 10.1016/j.jneuroim.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmetz NF, Manchester M. PEGylated viral nanoparticles for biomedicine: the impact of PEG chain length on VNP cell interactions in vitro and ex vivo. Biomacromolecules. 2009 Apr 13;10(4):784–92.. doi: 10.1021/bm8012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter PM, Caruthers SD, Yu X, Song SK, Chen J, Miller B, et al. Improved molecular imaging contrast agent for detection of human thrombus. Magn Reson Med. 2003 Aug;50(2):411–6.. doi: 10.1002/mrm.10532. [DOI] [PubMed] [Google Scholar]
- 25.Lanza GM, Wallace KD, Fischer SE, Christy DH, Scott MJ, Trousil RL, et al. High-frequency ultrasonic detection of thrombi with a targeted contrast system. Ultrasound Med Biol. 1997;23(6):863–70.. doi: 10.1016/s0301-5629(97)00046-x. [DOI] [PubMed] [Google Scholar]
- 26.Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R, et al. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol. 1999 Mar;33(3):867–75.. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton AJ, Huang SL, Warnick D, Rabbat M, Kane B, Nagaraj A, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004 Feb 4;43(3):453–60.. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119(6):777–84.. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res. 2009 Jul;124(3):306–10.. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laing ST, Moody M, Smulevitz B, Kim H, Kee P, Huang S, et al. Ultrasound-enhanced thrombolytic effect of tissue plasminogen activator-loaded echogenic liposomes in an in vivo rabbit aorta thrombus model — brief report. Arterioscler Thromb Vasc Biol. 2011 Jun;31(6):1357–9.. doi: 10.1161/ATVBAHA.111.225938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cormode DP, Chandrasekar R, Delshad A, Briley-Saebo KC, Calcagno C, Barazza A, et al. Comparison of synthetic high density lipoprotein (HDL) contrast agents for MR imaging of atherosclerosis. Bioconjug Chem. 2009 May 20;20(5):937–43.. doi: 10.1021/bc800520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skajaa T, Cormode DP, Jarzyna PA, Delshad A, Blachford C, Barazza A, et al. The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials. 2011 Jan;32(1):206–13.. doi: 10.1016/j.biomaterials.2010.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estey T, Kang J, Schwendeman SP, Carpenter JF. BSA degradation under acidic conditions: a model for protein instability during release from PLGA delivery systems. J Pharm Sci. 2006;95(7):1626–39.. doi: 10.1002/jps.20625. [DOI] [PubMed] [Google Scholar]
- 34.Costantino HR, Firouzabadian L, Wu C, Carrasquillo KG, Griebenow K, Zale SE, et al. Protein spray freeze drying. 2. Effect of formulation variables on particle size and stability. J Pharm Sci. 2002 Feb;91(2):388–95.. doi: 10.1002/jps.10059. [DOI] [PubMed] [Google Scholar]
- 35.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide). Nat Biotechnol. 2000 Jan;18(1):52–7.. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 36.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010 Aug 30;62(11):1052–63.. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005 Dec 14;57(15):2215–37.. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Theoharis S, Krueger U, Tan PH, Haskard DO, Weber M, George AJ. Targeting gene delivery to activated vascular endothelium using anti E/P-Selectin antibody linked to PAMAM dendrimers. J Immunol Methods. 2009 Apr 15;343(2):79–90.. doi: 10.1016/j.jim.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Herborn CU, Barkhausen J, Paetsch I, Hunold P, Mahler M, Shamsi K, et al. Coronary arteries: contrast-enhanced MR imaging with SH L 643A — experience in 12 volunteers. Radiology. 2003 Oct;229(1):217–23.. doi: 10.1148/radiol.2291021033. [DOI] [PubMed] [Google Scholar]
- 40.Nahrendorf M, Waterman P, Thurber G, Groves K, Rajopadhye M, Panizzi P, et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol. 2009 Oct;29(10):1444–51.. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devaraj NK, Keliher EJ, Thurber GM, Nahrendorf M, Weissleder R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjug Chem. 2009 Feb;20(2):397–401.. doi: 10.1021/bc8004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008 Aug;25(8):1815–21.. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2050–5.. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010 Feb;31(6):1392–402.. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Eckstein EC, Tilles AW, Millero FJ., 3rd Conditions for the occurrence of large near-wall excesses of small particles during blood flow. Microvasc Res. 1988 Jul;36(1):31–9.. doi: 10.1016/0026-2862(88)90036-2. [DOI] [PubMed] [Google Scholar]
- 46.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008 Jul-Aug;5(4):505–15.. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids Surf B Biointerfaces. 2008 Oct 15;66(2):274–80.. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Ananta JS, Godin B, Sethi R, Moriggi L, Liu X, Serda RE, et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast. Nat Nanotechnol. 2010 Nov;5(11):815–21.. doi: 10.1038/nnano.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maranhão RC, Tavares ER, Padoveze AF, Valduga CJ, Rodrigues DG, Pereira MD. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis. 2008 Apr;197(2):959–66.. doi: 10.1016/j.atherosclerosis.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 50.Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, et al. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardio. 1998 Dec;32(7):2072–9.. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]


