Abstract
Cardiovascular disease remains the leading cause of death in the world and continues to serve as the major contributor to healthcare costs. Likewise, there is an ever-increasing need and demand for novel and more efficient diagnostic tools for the early detection of cardiovascular disease, especially at the point-of-care (POC). This article reviews the programmable bio-nanochip (P-BNC) system, a new medical microdevice approach with the capacity to deliver both high performance and reduced cost. This fully integrated, total analysis system leverages microelectronic components, microfabrication techniques, and nanotechnology to noninvasively measure multiple cardiac biomarkers in complex fluids, such as saliva, while offering diagnostic accuracy equal to laboratory-confined reference methods. This article profiles the P-BNC approach, describes its performance in real-world testing of clinical samples, and summarizes new opportunities for medical microdevices in the field of cardiac diagnostics.
Keywords: programmable bio-nanochips, P-BNC, bio-nanochips, point of care testing, cardiovascular disease, in vitro diagnostics, cardiac biomarkers, lab-on-a-chip technologies

Introduction
Cardiovascular disease (CVD), a diagnostic class that includes several separate diseases of the heart and the circulatory system, is the leading cause of death in the United States and globally. Despite recent remarkable and continuing declines in mortality rates, CVD statistics remain staggering. In 1997, nearly 1 million people died of CVD in the United States, constituting about 40% of all deaths for that year. In 1998, 460,390 Americans died of coronary heart disease (CHD) and 158,060 of stroke. In 2006, CHD caused approximately one of every six deaths in the United States.1 In 2010, it was estimated that 785,000 Americans would have a new coronary attack and approximately 470,000 would suffer a recurrent attack. An additional 195,000 silent first myocardial infarctions are estimated to occur each year. Approximately every 25 seconds, an American will have a coronary event; approximately every minute, someone will die of one. The estimated direct and indirect cost of CVD for 2010 was $503.2 billion, making CVD a continuing major contributor to the escalating cost of U.S. health care.2 3
CVD is more prevalent in developed countries; however, its incidence is growing in developing parts of the world. According to the World Health Organization, CVD kills an estimated 17 million people each year worldwide.4 For these individuals, tobacco smoking poses a major known risk factor for death due to its contribution to CVD. Other risk factors, including high levels of LDL-associated cholesterol, hypertension, diabetes, and obesity, continue to contribute to CVD morbidity and mortality.
Fortunately, CVD is preventable and treatable. Quick and accurate identification of cardiovascular problems is of utmost importance to avoid hospitalization and to reduce CVD morbidity and mortality rates. Thus, early detection is critical to the survival of the patient afflicted with this multi-factorial, high-impact condition. The introduction of improved diagnostics, treatments, medications, and interventional techniques has led to a significant decrease in total CVD mortality over the past few decades.5–7 However, the emerging driver for CVD diagnostics is the in vitro diagnostics (IVD) sector, with an increasing emphasis and demand for POC testing technologies. The P-BNCs reviewed here promise to fulfill this need.
Point-of-Care Testing
In the United States there are more than 8 million visits to emergency departments (EDs) annually for chest pain or other symptoms consistent with acute coronary syndrome (ACS).8
The challenge for clinicians is rapid identification of those patients who require admission for urgent management and those with a benign etiology who can be discharged directly from the ED. Likewise, ACS outcomes depend strongly on time-dependent intervention and therapies; indeed, time is muscle for the ACS patient and the attending ED physician. Recent guidelines by the American College of Cardiology and the American Heart Association for the diagnosis and treatment of ACS recommend that cardiac markers should be evaluated within 30 to 60 minutes from the time of ED presentation.9 Many EDs and central laboratories do not meet this recommendation, as processing of samples in these environments includes transport of blood from the ED to the lab, extraction of serum (via clotting), centrifugation, and long assay times (at best 20 minutes if an automated immunoassay analyzer is used).
Point-of-care testing addresses this demand for accelerated diagnostic information and reduction in result turnaround times. It can be performed simply, outside the laboratory and without the need for highly trained personnel, and quickly, thus helping accelerate the administration of lifesaving treatments. Further, POC tests can be performed in the ED setting, negating the need for sample transportation to a central laboratory and thereby reducing the risk for sample degradation, which allows for a more accurate diagnosis. Point-of-care device manufacturers emphasize in their design, research, and development efforts provisions for ease of use, portability, clinical benefits linked to accuracy, and reliability of POC results.
Various POC devices have been reviewed elsewhere.10 Although POC testing for cardiac markers is developing rapidly and increasingly accepted by hospitals and patients, it still faces some challenges. The National Academy of Clinical Biochemistry (NACB) has recommended that performance specifications and characteristics for POC testing should be consistent with those for central laboratory testing.11 However, POC cardiac tests continue to yield inferior performance characteristics relative to the tests completed in central laboratories at the expense of speedy results. Likewise, the need for POC technologies that facilitate multiplexed POC biomarker panels without sacrificing analytical accuracy and precision remains largely unmet.
Biomarkers of CVD
Equally important to the POC technology are biomarkers, substances that can be measured to detect the presence or absence of disease. All aspects of care regarding CVD — including diagnosis, prognosis, monitoring, risk stratification, and guidance for therapeutic interventions of patients with suspected acute myocardial infarction (AMI) — rely strongly on cardiac biomarkers that offer high clinical sensitivity and specificity, quick release for early diagnosis, and stability so as to remain measurable within the relevant diagnostic window.
To date, commercial cardiac biomarker POC devices have focused on the detection of FDA-approved myoglobin, CK-MB (creatine kinase MB isoenzyme), and cardiac troponins.10 12 The feasibility and specificity of measuring troponins drives a trend toward earlier POC technology implementation in ED decision-making and risk stratification. Markers of cardiac injury at the POC are expected to enable diagnosis of myocardial infarction with high sensitivity and specificity, efficiently allowing for the prescription of appropriate and effective treatments in critical periods and thereby saving lives and significantly reducing healthcare costs.
It has been proposed that multiple biomarkers indicative of different underlying pathophysiologic conditions are independently predictive of increased adverse events in patients with ACS. Here, markers of myocardial necrosis (troponins) in conjunction with markers of neurohormonal activation (brain natriuretic peptide [BNP] and N-terminal-proBNP) and markers of systemic inflammation have been suggested for diagnosis, risk stratification, and guidance of ACS therapy.13–15
Inflammation has been linked to all stages of vulnerable plaques, from initial deposition of lipids to plaque destabilization and rupture, platelet activation, and thrombus formation.10 Likewise, with increased understanding of the pathobiology and the inflammatory nature of CVD, biomarkers of inflammation may serve in a panel to assess risk for both primary and secondary cardiac events and for CVD-related death. Early identification of risk would allow for the timely implementation of lifestyle changes and effective treatment regimens that could help slow down progression of or even reverse CVD.
Programmable Bio-nanochips for POC Testing
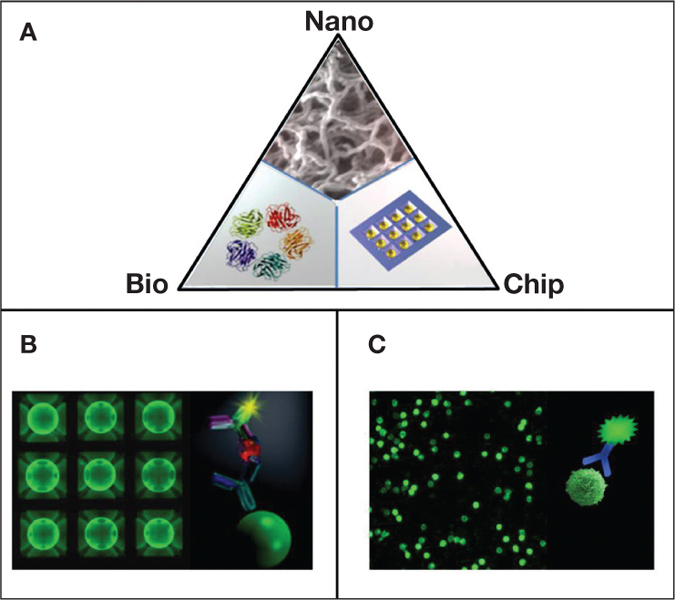
Nanotechnology is poised to have an increasing effect on cardiovascular health in coming years.16 Likewise, the ability to rapidly secure sensitive, reliable simultaneous measurements of multiple key cardiac biomarkers at the POC promises to revolutionize clinical diagnostics. Toward this goal, we have worked to improve the current state of POC IVD through the development, validation, and implementation of P-BNCs (Figure 1A).17–24 The “programmability” feature of the system refers to the capacity of the sensor ensemble to function as a standard platform that can be reprogrammed to serve a new application by inserting a molecular-level code (i.e., the biomarker-specific reagents). The “bio” terminology refers to the capacity to measure and extract the bio-signatures associated with the disease progression. The “nano” element describes the capacity to miniaturize the system, which is embodied in the use of nano-nets for efficient and rapid biomarker capture as well as quantum dots for increased signal generation. The “chip” term emphasizes the capacity to mass-produce the sensor elements in a way similar to those used by the microelectronics industry that ultimately leads to high performance at reduced cost. The same sensor platform has the capacity to measure both soluble analytes using bead microreactors (Figure 1B)17–24 as well as cell counting, typing, and differentiation using membrane microstructures (Figure 1C).25–27
Figure 1.
(A) P-BNCs are programmed to measure medically relevant species in complex biological samples through the employment of three-dimensional “nano-nets” composed of agarose strands supported within 280 μm bead “micro-sponges,” which are strategically supported within an enclosed mini flow chamber. Two assay platforms hosted by P-BNCs include (B) a bead sensor array that is dedicated to the analysis of general chemistries, small molecules, genomic, and proteomic targets, and (C) a membrane-based platform for cellular analyses.
These two distinct types of assay platforms are packaged with in a disposable, single-use P-BNC sensor lab card (Figure 2). The lab card — with built-in incipient-stabilized detection reagents, fluid mixing and partitioning compartments, and a sample-loading dock and self-contained bio-waste compartment — is inserted in the light-emitting diode/charge-coupled, device-equipped, mechano-optical analyzer to complete entire assay sequences in an automated manner. The toaster-size portable analyzer serves as a universal-interface portable smart device that includes an embedded PC. It also provides for mechanical, optical, and software capabilities operated through a user interface that functions upon insertion of the lab card and concludes with an easy-to-interpret liquid crystal display readout of the test result.
Figure 2.
From macro to nano. (A) In the P-BNC system, interface with the human user occurs via a portable, self-contained analyzer that (A/B) processes a compact, modular, single-use/disposable lab card. (C) The lab card houses the diagnostic core of the P-BNC, which for the “chemical processing unit” is a microchip-supported array of beads. Each bead produces a fluorophore-derived signal with intensity proportional to the concentration of the analyte for which it is sensitized to capture. (D) The fluorophores are densely functionalized throughout the micrometer-sized bead, as the analyte is efficiently captured and detected using (E) a “sandwich” type immunoassay on nanometer agarose strands.
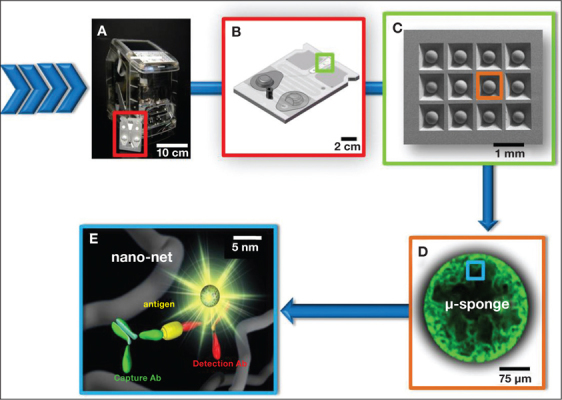
Compared to gold standard methods such as enzyme-linked immunoassay (ELISA), P-BNCs exhibit assay times in minutes instead of hours, a limit of detection (LOD) 2 or more orders of magnitude lower, and a proven capacity to multiplex 10 or more concurrent analytes with appropriate internal controls and calibrators.
The strong analytical performance of the P-BNCs may be attributed to the porous nature of its bead sensors, the active transport mode of delivery of the sample and detection reagents, and the highly stringent washes associated with this microfluidic approach. Like ELISA, the bead-based P-BNCs complete 2-site immunometric as well as competitive immunoassays. Unlike ELISA, which limits the diffusion-mediated antigen-antibody (Ag-Ab) binding to a 2-dimensional planar surface at the bottom of the well, P-BNCs provide a roughly 1000-fold to 10,000-fold increase in surface area on the 3-dimensional (3D) bead sensor. This 3D reactor allows for significantly increased contact area and on-off-on again, higher avidity Ag-Ab interactions. All of the aforementioned features contribute to the generation of high signal-to-noise ratios that ultimately translate into the advanced detection capabilities of the P-BNCs.21
As mentioned above, the P-BNC sensor device leverages microelectronic components and microfabrication techniques and, similar to a personal computer, is programmable. As such, it may be programmed to detect various panels of target proteins, antibodies, toxins, and drugs of abuse in biological fluids (Table 1). Furthermore, the portable P-BNC device can simultaneously test for multiple biomarkers, consistently offering ultra-sensitive detection of low-abundance proteins in complex fluids such as noninvasive saliva, which bodes well for POC applications.
Table 1.
Biomarker (analyte) diversity on the P-BNC bead-based platform.
| Cytokines | Cardiac markers | Cancer biomarkers |
| TNF-α, IL-1β, IL-6 | CRP, MPO, cTnl, Myo, CK-MB, D-dimer, apoA1, apoB, BNP, NT-proBNP, sCD40L, MCP-1, adiponectin | CEA, CA125, Her2-neu, PSA (free and complexed) |
| Antibodies | Drugs of abuse | Other |
| total lgE, allergen- specific lgE (>10 allergens), total lgG, HepA-, HepB- and HepC-specific lgG | cocaine, diazepam, THC, amphetamine, methamphetamine | HSA, glycated albumin, transferrin |
Abbreviations: TNF-α: tumor necrosis factor-alpha; IL-1β: interleukin-1beta; IL-6: interleukin-6; sCD40L: soluble CD40 ligand; MCP-1: monocyte chemoattractant protein-1; CRP: C-reactive protein; MPO: myeloperoxidase; cTnl: cardiac troponin I; Myo: myoglobin; CK-MB: creatine kinase; apoA1: apolipoprotein A1; apoB: apolipoprotein B; BNP: brain natriuretic peptide; NT-proBNP: N-Terminal proBNP; CEA: carcinoembryonic antigen; CA125: cancer antigen 125; PSA: prostate specific antigen; lgE: immunoglobulin E; lgG:immunoglobulin G; Hep: hepatitis; THC: Δ9-tetrahydrocannabinol; HSA: human serum albumin
Bio-nanochips for CVD Diagnostics
We recently investigated the feasibility and utility of saliva as an alternative or complement to serum diagnostic fluid for identifying biomarkers of AMI. Applying Luminex and ELISA methodologies and the P-BNC approach, we measured the levels of 21 proteins in serum and expectorated unstimulated whole saliva that was procured from 41 AMI patients within 48 hours of chest pain onset and from 43 healthy controls.28 The majority of these proteins had literature precedence for a serum association with the cardiac disease cascade. In our case, distinct biomarkers demonstrated significant differences in median concentrations of both serum and saliva between patients with AMI and controls without AMI. For saliva, the top 10 biomarkers that yielded the most valuable information for diagnosis of AMI from a single salivary biomarker perspective included C-reactive protein (CRP), soluble intercellular adhesion molecule 1 (sICAM-1), soluble CD40 Ligand (sCD40L), myeloperoxidase (MPO), matrix metalloproteinase-9 (MMP-9), tumor necrosis factor-alpha (TNF-α), myoglobin (MYO), interleukin-1 beta (IL-1β), adiponectin, and RANTES.28
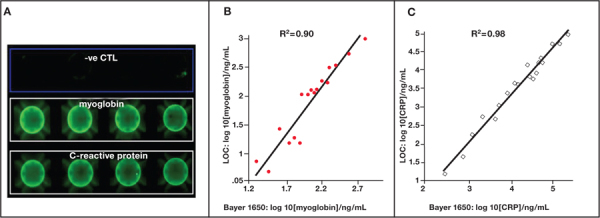
Further, logistic regression and area under curve (AUC) determined from receiver operating characteristic (ROC) analysis was applied to evaluate the AMI diagnostic utility of each biomarker or combinations of biomarkers. Here, salivary panels of CRP and MPO, CRP and MYO, and a panel involving CRP, MPO, and MYO yielded similar AUCs of 0.82, 0.85, and 0.85, respectively. Most importantly, the saliva-based biomarker panel of CRP and MYO exhibited significant diagnostic capability and, in conjunction with ECG, yielded strong screening capacity for AMI (AUC=0.94) that far exceeded the screening capacity of ECG alone (AUC approximately 0.6).28 Accordingly, we have adapted the two biomarkers, CRP and MYO, as a duplex test on the P-BNC platform; results achieved from the testing of clinical samples with this AMI chip correlate well to those obtained with a clinical reference analyzer (Figure 3).
Figure 3.
(A) A multiplexed assay for C-reactive protein and myoglobin has been developed on the P-BNC platform. This duplex assay was validated in a methods comparison study with the testing of clinical samples from patients with and without AMI. Here, samples were tested by both the P-BNC (lab on a chip) method and the Bayer 1650 Advia clinical analyzer system, and results for (B) myoglobin and (C) C-reactive protein were comparable.
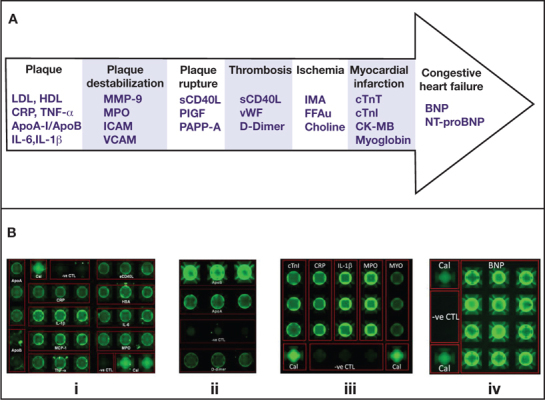
Additional P-BNC tests are being developed for dedicated cardiac diagnostic applications, including two multiplexed chips to assess cardiac risk for primary and secondary cardiac events, an expanded 5-plexed AMI diagnosis panel, and an assay for biomarker of congestive heart failure (CHF) BNP (Figure 4). A P-BNC assay for NT-proBNP, another biomarker of CHF, is also in development. Further, as recent reports have shown, the combined measurement of CRP concentrations and leukocyte counts provides one of the most accurate methods available to date to assess an individual’s risk for heart disease. To this end, we have combined the bead-based and membrane-based P-BNC platforms to provide a dual-function CRP and white blood cell cardiac risk measurement tool, thus making the P-BNC the only POC system amenable for the combined measurement of both cellular and proteomic biomarkers of cardiac risk.29–31
Figure 4.
(A) Specific molecules implicated in different stages of the cardiovascular disease cascade present themselves as putative diagnostic biomarkers for CVD. (B) Dedicated P-BNC diagnostic applications for CVD include: (i) risk for primary cardiac event chip: ApoA1, ApoB, CRP, IL-1β, MCP-1, TNF-α, sCD40L, HSA, IL-6 and MPO; (ii) risk for secondary cardiac events chip: ApoA1, ApoB, and D-dimer; (iii) expanded AMI diagnosis chip: cTnI, CRP, IL-1β, MPO, and MYO; (iv) congestive heart failure chip: BNP. Noted for each array is the redundancy of bead sensors per analyte, which contributes to more accurate and higher precision measurements. Also noted is the presence of calibrator beads (Cal) that serve as the baseline calibration of the P-BNC system, and negative control (-ve CTL) beads, coupled to antibodies irrelevant to any of the analytes targeted in each test, which serve as indicators of the specificity of the antigen-antibody reactions that take place within the lab card.
Our initial cross-sectional biomarker discovery study also demonstrated the potential utility for cTnI as a salivary biomarker of AMI despite its low concentration in this biological fluid. It must be noted that an essential prerequisite for the successful implementation of this biomarker in POC practice, whether in needle-prick-derived whole blood or saliva, depends on the availability of an ultra-sensitive method for its measurement. This is because the 99th percentile upper reference limit is the upper normal limit of the assay derived from a presumably normal healthy population. Levels below the 99th percentile upper reference limit are presumably normal, but this cutoff ultimately depends on the sensitivity and LOD of the assay. In the case of many current troponin assays, studies have shown that in actuality these 99th percentile reference limits include a heterogeneous patient population that comprises “true” normal but also other patients with low levels who have elevated cardiac risk.32–35 These studies suggest that higher-sensitivity troponin assays are necessary; likewise, the advantage of ultrasensitive troponins is based on the premise that lower cutoff levels achieve higher sensitivity that will allow earlier diagnosis, often within 90 minutes of presentation.
Traditional POC cTnI measurements have resulted in limits of detection that are on the order of ~1 ng/ml. The most advanced laboratory-based instruments yield LOD values 50× or more lower than this. Indeed, there is a strong drive to increase sensitivity of this test, raising the bar for what is required as a prerequisite performance for a POC cTnI test.
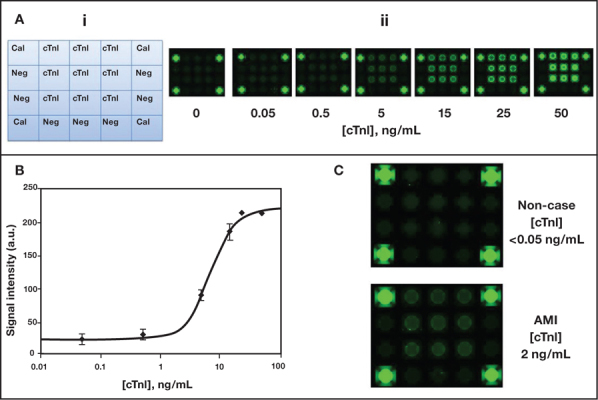
Sustained efforts have led to the development of an advanced P-BNC immunoassay for this gold standard of AMI diagnosis marker. With the P-BNC, cTnI capture on the beads is achieved through three distinct cTnI-specific antibody clones, and its detection is enhanced via an indirect signal amplification scheme. The 15-minute P-BNC cTnI assay demonstrates an LOD of 0.05 ng/mL and a precision associated with < 10% coefficient of variation. Indeed, this POC test allows for a most sensitive and specific detection of cTnI when challenged with clinical samples (Figure 5).
Figure 5.
Ultra-sensitive cTnI assay developed on the P-BNC sensor. (Ai) Schematic shows layout of bead array with calibrator beads (Cal), negative control beads (Neg), and cTnI bead sensors. (Aii) Images of cTnI bead array exposed to increasing concentrations of cTnI. (B) Dose curve for cTnI P-BNC assay, as derived from the automated analysis of images shown in Aii. (C) Images of the P-BNC array resulting from the testing of clinical samples collected from a non-case and an AMI case. Interpolation of the signal intensities of these images into the dose curve, as derived from dedicated automated data analysis macros, allows for the determination and ultimate reporting of cTnI levels.
Current AMI Biomarker Validation Studies
The biomarker discovery study described above focused on identifying biomarkers of interest for AMI screening. As such, this cross-sectional study cast a wide net that involved testing of serum and saliva samples collected from subjects with extreme phenotypes, in which the control group was composed of healthy individuals and the experimental group of AMI patients. Furthermore, to ensure timely detection of relevant biomarkers, samples in these initial studies were collected within a wide time frame (0 to 48 hours) from presentation to the ED, as the optimal time-point of elevation of the relevant biomarkers, at least in the oral fluid, was not known at this juncture. This pilot study was the first to demonstrate that biomarkers involved in the CVD cascade can be detected in oral fluids. Most importantly, the study derived the primary evidence of utility of oral fluids for the diagnosis of AMI, and it has confirmed the diagnostic utility of counterpart serum proteins.
It became essential to complete the next stage of a clinical study to validate these biomarkers in the context of the final application whereby chest pain patients arriving at the ED would be screened for AMI. Likewise, consistent with the POC testing at the ED, samples from these patients are being collected from within a more proximal time point from the onset of symptoms and within the timeframe of 1 to 12 hours from presentation to the ED. Furthermore, in contrast to the pilot study that involved collection of oral fluids through expectoration, this ongoing study incorporates a more convenient collection of salivary sample through a swab; it also involves a thorough dental examination in order to investigate the influence of dental health conditions on the use of biomarkers for diagnosing myocardial infarction.
Important septal ablation studies are also being completed so as to characterize the kinetics of biomarker release during the evolution of AMI. Alcohol septal ablation is used clinically to reduce the extent of left ventricular outflow obstruction among patients with hypertrophic cardiomyopathy. The ablation procedure causes myocardial necrosis via toxin-mediated cell death. Unlike the typical patient presenting with AMI, the timing of the event is known and the approximate size of AMI is consistent from patient to patient. This offers the opportunity to accurately characterize the kinetics of biomarker release both in serum and in oral fluids. Initial analysis of oral fluids from patients who underwent septal ablation showed a substantial change in triage biomarkers over time but to a lesser degree than was observed in serum. In addition, P-BNC testing of samples collected from chest pain patients en route to the ED (i.e., in the ambulance setting) confirm early elevations of select cardiac biomarkers, including myoglobin.
These essential biomarker validation studies promise to accelerate the bench-to-bedside translation activities for one of the most significant cardiac POC tests to date. Concentration thresholds for biomarkers of AMI and critical time course information are defined for optimal P-BNC tests to help rule in chest pain patients with AMI and rule out those without AMI with the highest level of clinical accuracy for the pre-hospital and ED setting.
Conclusion
A new era in CVD diagnostics is emerging, empowered by new advances in promising lab-on-a-chip technologies such as the P-BNC. The union between minimally invasive or noninvasive sampling methods with a portable microchip sensor device that performs sensitive and multiplexed analysis of CVD biomarkers may open up new avenues of more efficient and cost-effective clinical care for cardiac patients. Results achieved with this approach promise diagnostic accuracy of CVD equal to those achieved with traditional laboratory-based tests, only now this testing infrastructure can be more accessible to the patient, the ambulance, or the emergency room for the diagnosis of a cardiovascular condition. Similarly, a microchip-based test may be applied at the more frequently visited nearby pharmacy or primary physician’s or dentist’s office for early identification of cardiac risk. With this state-of-the-art P-BNC sensor system, biological signatures of cardiac disease may be obtained quickly, without a phlebotomist, and delivered to the cardiologist well before the patient is in need of critical care.
Conflict of Interest Disclosure: All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: Funding for this work was provided by the National Institutes of Health (NIH) through the National Institute of Dental and Craniofacial Research (Award Number 5U01 DE017793). The content is solely the responsibility of the authors and does not necessarily represent or reflect the official views of the NIH or the U.S. government.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart Disease and Stroke Statistics — 2007 Update, A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69–e171.. doi: 10.1161/CIRCULATIONAHA.106.179918. Epub 2006 Dec 28. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart Disease and Stroke Statistics — 2009 Update, A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181.. doi: 10.1161/CIRCULATIONAHA.108.191261. Epub 2008 Dec 15. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics — 2010 update, A report from the American Heart Association. Circulation. 2010;121:e46–e215.. doi: 10.1161/CIRCULATIONAHA.109.192667. Epub 2009 Dec 17. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization [Internet] Geneva Switzerland: World Health Organization; c2011. Programmes and projects: Global atlas on cardiovascular disease prevention and control; 2011 Sep 19 [cited 2011 Nov 21]. Available from: http://www.who.int/cardiovascular_diseases/en/ [Google Scholar]
- 5.Budoff MJ, Achenbach S, Duerinckx A. Clinical utility of computed tomography and magnetic resonance techniques for noninvasive coronary angiography. J Am Coll Cardiol. 2003;42:1867–78.. doi: 10.1016/j.jacc.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer S, Revel D, de Roos A, van Rossum A, von Schulthess G, Sechtem U, et al. Task Force of the European Society of Cardiology, in Collaboration with the Association of European Paediatric Cardiologists. Task Force Report: The clinical role of magnetic resonance in cardiovascular disease. Eur Heart Journ. 1998;19:19–39.. [PubMed] [Google Scholar]
- 7.Arad Y, Spadaro LA, Goodman K, Lledo-Perez A, Sherman S, Lerner G, et al. Predictive value of electron beam computed tomography of the coronary arteries: 19-month follow-up of 1173 asymptomatic subjects. Circulation. 1996 Jun 1;93(11):195–153.. doi: 10.1161/01.cir.93.11.1951. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention [Internet]. Atlanta GA: Centers for Disease Control and Prevention; c2011. FastStats Homepage: Emergency Department Visits; 2011 Oct 24 [cited 2011 Nov 21]. Available from: http://www.cdc.gov/nchs/fastats/ervisits.htm. [Google Scholar]
- 9.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction — 2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2002 Oct 1;106(14):1893–1900.. doi: 10.1161/01.cir.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Min Zhou D. Cardiac markers and their point-of- care testing for diagnosis of acute myocardial infarction. Clin Biochem. 2006 Aug;39(8):771–80.. doi: 10.1016/j.clinbiochem.2006.05.011. Epub 2006 Jun 7. [DOI] [PubMed] [Google Scholar]
- 11.Storrow AB, Apple FS, Wu AHB, Jesse R, Francis G, Christenson RH. Use of cardiac biomarkers for acute coronary syndromes. In: Nichols JH editor. Laboratory Medicine Practice Guidelines: Evidence based practice for point of care testing. The National Academy of Clinical Biochemistry [Internet]. Washington, D.C.: American Association for Clinical Chemistry, Inc.; c2006 [cited 2011 Nov 21]. Available from: http://www.aacc.org/SiteCollectionDocuments/NACB/LMPG/POCTLMPG.pdf. [Google Scholar]
- 12.Brogan GX, Jr, Bock JL. Cardiac marker point-of-care testing in the Emergency department and Cardiac Care Unit. Clin Chem. 1998 Aug;44(8 Pt 2):1865–69.. [PubMed] [Google Scholar]
- 13.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011 Apr 5;123(13):1367–76.. doi: 10.1161/CIRCULATIONAHA.110.005264. Epub 2011 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal SK, Avery CL, Ballantyne CM, Catellier D, Nambi V, Saunders J, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clin Chem. 2011 Jun;57(6):891–97.. doi: 10.1373/clinchem.2010.159350. Epub 2011 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo VY, De Lemos JA. Individualizing therapy in acute coronary syndromes: using a multiple biomarker approach for diagnosis risk stratification, and guidance of therapy. Curr Cardiol Rep. 2004 Jul;6:273–8.. doi: 10.1007/s11886-004-0076-x. [DOI] [PubMed] [Google Scholar]
- 16.Buxton DB. Current status of nanotechnology approaches for cardiovascular disease: a personal perspective. Nanobiotechnol. 2009 Mar-Apr;1(2):149–55.. doi: 10.1002/wnan.8. [DOI] [PubMed] [Google Scholar]
- 17.Lavigne JJ, Savoy S, Clevenger MB, Ritchie JE, McDoniel B, Yoo SJ, et al. Solution-based analysis of multiple analytes by a sensor array: toward the development of an ‘electronic tongue’. J Am Chem Soc. 1998 Jun 11;120(25):6429–30.. [Google Scholar]
- 18.Goodey A, Lavigne JJ, Savoy SM, Rodriquez MD, Curey T, Tsao A, et al. Development of multianalyte sensor arrays composed of chemically derivitized polymeric microspheres localized in micromachined cavities. J Am Chem Soc. 2001 Mar 21;123(11):2559–70.. doi: 10.1021/ja003341l. [DOI] [PubMed] [Google Scholar]
- 19.Christodoulides N, Tran M, Floriano PN, Rodriquez M, Goodey A, Ali M, et al. A microchip-based multianalyte assay system for the assessment of cardiac risk. Anal Chem. 2002 Jul 1;74(13):3030–6.. doi: 10.1021/ac011150a. [DOI] [PubMed] [Google Scholar]
- 20.Ali MF, Kirby R, Goodey AP, Rodriguez MD, Ellington AD, Neikirk DP, et al. DNA hybridization and discrimination of single-nucleotide mismatches using chip-based microbead arrays. Anal Chem. 2003 Sept 15;75(18):4732–9.. doi: 10.1021/ac034106z. [DOI] [PubMed] [Google Scholar]
- 21.Christodoulides N, Mohanty S, Miller CS, Langub MC, Floriano PN, Dharshan P, et al. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab Chip. 2005 Mar;5(3):261–9.. doi: 10.1039/b414194f. Epub 2005 Jan 13. [DOI] [PubMed] [Google Scholar]
- 22.Christodoulides N, Dharshan P, Wong J, Floriano PF, Neikirk D, McDevitt JT. A microchip-based assay for interleukin-6. Meth Mol Bio. 2007;385:131–44.. doi: 10.1007/978-1-59745-426-1_10. [DOI] [PubMed] [Google Scholar]
- 23.Christodoulides N, Floriano PN, Mohanty S, Dharshan P, Griffin M, Lennart A, et al. Lab-on-a-chip methods for point of care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007 Mar;1098:411–428.. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 24.Jokerst JV, Raamanathan A, Christodoulides N, Floriano PN, Pollard AA, Simmons GW, et al. Nano-bio-chips for high performance multiplexed protein detection: determinations of cancer biomarkers in serum and saliva using quantum dot bioconjugate labels. Biosens Bioelectron. 2009 Aug 15;24:3622–9.. doi: 10.1016/j.bios.2009.05.026. Epub 2009 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez WR, Christodoulides N, Floriano PN, Graham S, Mohanty S, Dixon M, et al. A microchip CD4 counting method for HIV monitoring in resource-poor settings. PLoS Med. 2005 Jul 19;2(7):e182.. doi: 10.1371/journal.pmed.0020182. Epub 2005 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floriano PN, Christodoulides N, Romanovicz D, Bernard B, Simmons GW, Cavell M, et al. Membrane-based on-line optical analysis system for rapid detection of bacteria and spores. Biosens Bioelectron. 2005 Apr 15;20(10):2079–88.. doi: 10.1016/j.bios.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 27.Weigum SE, Floriano PN, Christodoulides N, McDevitt JT. Cell-based sensor for analysis of EGFR biomarker expression in oral cancer. Lab Chip. 2007 Aug;7(8):995–1003.. doi: 10.1039/b703918b. Epub 2007 Jul 11. [DOI] [PubMed] [Google Scholar]
- 28.Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus S, Rose BG, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009 Aug;55(8):1530–8.. doi: 10.1373/clinchem.2008.117713. Epub 2009 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, et al. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med. 2005 Mar 14;165:500–8.. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 30.Christodoulides N, Floriano PN, Acosta SA, Michael-Ballard KL, Weigum SE, Mohanty S, et al. Toward the development of a lab-on-a-chip dual-function leukocyte and C-reactive protein analysis method for the assessment of inflammation and cardiac risk. Clin Chem. 2005;51(12):2391–5.. doi: 10.1373/clinchem.2005.054882. [DOI] [PubMed] [Google Scholar]
- 31.Jokerst J, Jacobson JW, Bhagwandin BD, Floriano PN, Christodoulides N, McDevitt JT. Programmable nano-bio-chip sensors: analytical meets clinical. Anal Chem. 2010 Mar 1;82:1571–9.. doi: 10.1021/ac901743u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH, Cannon CP, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine practice guidelines: analytical issues for biomarkers of acute coronary syndromes. Clin Chem. 2007 Jul 31;116(5):e95–8.. doi: 10.1161/CIRCULATIONAHA.107.185266. Epub 2007 Jul 14. [DOI] [PubMed] [Google Scholar]
- 33.Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem. 2003 Aug;49(8):1331–36.. doi: 10.1373/49.8.1331. [DOI] [PubMed] [Google Scholar]
- 34.Apple FS, Murakami MM. Serum and plasma cardiac troponin I 99th percentile reference values for 3 2nd-generation assays. Clin Chem. 2007 Aug;53(8):1558–60.. doi: 10.1373/clinchem.2007.087718. [DOI] [PubMed] [Google Scholar]
- 35.Collinson PO, Clifford-Mobley O, Gaze D, Boa F, Senior R. Assay imprecision and 99th-percentile reference value of a high-sensitivity cardiac troponin I assay. Clin Chem. 2009 Jul;55(7):1433–4.. doi: 10.1373/clinchem.2009.124925. Epub 2009 May 14. [DOI] [PubMed] [Google Scholar]


