Abstract
The use of stem cell therapy for the treatment of cardiovascular diseases has generated significant interest in recent years. Limitations to the clinical application of this therapy center on issues of stem cell delivery, engraftment, and fate. Nanotechnology-based cell labeling and imaging techniques facilitate stem cell tracking and engraftment studies. Nanotechnology also brings exciting new opportunities to translational stem cell research as it enables the controlled engineering of nanoparticles and nanomaterials that can properly relate to the physical scale of cell-cell and cell-niche interactions. This review summarizes the most relevant potential applications of nanoscale technologies to the field of stem cell therapy for the treatment of cardiovascular diseases.
Keywords: Nanotechnology, stem cell therapy, cardiovascular disease, translational research, nanoparticles, MRI labeling, optical labeling, multimodality imaging, nanomaterials

Introduction
Cardiovascular diseases (CVDs) claim more lives each year than cancer, chronic lower respiratory disease, and accidents combined. Clearly, there is a need for new therapies to treat this pervasive problem. The use of stem cell therapy in CVDs for protection, restoration, and regeneration has gathered momentum in the past few years.1–5 A variety of cell types have been considered as candidates.6 Currently available routes for delivering progenitor cells to the heart, which include intravenous (IV), intracoronary (IC), or direct epicardial injection and, more recently, injection in the coronary sinus, are inefficient due to low cell retention and a lack of targeted localization. Although IV delivery of cells is the least invasive of these methods, most of the delivered cells are trapped in the lungs, with less than 1% homing to the infarcted heart. During angioplasty, cells can be delivered by IC infusion directly to the region of interest. However, studies show that 50% to 90% of injected cells are lost by extrusion and that 90% of the remaining cells die within 1 week of implantation. Upon restoration of blood flow, the majority of cells are washed away from the region of interest, and only 3% of the delivered cells engraft into the heart. By comparison, some studies showed that direct intramuscular injection of cells into the heart wall resulted in a modest increase in the number of cells delivered to the myocardium, with 11% of the cells engrafting.7–9 Although the concept of cell repair is scientifically sound, there are extraordinary challenges that need to be overcome before realizing the full clinical potential of this therapeutic approach. The heart is a complex organ that has more than three dimensions since, unlike any other organ, it also displays rhythm and contractility. In addition, it is an asymmetric and anisotropic organ with variable anatomy. Furthermore, the infarcted myocardium is a hypoxic environment that is not favorable for cell survival. The use of nanotechnology brings new, exciting opportunities to address these challenges through stem cell research and development.
Nanotechnology involves the development of materials and functional structures with at least one characteristic dimension measured in nanometers. Due to the size of their constituent particles, these materials can be manipulated to exhibit new and enhanced physical, chemical, and biological properties, creating unique advantages when compared with both macroscopic materials and molecular systems. Nanoscopic objects can be designed to optimize the balance of internal volume and external surface area, and many functionalities can be added to their surface and interior, making them ideal vessels for transport and tissue-selective targeting. The application of nanotechnology in stem cell research and development has become a new interdisciplinary frontier in materials science and regenerative medicine. This review presents several prospective applications of various nanoscale technologies applied to the field of stem cell therapy for the treatment of CVDs.
Application of Nanoparticles in Imaging and Tracing of Stem Cells
For the development of stem cell therapies, novel imaging techniques to study stem cell engraftment dynamics after cell delivery are essential to monitor the cells’ fate in a noninvasive and real-time fashion over a reasonably long observation period in both animal models and in clinical trials.10 11 Nyolczas et al. have reviewed the current results for tracking the fate of stem cells delivered to the heart.12 To date, imaging techniques including bioluminescence, magnetic resonance imaging (MRI), contrast agents, near infrared fluorescence, radioactive substrates, and post-mortem histological analysis have been used to detect migration and homing of the transplanted cells.7 13–25
MRI Labeling
There are several types of iron oxide nanoparticles (IONPs) that are used to label stem cells, including superparamagnetic iron oxide nanoparticles (SPIONs), which are 50 nm to 200 nm in diameter, and ultra-small superparamagnetic iron oxide (USPIO) nanoparticles, approximately 35 nm in diameter. The major limitation of SPIONs for labeling mesenchymal stem cells (MSCs) is their low intracellular labeling efficiency. The MRI signal hypointensity caused by those particles does not reflect the actual cell count after several rounds of cell division due to particle dilution. Furthermore, the signal from iron oxide nanoparticles is independent of cell viability, with no discrimination by MRI between live and dead cells. If the transplanted cells die, magnetic nanoparticles could persist in the tissue; dead cells could also be phagocytosed by macrophages and produce a misleading MRI signal.26 Amsalem et al. examined the functionality of SPION-labeled MSCs in the injured myocardium by injecting the stem cells directly into immunocompetent Sprague-Dawley rat hearts after ischemic injury. Upon MRI analysis 4 weeks after delivery, the SPIONs were only observed in cardiac macrophages and not within MSCs.27 Also, macrophages loaded with hemosiderin from hemorrhage can often be found in infarcted myocardium, and their hypointense signals may not be distinguishable from labeled cells.27 28 After intracellular labeling, commercially available MRI contrast agents of a large size (120–180 nm) usually tend to be biodegraded by intracellular enzymes and acids and then diluted by rapid cell division. To solve this problem, MSCs need to be labeled with a larger number of nanoparticles of a smaller size, so that after cell proliferation the nanoparticles will be numerous enough to be distributed within the daughter cells; they also need to be coated with chemically inert substances that are resistant to intracellular enzymes and acid. The previously available SPIONs, Feridex and Endorem, were discontinued at the end of 2008 and are no longer commercially available in the United States. Resovist has now also been taken off the market. New types of iron oxide nanoparticles have been studied since then but are currently not approved for clinical use. BioPAL Inc (Worcester, Massachusetts) produces iron oxide nanoparticles including FeREX (USPIO, 50-150 nm) and Molday ION products (approximately 30 nm). Recently it has been shown that, despite the initial belief in the noncytotoxic properties of IONPs, the physico-chemical properties of nanoparticles and the high intracellular concentrations of IONPs required for efficient MRI can alter cell homeostasis. Soenen et al. reported that high intracellular concentrations of IONPs affected the actin cytoskeleton, resulting in diminished cell proliferation.29 SPIONs are prone to aggregation, which can be reduced by coating the particles with dextran or other polymers. It has also been shown that without a transfecting agent, dextran-coated SPIONs do not exhibit sufficient cellular uptake to enable tracking of nonphagocytic cells. The cellular uptake of SPIONs by nonphagocytic cells can be facilitated by cationic compounds such as poly-L-lysine (PLL) and protamine sulfate due to their interaction with the negatively charged cell surface and subsequent endosomal uptake.30 PLL is a cationic synthetic polymer used in vitro. Since PLL is toxic in high concentrations, it has not yet been approved for clinical use. Protamines are low-molecular-weight, arginine-rich proteins (~4000 Da) purified from the mature testes of fish. Protamine sulfate is an FDA-approved polycationic peptide used routinely in patients for heparin anticoagulation reversal after cardiopulmonary bypass.
Nanomaterials for targeted imaging are capable of delivering large numbers of contrast agents per targeted molecular recognition event to achieve high-sensitivity imaging. Nanovectors can also simultaneously deliver different types of imaging agents to enable imaging. Tran et al. studied gadonanotubes (GNTs), short (20–80 nm) segments of single-walled carbon nanotubes encapsulating small clusters of gadolinium ions, as magnetic nanolabels. They showed that the magnetic labeling of MSCs with GNTs in vitro did not affect the differentiation potential of the MSCs; however, cell adhesion properties of the MSCs were impaired.31 Sanchez-Antequera et al. developed a novel methodology for performing genetic modification and cell isolation in a single standardized procedure that they called “magselectofection,” which integrated clinically approved nanomagnetic cell separation and magnetofection, nanomagnetically guided nucleic acid delivery. It was shown that the performance of cell sorting and cell recovery is not affected by magselectofection and that the function, viability, and differentiation of cells are not diminished.32
Optical Labeling
Optical labeling (OL) involves introducing a fluorescent signal to the cells, primarily in the near-infrared region. The method is based on ex vivo labeling of cells with a fluorescent tag, subsequent engraftment of the labeled cells, and visualization of their accumulation in specific target organelles of interest. OL is as sensitive as radiolabel-based imaging techniques but without any exposure to irradiation. OL provides an effective means of repeatedly tracking cells noninvasively, thereby providing insight into cell migration to the target site. Cell labeling efficiency is usually improved if the cells are incubated with the fluorescent dye in serum-free media as opposed to incubation in serum-containing media. One major disadvantage of OL is the limited tissue penetration of fluorescent labels in vivo. Tracer accumulation in deep tissues, more than about 4 cm to 10 cm from the skin surface, may not be detected. Nanomaterial-based cellular labels like quantum dots have made OL a relatively low-cost method, and it has become an indispensable tool in small animal studies.33
Multimodality Imaging
The combination of several molecular imaging modalities can offer synergistic advantages over any one modality alone. Combining an optical imaging modality with 3D tomographic techniques such as positron emission tomography, single-photon emission computed tomography, or MRI can allow for noninvasive imaging in living subjects with higher sensitivity and/or accuracy with the needed resolution. Shi et al. developed bifunctional anionic Eu3+-doped Gd2O3 hybrid nanoparticles as a luminescent and T1-weighted MRI contrast agent for stem cell labeling. Cellular uptake of these nanoparticles into human MSCs was confirmed by confocal laser scanning microscopy after 2 hours of nanoparticle incubation.34
Application of Three-Dimensional (3D) Nanostructures in Stem Cell Tissue Engineering
Great potential resides in the creation of well-controlled, engineered nanodimensional constructs and nanoarchitectures in an attempt to mimic the natural physical and biological environment that promotes tissue regeneration and growth through improved cell differentiation and functionality. Langer has defined tissue engineering as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function.”35 The fundamental concept in tissue engineering is the seeding of a scaffold with specific cells in order to drive their growth and development through the application of specific signaling agents including hormones, proteins, growth media, and environmental stimuli (Figure 1).36 A scaffold is a 3D precise space that supports the cells and allows them to proliferate and differentiate. By developing specifically tailored nanomaterials with enhanced properties, it is hypothesized that the scaffold will play a pivotal role in the growth and differentiation of the seeded cell populations. The extracellular matrix (ECM) is defined as any tissue that is not part of a cell. The main components of the ECM are glycoproteins (the most abundant being collagens), proteoglycans, and hyaluronic acid that are hierarchically arranged in a complex topography in the nanometer range.37–39 The scaffold itself is merely an imitation of the ECM found within the body, and it provides a framework for cell-cell interaction and the finite space that transforms and organizes the cells into 3D tissues and organs (Figure 2).40 Nutrient transport within the scaffold is mainly a function of diffusion and is of extreme importance in that it controls how the cells proliferate and differentiate. The rate and capacity of the transfer is based on the size, geometry, orientation, interconnectivity, branching, and surface chemistry associated with the pores and channels, which in turn are dictated by the material composition, fabrication, and physical arrangement. Conventional polymer-processing techniques have difficulty producing fibers smaller than 10 μm in diameter, which are several orders of magnitude larger than the native ECM topography (50–500 nm) (Figure 3).36 41 Nanofibers with diameters less than 1 μm that have been loaded with suitable growth factors, cells, or bioactive agents have great potential for use in tissue regeneration by providing cells with the necessary physical and chemical cues that drive stem cell fate decisions.41 It may be possible to incorporate these cues into the design of future 3D microenvironments to optimize and facilitate tissue repair and regeneration. These cues include soluble/immobilized factors, chemical and physical signals from the ECM, cell morphology, and external stresses. Due to their extremely high surface-to-mass ratio, nanofibers possess several novel properties such as low density, high pore volume, variable pore size, and exceptional mechanical properties. Cellular signal processing often occurs in small “nano-domains” where proteins and protein complexes interact at spatial dimensions ranging from 1s to 10s of nanometers.42 Specifically, the coupling of cell adhesion molecules (such as integrins) to the cytoskeleton and the formation of focal adhesion complexes is highly dependent on matrix stiffness in both differentiated and undifferentiated cells. The interplay of adhesion ligands and stiffness was investigated in one study to determine possible synergistic effects of the two factors on MSC differentiation. Myogenesis, while not as stiffness-dependent as osteogenesis, required a threshold stiffness (>9 kPa) before sufficient cell spreading and upregulation in MyoD1 occurred.43 Three distinct techniques have proven successful in routinely creating nanofibrous tissue engineering structures: self-assembly, phase separation, and electrospinning.44–48 Table 1 summarizes some of the materials used and the fibers obtained.49
Figure 1.
An example of a tissue engineering concept that involves seeding cells within porous biomaterial scaffolds. (A) Cells are isolated from the patient and may be cultivated in vitro (B) on two-dimensional surfaces for efficient expansion. (C) Next, the cells are seeded in porous scaffolds together with growth factors, small molecules, and micro- and/or nanoparticles. The scaffolds serve as a mechanical support and a shape-determining material, and their porous nature provides high mass transfer and waste removal. (D) The cell constructs are further cultivated in bioreactors to provide optimal conditions for organization into a functioning tissue. (E) Once a functioning tissue has been successfully engineered, the construct is transplanted on the defect to restore function. Reprinted with permission from Macmillan Publishers Ltd: Nature Nanotechnology, copyright 2011.36
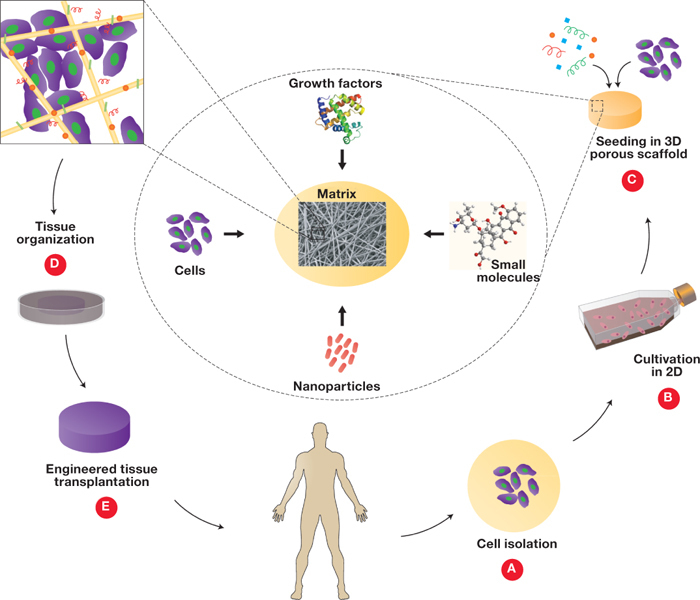
Figure 2.
The nesting cell. (A, B) A stem cell is exposed to multivariate cues including cell-cell interactions, cell-ECM interaction, soluble factors, and biophysical factors such as substratum rigidity, topography, shear stress, oxygen, and pH. (C) Novel techniques such as nanoengineering can mimic the microenvironmental conditions that a cell experiences in vivo and allow more precise control of experimental parameters such as shear stress, biochemical gradients, substrate rigidity and nanotopography, and cell positioning. Reprinted from Gupta et al. with permission from Elsevier.40
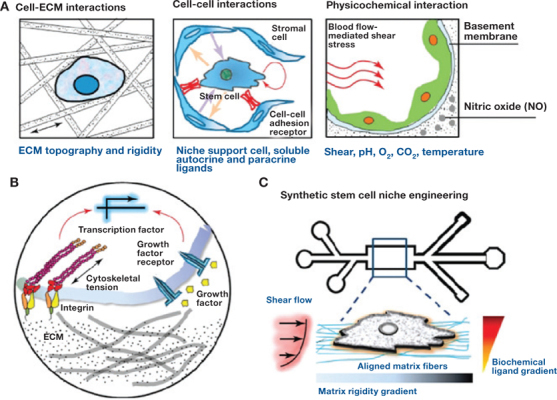
Figure 3.
(A) Illustrations of the heart at the level of organ (left) and tissue and cell/matrix interaction (center), followed by scanning electron micrographs of engineered scaffolds (right). The ECMs of various tissues have different composition and spatial organization of molecules to maintain specific tissue morphologies. The ECM of muscle tissues, such as the heart, forces the heart cells (cardiomyocytes) to couple mechanically to each other and to form elongated and aligned cell bundles that create an anisotropic syncytium. Reprinted with permission from Macmillan Publishers Ltd: Nature Nanotechnology, copyright 2011.36 (B) Nanogrooved surfaces (SEM image) are suitable matrices for cardiac tissue engineering because they force cardiomyocytes to align.41
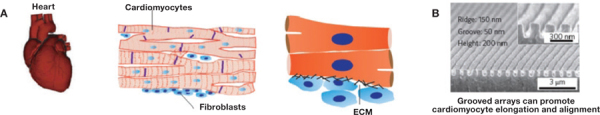
Table 1.
Most common types of nanofibers for medical applications.49
| Process | Lab/industrial application | Ease of processing | Advantages | Limitations |
| Self-assembly | Lab | Difficult |
|
|
| Phase separation | Lab | Easy |
|
|
| Electrospinning | Lab/industrial | Easy |
|
|
ECM: extracellular matrix. Reprinted from Barnes et al. with permission from Elsevier.49
Phase separation is based on the thermodynamic demixing of a homogeneous polymer-solvent solution into a polymer-rich and polymer-poor phase, thereby obtaining a porous nanofibrous matrix.
Electrospinning is a simple and cost-effective fabrication process that uses an electric field to control the deposition of polymer fibers onto a target substrate. This system can produce fibers with diameters ranging from several microns down to 100 nm or less. The generated fibers can mimic the structural profile of the proteins found in the native ECM. Different materials have been used to generate such fibers: synthetic biodegradable polymers, such as poly-L-lactic acid (PLLA), ε-caprolactone (PCL), poly(glycolic acid) (PGA), and also natural polymers such as collagen, silk, and DNA. The combination of natural and synthetic fibers has been achieved as well. In addition, electrospinning is able to produce both random and aligned networks. This prospect of controlling the orientation of fibers is a pre-requirement for biomimicking natural tissues. Altering the concentration/viscosity of the polymer solution affects fiber diameter: the higher the concentration, the larger the diameter of the fibers. Its simplicity allows electrospinning to be used in a laboratory setting and used successfully in scale-up and mass production. Stem cells grown on fibrous scaffolds have also shown differentiation-dependent behavior in terms of the fiber chemistry, size, and alignment. For example, MSCs grown on electrospun-aligned PCL scaffolds showed preferential differentiation to a chondrogenic lineage on nanoscale versus microscale fibers. While cells aligned in the direction of the fibers for both nano- and microscale scaffolds, the nanofibers (<500 nm diameter) promoted higher levels of glycosaminoglycan production and mRNA expression of collagen II and aggrecan. Electrospun nanofiber matrices show morphological similarities to the natural ECM, characterized by ultrafine continuous fibers with a high surface-to-volume ratio.
Hosseinkhani et al. demonstrated that PGA/collagen nanofibers fabricated through electrospinning significantly enhanced cell adhesion compared with PGA/collagen microfibers.50 Furthermore, different scaffold architectures may have varying influence on cell function. Generally, electrospinning produces a 3D mesh of nonwoven nano/micro fibers. Influencing cellular function using electrospun scaffolds remains a challenge, as the scaffold must mimic some of the components that make up the natural ECM while providing the appropriate biochemical and mechanical inputs for the cellular microenvironment. Chemical cues in the form of various biomolecules (nanometer scale), such as adhesive protein or growth factors, also significantly influence cell behavior.45 49 51
Self-assembly involves the spontaneous organization of individual components into an ordered and stable structure with noncovalent bonds.52 The most common particles used in self-assembly for medical purposes are amphiphilic particles that interact in solution, driven by shielding of hydrophobic regions, hydrogen bonding, and electrostatic repulsing forces. Self-assembly is a rather complex laboratory procedure that is limited to only a select few polymer configurations. This technique generally creates nanofibers that are 5 nm to 8 nm in diameter and 1 μm in length. In a rat model of myocardial infarction, Guo et al. demonstrated that survival was improved when stem cells were delivered with a self-assembling peptide nanoscaffold.53 The differentiation of bone marrow-derived MSCs on nanofibrous membranes or hydrogels could be another area of research that might accelerate the cardiac regeneration process. Use of an engineered cardiac construct with incorporated cytokines and growth factors not only provides physical support but also allows for the differentiation of stem cells to cardiomyogenic lineages, thus enhancing the myocardial regeneration. The use of nanofibers as scaffolds to replace the natural ECM has several advantages. Nanofibers have a high surface area and a highly interconnected porous architecture, which facilitate the colonization of cells in the scaffold and the efficient exchange of nutrients and metabolic waste between the scaffold and its environment. These nanofibers can be made of synthetic or natural materials or a combination thereof. Poly(ethylene glycol) (PEG) hydrogels were patterned with nanoscale topographical features that mimic the architecture of matrix fibers found in the ECM of the native heart. Cells grown on patterned gels exhibited significantly improved organization, contraction strength, and conduction velocity, suggesting that nanoscale features may exercise important influences on cardiac cells. Nanoparticles are also useful for the delivery of molecules to stem cells. Since stem cells undergoing lineage commitment require a specific spatio-temporal presentation of factors, efforts have been made to incorporate these particles into biomaterials for controlled release rates.
Controlled Presentation and Delivery of Differentiation Factors
To promote vascularization, vascular growth factors (VGF) incorporated by the gene delivery techniques and an optimal stem cell type (i.e., MSCs) could be applied to engineer the constructs. Two growth factors intimately involved in the process of vascularization are vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF). However, it is not only the presence of these two factors that influences angiogenesis but also their temporal presentation. VEGF is responsible for the initiation of angiogenesis and involves endothelial cell activation and proliferation, while PDGF is required after VEGF activation to allow for blood vessel maturation through recruitment of smooth muscle cells. Richardson et al. developed a dual growth factor release system in which VEGF encapsulated in poly(lactic/glycolic acid) (PLGA) microspheres was dispersed throughout the scaffold.54 Based on release kinetics, they demonstrated an initial rapid release of VEGF and a delayed release of PDGF, which contributed to greater maturation of vessels as evidenced by α-smooth muscle actin compared to VEGF or PDGF factor addition only.
In a recent pig model study, Lin et al. directly injected bone marrow mononuclear cells (MNCs) and a self-assembling peptide nanofiber (NFs) scaffold.55 They also injected the scaffold or the cells alone. Injection of the nanofibers after myocardial infarction (MI) restrained scar extension and prevented further harmful fibrosis at the remote zone. Moreover, reduction in global cardiac remodeling and diastolic dysfunction after MI were achieved. The injection of MNCs along with NFs showed even better amelioration of cardiac function. The authors attributed these results to the ability of the nanofibers to increase cell retention. The presence of nanofibers did not alter the viability of MNCs, which remained approximately 95% viable before injection and more readily differentiated into endothelial cells and smooth muscle cells. The proposed explanation was that NFs may act as a scaffold that provides a suitable microenvironment for the MNCs to adhere and perform normal cellular functions. Their results show the synergistic effect of NF and MNC injection. In another study involving MSCs in the process of differentiating into cardiac muscle cells, co-culture of these predifferentiated cells on aligned substrates with cardiomyocytes resulted in greater electrical conduction and upregulation of cardiogenic markers of differentiation as compared to co-cultures on isotropic substrates. While adhesion to specific molecules can initiate a differentiation program, the presentation of these adhesion sites allows for proper coupling of cell morphological and signal transduction pathways. Chen et al. concluded that surfaces modified with cell affinity molecules can be considered as “cellphilic,” and this effect may be enhanced by particular micro/nanoscale topography even to a “supercellphilic” state.56
Cardiac Graft
The requirements involved in fabricating cardiac grafts are much more demanding than those faced in producing vascular grafts. The scaffolds must be designed not only to withstand pulsation and the high pressure and flow rate of the bloodstream but also with attention to the diastolic property (expansive) loads, otherwise a “rigid” graft might negatively affect the diastolic functioning. Engineered heart muscle must develop systolic (contractive) force as the material used for the construct has no ability to beat without cells. The contractile movement is driven by the seeded cells. Moreover, a cardiac patch is required to integrate well into the electrical rhythm of the host myocardium, and it should not cause arrhythmia. Once the scaffold is implanted in vivo, the thick tissue will need to be vascularized to ensure adequate cellular nutrition and waste product removal. Electrospinning offers the potential to fabricate highly porous scaffolds to promote the transportation of nutrients and waste and encourage blood vessel formation. This technique has been investigated as a potential method of fabricating cardiac grafts. Primary cardiomyocytes (CMs) cultured on electrospun PLLA and PLGA scaffolds make use of external cues for isotropic and anisotropic growth. These studies suggest that a desirable scaffold for cardiac grafts should consist of aligned fibers to provide contact guidance cues, and it should have “adequate” porosity to allow the cells to respond to external cues and allow for the transportation of nutrients and waste in and out of the scaffold. Vacanti’s group demonstrated the formation of contractile cardiac grafts in vitro using a nanofibrous PCL mesh with ECM-like topography, which was produced by the electrospinning technique.57 The average fiber diameter of the scaffold was about 250 nm, well below the size of an individual cardiomyocyte. Following seeding of neonatal rat cardiomyocytes in the nanofibrous mesh, the construct was cultured while being suspended across a wire ring that acted as a passive load to contracting cardiomyocytes. The cardiomyocytes started beating after 3 days and were cultured in vitro for 14 days. The cardiomyocytes attached well to the PCL mesh and expressed cardiac-specific proteins such as alpha-myosin heavy chain, connexin 43, and cardiac troponin I. This work indicated that by using nanofibrous PCL mesh with ECM-like topography, cardiac grafts can be matured in vitro to obtain sufficient function prior to implantation. The same group subsequently demonstrated the formation of thick cardiac grafts in vitro and the versatility of biodegradable electrospun meshes for cardiac tissue engineering.58 To construct 3D cardiac grafts, the cell-seeded cardiac nanofibrous PCL meshes were overlaid between days 5 and 7 of the in vitro culture period. In addition to well-attached and strongly beating cells throughout the experimental period, constructs with up to five layers could be cultured without any incidence of core necrosis. The layers adhered intimately, with morphologic and electrical communication being established between the layers as verified by histology, immunohistochemistry, and synchronized beating. We envision that cardiac grafts with clinically relevant dimensions can be created by using this approach and combining it with new technologies to induce vascularization.
Conclusions
Although the development of nanomaterials seems to hold great potential for several biomedical fields, only modest progress has been made in its effective application to current human therapy. Encompassing nanoscale science, engineering, and technology, nanotechnology enables much finer control of the culture, separation, differentiation, tracking, delivery, and engraftment of stem cells for future cell-based therapies. Nanotechnology provides the ability to produce surfaces, materials, and constructs with nanoscale features that can mimic the natural environment of cells to promote certain functions, such as cell adhesion and cell differentiation. In the near future, it will allow labeling and tracking of the stem cells in vivo. In the long term, it is possible to envision the use of nanomaterials as a suitable 3D construct that induces the stem cell to engraft in the target site and directs the cell’s differentiation toward the desired specific lineage. Eventually, nanoparticles will be able to deliver a variety of factors, including growth factors, within the nanoscaffold in a controlled spatiotemporal manner. Nanosensors embedded in the 3D construct will control the release of desired cues.
Conflict of Interest Disclosure: The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: The author has no funding disclosures.
References
- 1.Zhang C, Sun A, Zhang S, Yao K, Wu C, Fu M, et al. Efficacy and safety of intracoronary autologous bone marrow-derived cell transplantation in patients with acute myocardial infarction: insights from randomized controlled trials with 12 or more months follow-up. Clin Cardiol. 2010 Jun;33(6):353–60. doi: 10.1002/clc.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002 Feb;34(2):107–16. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 3.Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, et al. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J Card Fail. 2011 Apr;17(4):272–81. doi: 10.1016/j.cardfail.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Fischer-Rasokat U, Assmus B, Seeger FH, Honold J, Leistner D, Fichtlscherer S, et al. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circulation. 2009 Sep;2(5):417–23. doi: 10.1161/CIRCHEARTFAILURE.109.855023. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001 May;33(5):907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 6.Alaiti MA, Ishikawa M, Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res. 2010 Sep;156(3):112–29. doi: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005 Sep 6;112(10):1451–61. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003 Aug 19;108(7):863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 9.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005 Aug 30; 112(9 Suppl):I150–6. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira L, Karp JM, Nobre L, Langer R. New opportunities: the use of nanotechnologies to manipulate and track stem cells. Cell Stem Cell. 2008 Aug 7;3(2):136–46. doi: 10.1016/j.stem.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira L. Nanoparticles as tools to study and control stem cells. J Cell Biochem. 2009 Nov 1;108(4):746–52. doi: 10.1002/jcb.22303. [DOI] [PubMed] [Google Scholar]
- 12.Nyolczas N, Charwat S, Posa A, Hemetsberger R, Pavo N, Hemetsberger H, et al. Tracking the migration of cardially delivered therapeutic stem cells in vivo: state of the art. Regen Med. 2009 May;4(3):407–22. doi: 10.2217/rme.09.14. [DOI] [PubMed] [Google Scholar]
- 13.Bulte JW, Kostura L, Mackay A, Karmarkar PV, Izbudak I, Atalar E, et al. Feridex-labeled mesenchymal stem cells: cellular differentiation and MR assessment in a canine myocardial infarction model. Acad Radiol. 2005 May;12 Suppl 1:S2–6. doi: 10.1016/j.acra.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006 Feb;55(2):242–9. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 15.Rogers WJ, Meyer CH, Kramer CM. Technology insight: in vivo cell tracking by use of MRI. Nat Clin Pract Cardiovasc Med. 2006 Oct;3(10):554–62. doi: 10.1038/ncpcardio0659. [DOI] [PubMed] [Google Scholar]
- 16.Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R. Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small (Weinheim an der Bergstrasse Germany). 2006 Dec;2(12):1412–7. doi: 10.1002/smll.200600218. [DOI] [PubMed] [Google Scholar]
- 17.Shah BS, Clark PA, Moioli EK, Stroscio MA, Mao JJ. Labeling of mesenchymal stem cells by bioconjugated quantum dots. Nano Lett. 2007 Oct;7(10):3071–9. doi: 10.1021/nl071547f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao JK, Tai MF, Chu HH, Chen ST, Li H, Lai DM, et al. Magnetic nanoparticle labeling of mesenchymal stem cells without transfection agent: cellular behavior and capability of detection with clinical 1.5 T magnetic resonance at the single cell level. Magn Reson Med. 2007 Oct;58(4):717–24. doi: 10.1002/mrm.21377. [DOI] [PubMed] [Google Scholar]
- 19.Sitharaman B, Tran LA, Pham QP, Bolskar RD, Muthupillai R, Flamm SD, et al. Gadofullerenes as nanoscale magnetic labels for cellular MRI. Contrast Media Mol Imaging. 2007 May-Jun;2(3):139–46. doi: 10.1002/cmmi.140. [DOI] [PubMed] [Google Scholar]
- 20.Song YS, Ku JH. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol Urodyn. 2007;26(4):584–93.. doi: 10.1002/nau.20351. [DOI] [PubMed] [Google Scholar]
- 21.Thorek DL, Tsourkas A. Size charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials. 2008 Sep;29(26):3583–90. doi: 10.1016/j.biomaterials.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraitchman DL, Bulte JW. Imaging of stem cells using MRI. Basic Res Cardiol. 2008 Mar;103(2):105–13. doi: 10.1007/s00395-008-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park B-H, Jung J-C, Lee G-H, Kim T-J, Lee Y-J, Kim J-Y, et al. Comparison of labeling efficiency of different magnetic nanoparticles into stem cell. Colloids Surf A. 2008;313(0):145–9.. [Google Scholar]
- 24.Wang L, Neoh KG, Kang ET, Shuter B, Wang SC. Biodegradable magnetic-fluorescent magnetite/poly(dl-lactic acid-co-alpha beta-malic acid) composite nanoparticles for stem cell labeling. Biomaterials. 2010 May;31(13):3502–11. doi: 10.1016/j.biomaterials.2010.01.081. [DOI] [PubMed] [Google Scholar]
- 25.Tseng CL, Shih IL, Stobinski L, Lin FH. Gadolinium hexanedione nanoparticles for stem cell labeling and tracking via magnetic resonance imaging. Biomaterials. 2010 Jul;31(20):5427–35. doi: 10.1016/j.biomaterials.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008 Mar 25;117(12):1555–62. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 27.Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation. 2007 Sep 11;116(11 Suppl):I38–45. doi: 10.1161/CIRCULATIONAHA.106.680231. [DOI] [PubMed] [Google Scholar]
- 28.van den Bos EJ, Baks T, Moelker AD, Kerver W, van Geuns RJ, van der Giessen WJ, et al. Magnetic resonance imaging of haemorrhage within reperfused myocardial infarcts: possible interference with iron oxide-labelled cell tracking? Eur Heart J. 2006 Jul;27(13):1620–6.. doi: 10.1093/eurheartj/ehl059. [DOI] [PubMed] [Google Scholar]
- 29.Soenen SJ, De Cuyper M. Assessing cytotoxicity of (iron oxide-based) nanoparticles: an overview of different methods exemplified with cationic magnetoliposomes. Contrast Media Mol Imaging. 2009 Sep-Oct;4(5):207–19. doi: 10.1002/cmmi.282. [DOI] [PubMed] [Google Scholar]
- 30.Arbab AS, Yocum GT, Rad AM, Khakoo AY, Fellowes V, Read EJ, et al. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005 Dec;18(8):553–9. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 31.Tran LA, Krishnamurthy R, Muthupillai R, Cabreira-Hansen Mda G, Willerson JT, Perin EC, et al. Gadonanotubes as magnetic nanolabels for stem cell detection. Biomaterials. 2010 Dec;31(36):9482–91. doi: 10.1016/j.biomaterials.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Antequera Y, Mykhaylyk O, van Til NP, Cengizeroglu A, de Jong JH, Huston MW, et al. Magselectofection: an integrated method of nanomagnetic separation and genetic modification of target cells. Blood. 2011 Apr 21;117(16):e171–81. doi: 10.1182/blood-2010-08-302646. [DOI] [PubMed] [Google Scholar]
- 33.Emerich DF, Thanos CG. Nanotechnology and medicine. Expert Opin Biol Ther. 2003 Jul;3(4):655–63. doi: 10.1517/14712598.3.4.655. [DOI] [PubMed] [Google Scholar]
- 34.Shi Z, Neoh KG, Kang ET, Shuter B, Wang SC. Bifunctional Eu(3+)-doped Gd(2)O(3) nanoparticles as a luminescent and T(1) contrast agent for stem cell labeling. Contrast Media Mol Imaging. 2010 Mar-Apr;5(2):105–11. doi: 10.1002/cmmi.373. [DOI] [PubMed] [Google Scholar]
- 35.Langer R, Vacanti JP. Tissue engineering. Science. 1993 May 14;260(5110):920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 36.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011 Jan;6(1):13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams JC. Cell-matrix contact structures. Cell Mol Life Sci. 2001 Mar;58(3):371–92. doi: 10.1007/PL00000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999 Oct;18(5):417–26. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 39.Watt FM. The Extracellular-Matrix and Cell-Shape. Trends Biochem Sci. 1986 Nov;11(11):482–5. [Google Scholar]
- 40.Gupta K, Kim DH, Beebe DJ, Levchenko A. Micro- and nanoengineering for stem cell biology: the promise with a caution. Trends Biotechnol. 2011 Aug;29(8):399–408. doi: 10.1016/j.tibtech.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):565–70. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winslow RL, Greenstein JL. Cardiac myocytes and local signaling in nano-domains. Prog Biophys Mol Biol. 2011 Oct;107(1):48–59. doi: 10.1016/j.pbiomolbio.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol. 2008 Oct;295(4):C1037–44. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 44.Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008 Sep;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 45.Jayaraman K, Kotaki M, Zhang Y, Mo X, Ramakrishna S. Recent advances in polymer nanofibers. J Nanosci Nanotechnol. 2004 Jan-Feb;4(1-2):52–65. [PubMed] [Google Scholar]
- 46.Smith LA, Ma PX. Nano-fibrous scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2004 Dec 10;39(3):125–31. doi: 10.1016/j.colsurfb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Wen X, Shi D, Zhang N. Applications of nanotechnology in tissue engineering. In: Nalwa H editor. Handbook of Nanostructured Biomaterials and their Applications in Nanbiotechnology. Stevenson Ranch, CA: American Scientific Publishers; 2005. p1-23. [Google Scholar]
- 48.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005 Jan-Feb;11(1-2):101–9. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 49.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007 Dec 10;59(14):1413–33. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 50.Hosseinkhani H, Hosseinkhani M, Hattori S, Matsuoka R, Kawaguchi N. Micro and nano-scale in vitro 3D culture system for cardiac stem cells. J Biomed Mater Res. 2010 Jul;94(1):1–8. doi: 10.1002/jbm.a.32676. [DOI] [PubMed] [Google Scholar]
- 51.Engel E, Michiardi A, Navarro M, Lacroix D, Planell JA. Nanotechnology in regenerative medicine: the materials side. Trends Biotechnol. 2008 Jan;26(1):39–47. doi: 10.1016/j.tibtech.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94(1):1–18.. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo HD, Cui GH, Wang HJ, Tan YZ. Transplantation of marrow-derived cardiac stem cells carried in designer self-assembling peptide nanofibers improves cardiac function after myocardial infarction. Biochem Biophys Res Commun. 2010 Aug 13;399(1):42–8. doi: 10.1016/j.bbrc.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 54.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001 Nov;19(11):1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 55.Lin YD, Yeh ML, Yang YJ, Tsai DC, Chu TY, Shih YY, et al. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation. 2010 Sep 14;122(11 Suppl):S132–41. doi: 10.1161/CIRCULATIONAHA.110.939512. [DOI] [PubMed] [Google Scholar]
- 56.Chen L, Han D, Jiang L. On improving blood compatibility: from bioinspired to synthetic design and fabrication of biointerfacial topography at micro/nano scales. Colloids Surf B Biointerfaces. 2011 Jun 15;85(1):2–7. doi: 10.1016/j.colsurfb.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 57.Shin M, Ishii O, Sueda T, Vacanti JP. Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials. 2004 Aug;25(17):3717–23. doi: 10.1016/j.biomaterials.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 58.Ishii O, Shin M, Sueda T, Vacanti JP. In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. J Thorac Cardiovasc Surg. 2005 Nov;130(5):1358–63. doi: 10.1016/j.jtcvs.2005.05.048. [DOI] [PubMed] [Google Scholar]


