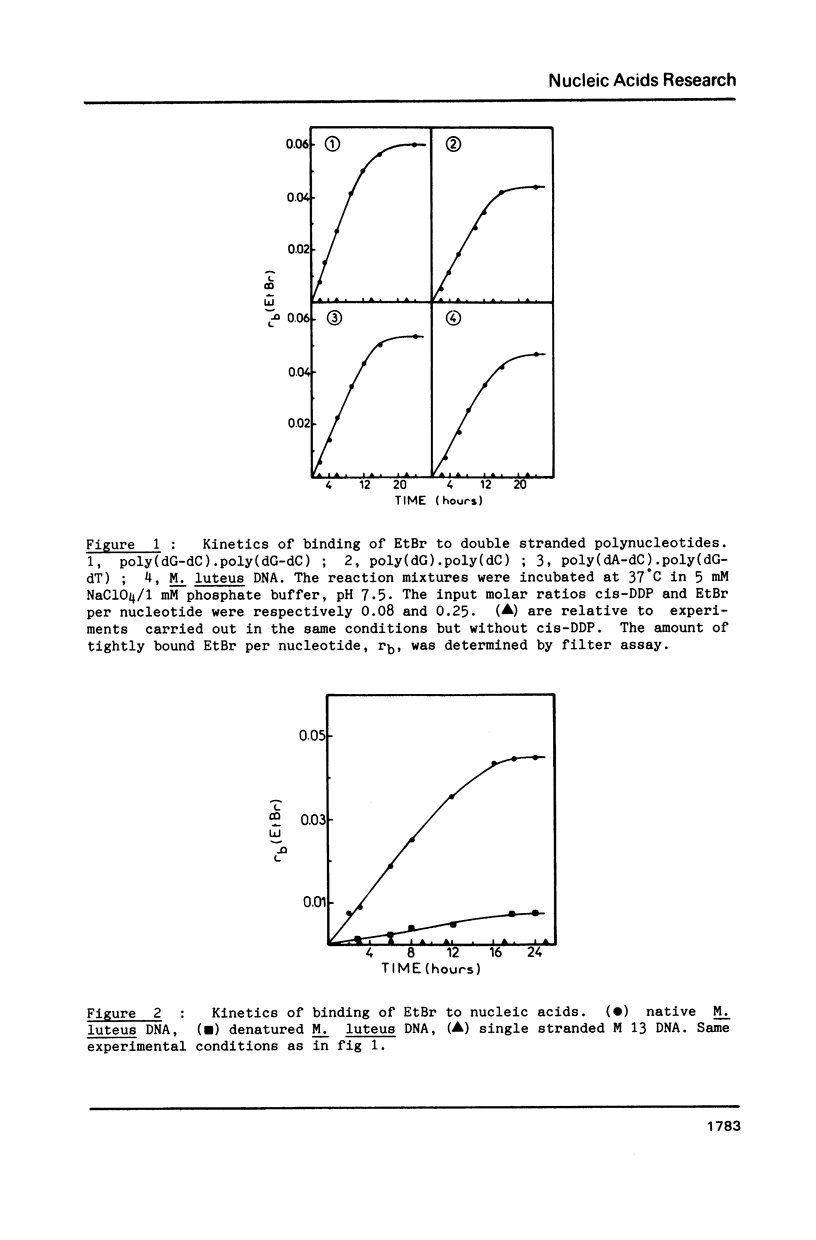
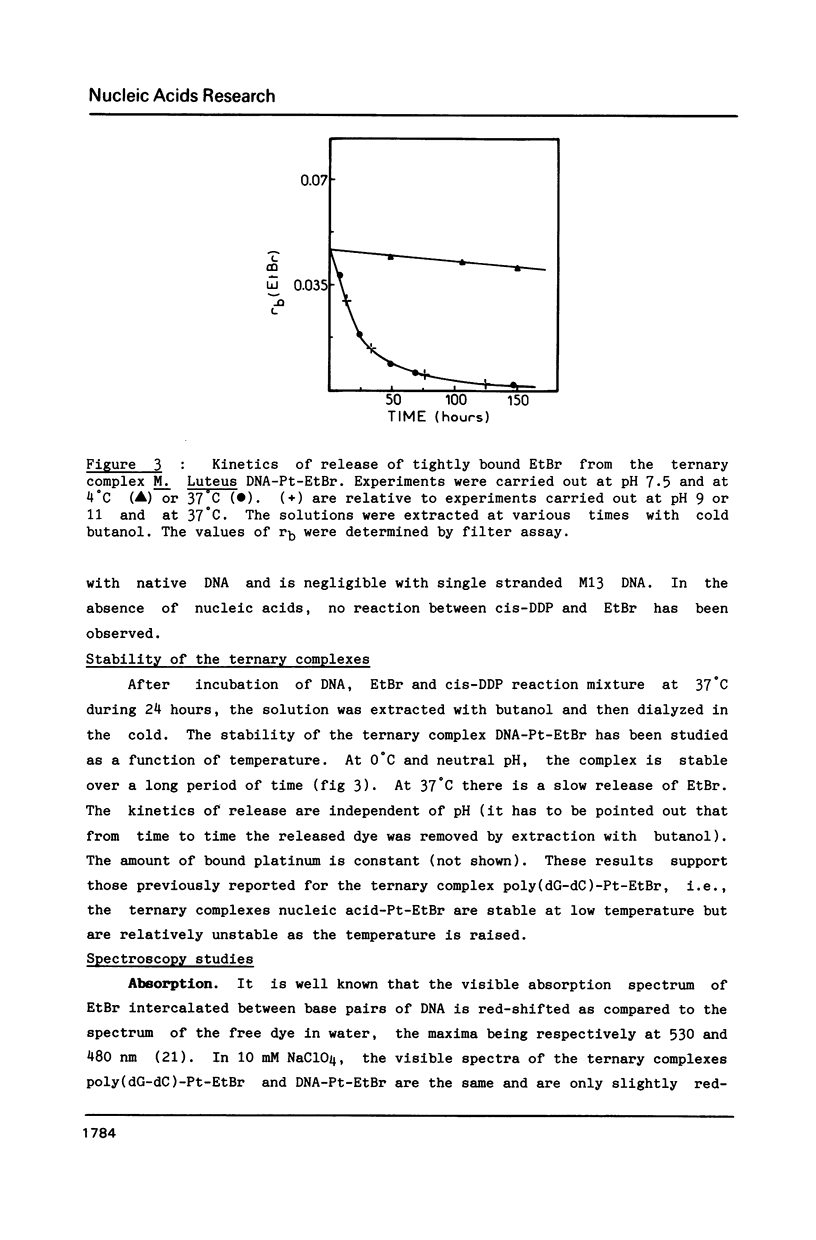
Abstract
The purpose of this study was to characterize the ternary complexes formed in the reaction of cis-diamminedichloroplatinum (II) (cis-DDP) and nucleic acids, in the presence of the intercalating compound ethidium bromide (EtBr). In these ternary complexes, some EtBr is tightly bound to the nucleic acids. Tight binding is defined by resistance to extraction with butanol, assayed by filtration at acid pH or thin layer chromatography at basic pH. These ternary complexes are formed with double stranded but not with single stranded nucleic acids. They are not formed if cis-DDP is replaced by transdiamminedichloroplatinum(II). The amount of tightly bound EtBr depends upon the sequence of the nucleic acid, being larger with poly (dG-dC).poly(dG-dC) than with poly(dG).poly(dC). Spectroscopic results support the hypothesis that the tight binding of the dye is due to the formation of a bidentate adduct (guanine-EtBr)cis-platin. The visible spectrum of the ternary complexes is blue-shifted as compared to that of EtBr intercalated between the base pairs of unplatinated DNA and it depends upon the conformation of the ternary complex. The fluorescence quantum yield of the ternary complexes is lower than that of free EtBr in water. Tightly bound EtBr stabilizes strongly the B form versus the Z form of the ternary complex poly(dG-dC)-Pt-EtBr and slows down the transition from the B form towards the Z form. The sequence specificity of cis-DDP binding to a DNA restriction fragment in the absence or presence of EtBr is mapped by means of the 3'----5' exonuclease activity of T4 DNA polymerase. In the absence of the dye, all the d(GpG) sites and all the d(ApG) sites but one in the sequence d(TpGpApGpC) are platinated. The d(GpA) sites are not platinated. In the presence of EtBr, some new sites are detected. These results might help to explain the synergism for drugs used in combination with cis-DDP and in the design of new chemotherapeutic agents.
Full text
PDF



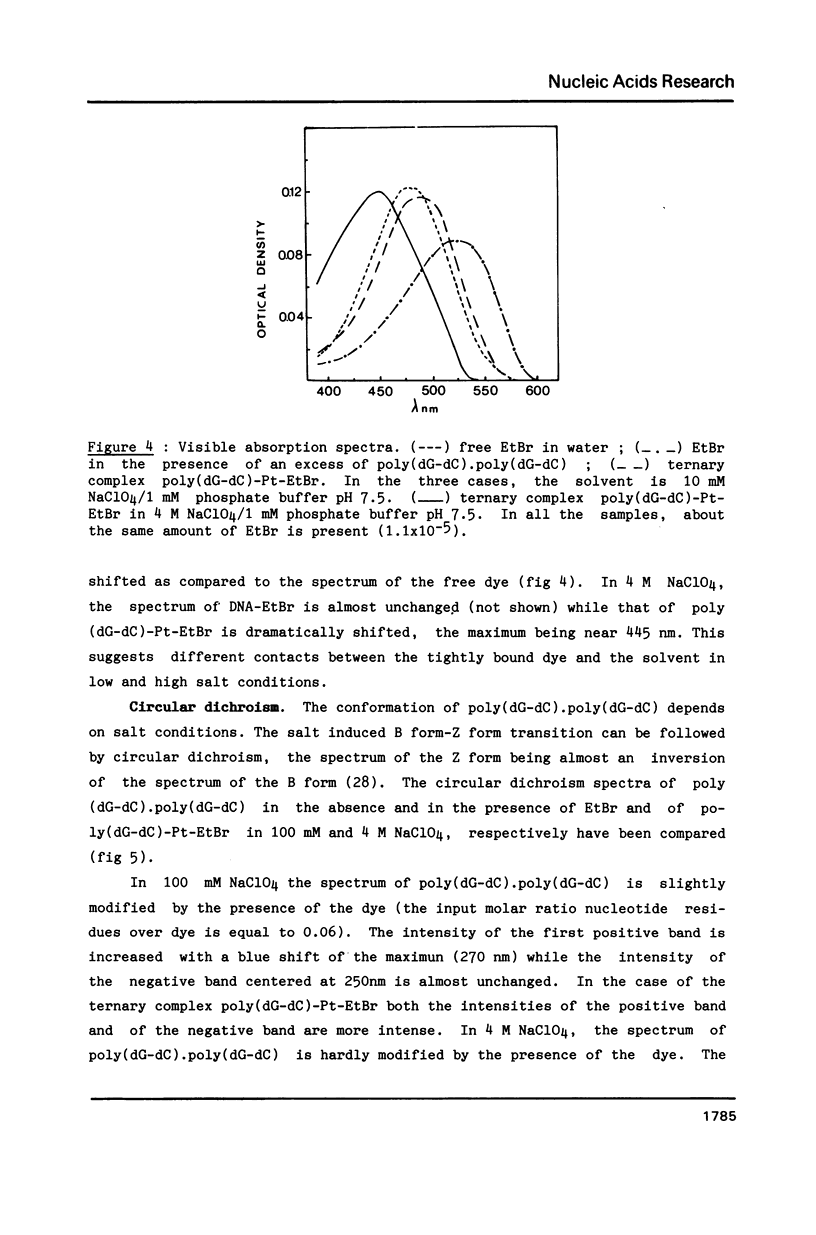
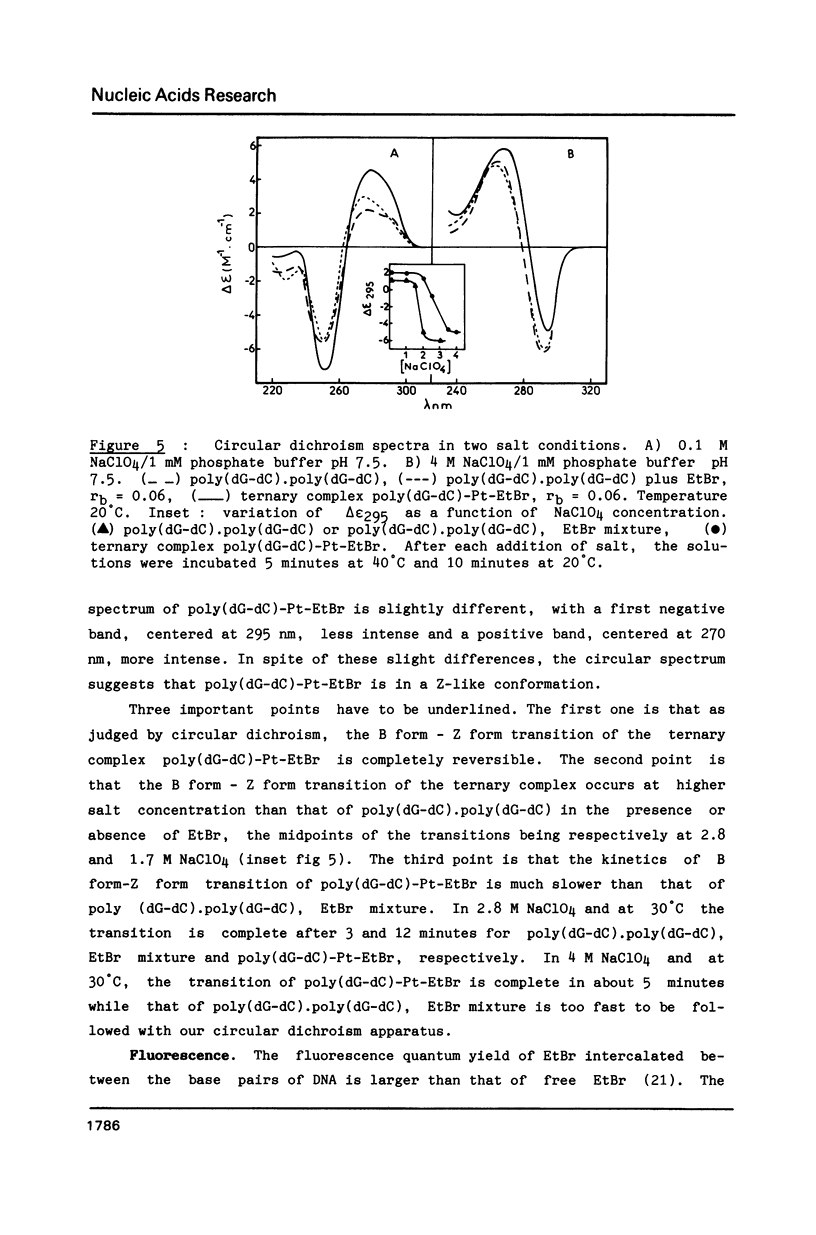
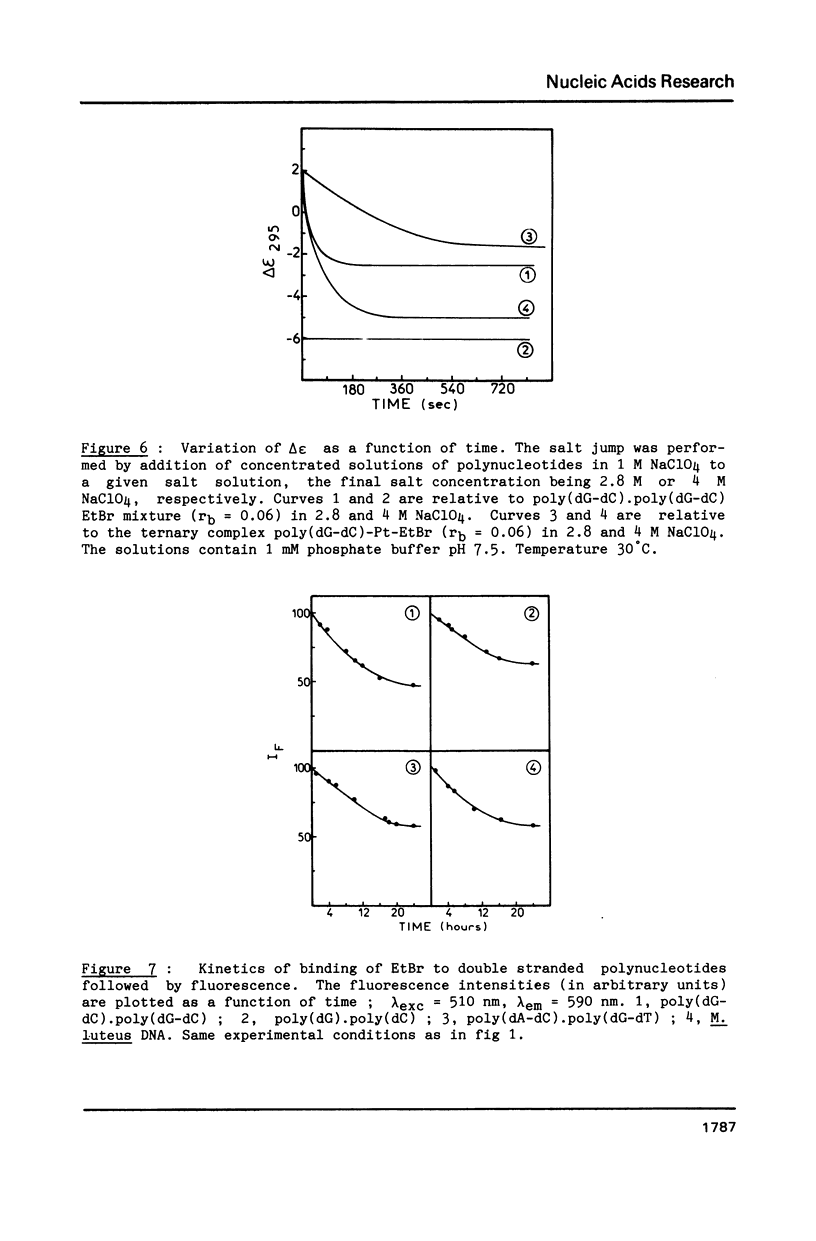
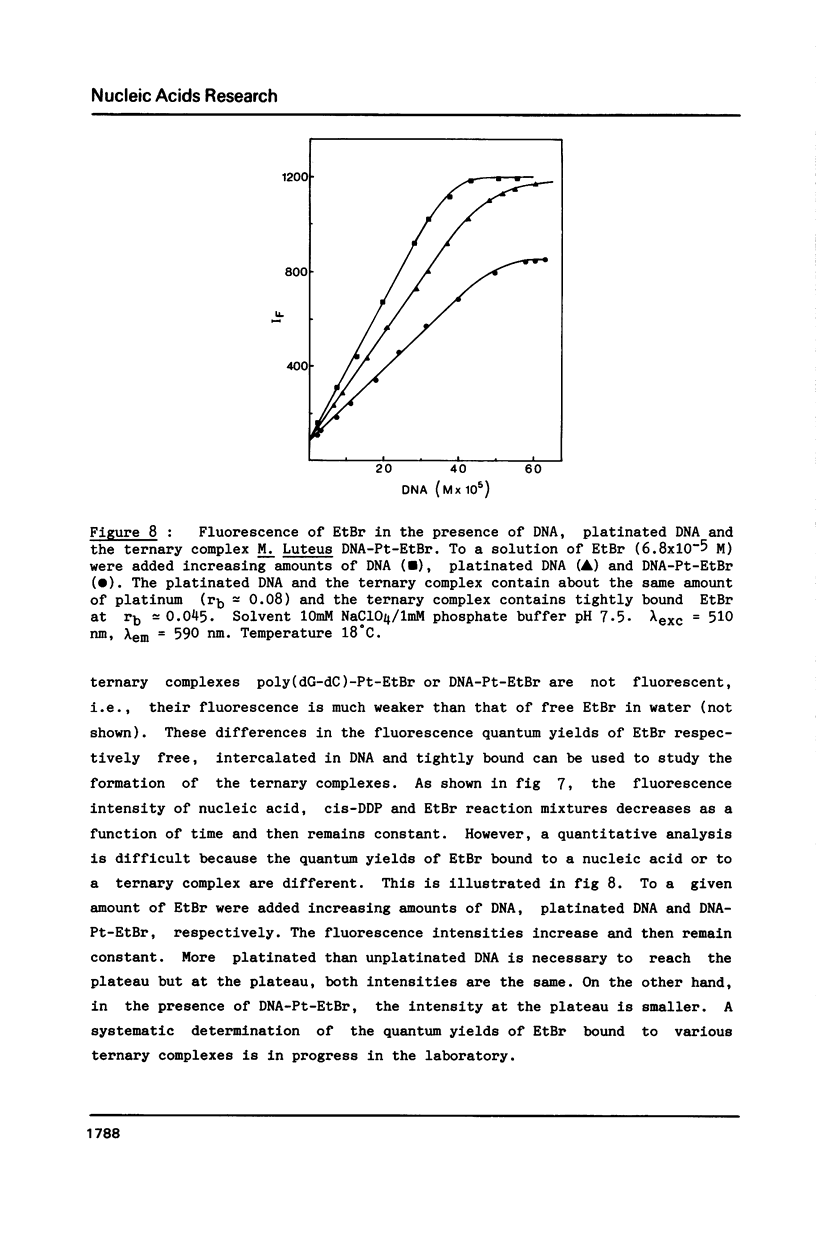

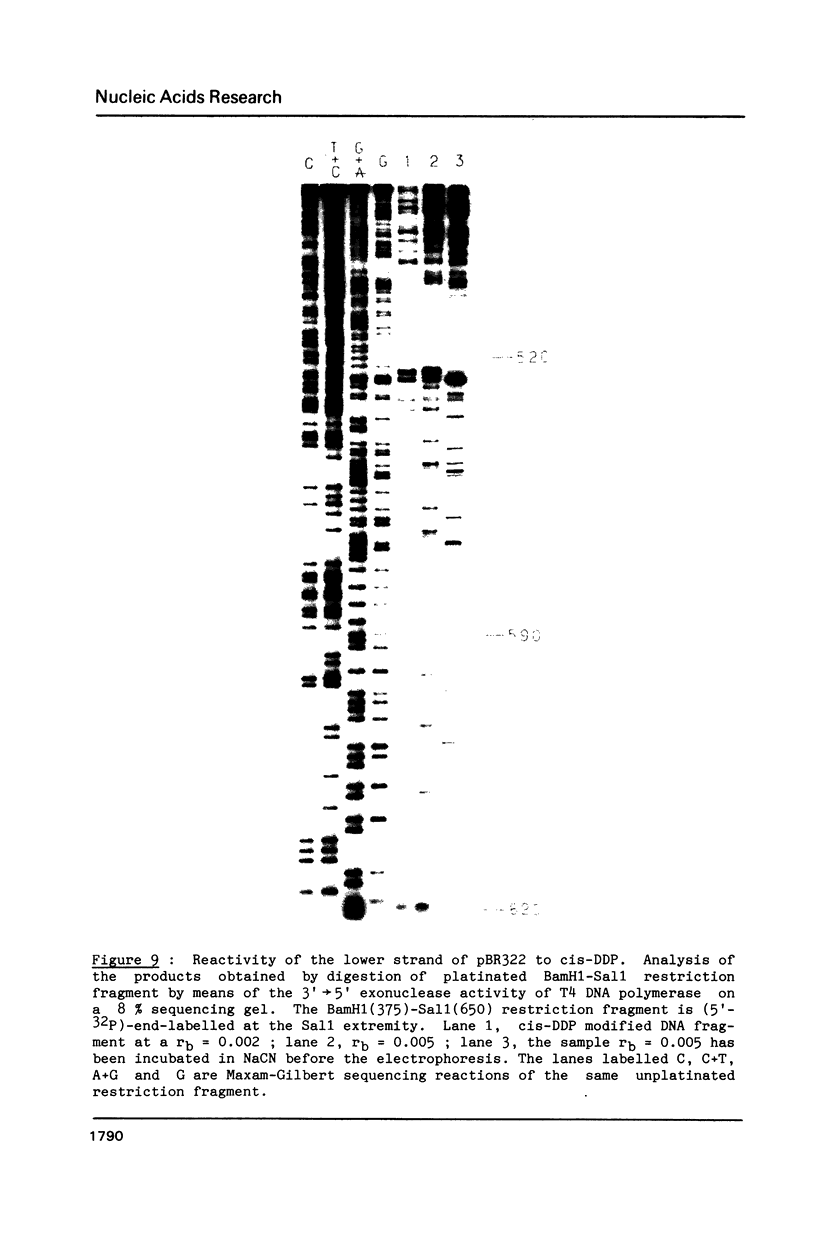







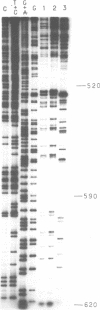
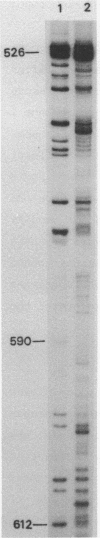
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowler B. E., Lippard S. J. Modulation of platinum antitumor drug binding to DNA by linked and free intercalators. Biochemistry. 1986 May 20;25(10):3031–3038. doi: 10.1021/bi00358a044. [DOI] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Eastman A. Interstrand cross-links and sequence specificity in the reaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1985 Sep 10;24(19):5027–5032. doi: 10.1021/bi00340a011. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., Lohman P. H., Reedijk J. Detection and quantification of adducts formed upon interaction of diamminedichloroplatinum (II) with DNA, by anion-exchange chromatography after enzymatic degradation. Nucleic Acids Res. 1982 Sep 11;10(17):5345–5356. doi: 10.1093/nar/10.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. DNA binding spectrum of the carcinogen N-acetoxy-N-2-acetylaminofluorene significantly differs from the mutation spectrum. J Mol Biol. 1984 Jul 25;177(1):173–180. doi: 10.1016/0022-2836(84)90063-9. [DOI] [PubMed] [Google Scholar]
- Galley W. C., Purkey R. M. Spin-orbital probes of biomolecular structure. A model DNA-acridine system. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2198–2202. doi: 10.1073/pnas.69.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger-Schnarr M., Daune M. P., Fuchs R. P. Specificity of N-acetoxy-N-2-acetylaminofluorene-induced frameshift mutation spectrum in mismatch repair deficient Escherichia coli strains mutH, L, S and U. J Mol Biol. 1986 Aug 5;190(3):499–507. doi: 10.1016/0022-2836(86)90018-5. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Hartmann B., Leng M. The B goes to Z transition of poly(dG-dC) . poly(dG-dC) modified by some platinum derivatives. Nucleic Acids Res. 1981 Nov 11;9(21):5659–5669. doi: 10.1093/nar/9.21.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfoy B., Hartmann B., Macquet J. P., Leng M. Immunochemical studies of DNA modified by cis-dichlorodiammineplatinum(II) in vivo and in vitro. Cancer Res. 1981 Oct;41(10):4127–4131. [PubMed] [Google Scholar]
- Malinge J. M., Leng M. Reaction of cis-diamminedichloroplatinum (II) and DNA in B or Z conformation. EMBO J. 1984 Jun;3(6):1273–1279. doi: 10.1002/j.1460-2075.1984.tb01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinge J. M., Leng M. Reaction of nucleic acids and cis-diamminedichloroplatinum(II) in the presence of intercalating agents. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6317–6321. doi: 10.1073/pnas.83.17.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merkel C. M., Lippard S. J. Ethidium bromide alters the binding mode of cis-diamminedichloroplatinum(II) to pBR322 DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):355–360. doi: 10.1101/sqb.1983.047.01.041. [DOI] [PubMed] [Google Scholar]
- Mirau P. A., Kearns D. R. The effect of intercalating drugs on the kinetics of the B to Z transition of poly(dG-dC). Nucleic Acids Res. 1983 Mar 25;11(6):1931–1941. doi: 10.1093/nar/11.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780(3):167–180. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Rahmouni A., Malinge J. M., Schwartz A., Leng M. Comparison between poly(dG-dC).poly(dG-dC) and DNA modified by cis-diamminedichloroplatinum (II): immunological and spectroscopic studies. J Biomol Struct Dyn. 1985 Oct;3(2):363–375. doi: 10.1080/07391102.1985.10508423. [DOI] [PubMed] [Google Scholar]
- Rio P., Leng M. Preferential binding of the chemical carcinogen N-hydroxy-2-aminofluorene to B-DNA as compared to Z-DNA. Nucleic Acids Res. 1983 Jul 25;11(14):4947–4956. doi: 10.1093/nar/11.14.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer R. H., Brown S. C., Delbarre A., Wade D. Binding of ethidium and bis(methidium)spermine to Z DNA by intercalation. Nucleic Acids Res. 1984 Jun 11;12(11):4679–4690. doi: 10.1093/nar/12.11.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. J., Kelman A. D., Sinex F. M. Resolution of alpha, beta and gamma DNA of Saccharomyces cerevisiae with the antitumor drug cis-Pt (NH3)2CL2. Evidence for preferential drug binding by GpG sequences of DNA. J Mol Biol. 1976 Jul 15;104(4):793–801. doi: 10.1016/0022-2836(76)90182-0. [DOI] [PubMed] [Google Scholar]
- Stone P. J., Kelman A. D., Sinex F. M. Specific binding of antitumour drug cis-Pt(NH3)2C12 to DNA rich in guanine and cytosine. Nature. 1974 Oct 25;251(5477):736–737. doi: 10.1038/251736a0. [DOI] [PubMed] [Google Scholar]
- Tullius T. D., Lippard S. J. Ethidium bromide changes the nuclease-sensitive DNA binding sites of the antitumor drug cis-diamminedichloroplatinum(II). Proc Natl Acad Sci U S A. 1982 Jun;79(11):3489–3492. doi: 10.1073/pnas.79.11.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushay H. M., Santella R. M., Caradonna J. P., Grunberger D., Lippard S. J. Binding of [(dien)PtCl] Cl to poly(dG-dC)-poly(dG-dC) facilitates the B goes to Z conformational transition. Nucleic Acids Res. 1982 Jun 11;10(11):3573–3588. doi: 10.1093/nar/10.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]