Summary
OBJECTIVE
The cardiovascular risk factors which comprise the metabolic syndrome are associated with increased hypothalamic–pituitary–adrenal axis (HPAA) activity in some Caucasian populations. South Asians have high rates of cardiovascular disease and its risk factors. We have investigated the relationships between HPAA activity, adiposity and the metabolic syndrome in a South Asian population.
DESIGN
Cross-sectional cohort study.
PARTICIPANTS
A total of 509 men and women born at the Holdsworth Memorial Hospital, Mysore, South India between 1934 and 1954 and still living in the area.
MEASUREMENTS
Fasting 09·00 h cortisol and corticosteroid-binding globulin. The cohort had previously been investigated for features of the metabolic syndrome.
RESULTS
At 09·00 h, cortisol concentration was strongly associated with systolic and diastolic blood pressure (r = 0·25 and r = 0·24, respectively; P < 0·001), fasting glucose concentration (r = 0·26; P < 0·001), insulin resistance (r = 0·20; P < 0·001) and fasting triglyceride concentration (r = 0·17; P < 0·001). In general, higher cortisol concentrations added to the effect of adiposity in increasing cardiovascular risk factors, but there was evidence of an interaction between cortisol and adiposity in determining fasting glucose concentration (P = 0·045) and insulin resistance (P = 0·006).
CONCLUSIONS
Associations between 09·00 h cortisol concentration and cardiovascular risk factors in this South Asian cohort were stronger than those previously shown in Caucasian populations, despite similar mean cortisol concentrations, and were amplified by adiposity. This suggests that increased glucocorticoid action may contribute to ethnic differences in the prevalence of the metabolic syndrome, particularly among men and women with a higher body mass index.
The metabolic syndrome, a cluster of abnormalities including glucose intolerance, hypertension and dyslipidaemia (Reaven, 1988), has many similarities with Cushing’s syndrome which has led to the suggestion that a milder degree of hypothalamic–pituitary–adrenal (HPA) axis dysregulation may underlie this phenotype. This is supported by cross-sectional studies which show that individuals with raised blood pressure, glucose intolerance or other features of the metabolic syndrome have raised fasting plasma cortisol concentrations and increased reactivity of the HPA axis (Filipovsky et al., 1996; Phillips et al., 1998; Lee et al., 1999).
The interplay between obesity and the HPA axis is complex. Tissue steroid metabolism, principally reactivation of cortisol by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in the liver, is altered in obese individuals leading to increased metabolic clearance of cortisol (Andrew et al., 1998; Stewart et al., 1999; Rask et al., 2001, 2002). As a result, although cortisol secretion is enhanced somewhat, circulating cortisol concentrations are generally normal or low in obese subjects. However, some studies suggest that a subset of individuals who maintain raised plasma cortisol concentrations in the face of obesity have a high prevalence of the metabolic syndrome. In these subjects, increased plasma cortisol concentrations or HPA reactivity add to the effect of adiposity in determining the risk of glucose intolerance, raised blood pressure and the metabolic syndrome (Phillips et al., 2000; Walker et al., 2000; Reynolds et al., 2001).
South Asians have a high morbidity and mortality from coronary heart disease and the cardiovascular risk factors which comprise the metabolic syndrome are prevalent. Although South Asians have a relatively high proportion of fat, particularly visceral fat, across the body mass index (BMI) range compared with Caucasian populations, the increase in adiposity does not fully explain the high prevalence of the metabolic syndrome (McKeigue et al., 1991; Shelgikar et al., 1991; Banerji et al., 1999). A greater understanding of the mechanisms underlying this increased cardiovascular risk may allow new therapies to be developed. The role of glucocorticoids has not previously been investigated in this ethnic group.
We have investigated the relationship between fasting 09·00 h cortisol concentration and cardiovascular risk factors in 509 adults from Mysore, South India to investigate the hypothesis that the high prevalence of the metabolic syndrome in South Asians is mediated by HPA axis hyperactivity.
Subjects and methods
Participants and data collection
In 1993, 517 adults born between 1934 and 1954 at the Holdsworth Memorial Hospital, Mysore, South India and still living in Mysore were traced and attended a clinic as part of a study assessing the influence of size at birth on cardiovascular risk factors (Stein et al., 1996; Fall et al., 1998). Detailed information on the subjects’ medical and social history was collected and they had standardized anthropometric measurements, including skinfold thickness at four sites, and blood pressure and electrocardiogram (ECG) recordings. Blood samples, taken between 08·00 and 09·30 h after a 12-h fast, were previously analysed for lipids and paired glucose, and insulin concentrations were available from 0, 30 and 120 min during a standard 75 g oral glucose tolerance test.
For the current study, stored fasting sera from 509 of these subjects were analysed for cortisol and corticosteroid-binding globulin (CBG) in the Regional Endocrine Laboratory at Southampton General Hospital. Subjects without a stored sample (n = 2) and subjects on oral glucocorticoid therapy (n = 6) were excluded. The study was approved by the local ethics committee and the subjects gave informed consent at the time of the original study.
Laboratory assays
Cortisol was measured using an in-house radioimmunoassay (RIA), interassay coefficient of variation (CV) 7·4–10·3%. CBG was assayed with a commercial assay (Medgenix Diagnostics, Fleurus, Belgium), intra-assay CV 3·3–7·7%, interassay CV 4·5–5·0%. Plasma glucose, insulin, total cholesterol, high-density lipoprotein (HDL)-cholesterol and triglyceride were measured as described previously (Stein et al., 1996; Fall et al., 1998).
Data analysis
Generalized obesity was defined as a BMI greater than 25 kg/m2 and central obesity as a waist–hip ratio (WHR) of 0·9 in men and 0·85 in women. These cut-offs have previously been defined for urban Indian populations (Ramachandran et al., 2001). Total body fat (%) was calculated from the skinfolds according to the equations of Durnin & Womersley (1974). Free cortisol index represents a ratio of cortisol to CBG. Insulin resistance and insulin secretion were estimated using the homeostasis model assessment (HOMA; Matthews et al., 1985). Glucose, insulin and triglyceride concentrations and HOMA variables had skewed distributions and were transformed to normality using logarithms. The metabolic syndrome was identified in those subjects with a systolic blood pressure ≥ 150 mmHg, a 2-h glucose ≥ 7·8 mmol/l and a triglyceride concentration above the median value (≥ 1·8 mmol/l in men or ≥ 1·4 mmol/l in women; Fall et al., 1998).
The relationships between cortisol, CBG and free cortisol index and the components of the metabolic syndrome were examined using partial correlation coefficients and multiple linear and logistic regression, correcting for age, sex, adiposity and adult lifestyle factors (smoking and alcohol intake). Outcomes and predictors were used as continuous variables where possible. Statistical analyses were performed using SPSS statistical software version 10·0 (Chicago, IL, USA). A P-value of less than 0·05 was considered to be statistically significant.
Results
The characteristics of the cohort have previously been described in detail and are summarized in Table 1 (Stein et al., 1996). In this relatively young population (mean age 47 years) the prevalence of obesity was high; 29·5% of men and 48·6% of women had a BMI greater than 25 kg/m2, a cut-off that has been used in previous studies in urban Indian populations. Both men and women had high waist-to-hip ratios (WHR above 0·9 in 66·3% of men and above 0·85 in 45·4% of women). Overall, 14·5% of the subjects had type 2 diabetes (mean BMI 25·3 kg/m2) and 8·6% were identified as having the metabolic syndrome (mean BMI 26·1 kg/m2). It is noteworthy that only 43 of the 509 subjects (8·4%) had a BMI > 30 kg/m2, the WHO definition of obesity, despite the high prevalence of type 2 diabetes.
Table 1.
Baseline characteristics, fasting 09·00 h cortisol and CBG concentrations and free cortisol index in 509 adults from Mysore, India
| Men n = 258 | Women n = 251 | All n = 509 | |
|---|---|---|---|
| Age (years) | 47·0 (4·7) | 47·0 (4·7) | 47·0 (4·7) |
| BMI (kg/m2) | 22·9 (4·1) | 24·8 (4·9) | 23·8 (4·6) |
| WHR | 0·91 (0·06) | 0·83 (0·06) | 0·87 (0·07) |
| Total body fat (%) | 23·1 (6·7) | 35·7 (5·6) | 29·2 (8·8) |
| Generalized obesity (n,%)* | 76 (29·5) | 12 (48·6) | 198 (38·9) |
| Central obesity (n,%)* | 171 (66·3) | 114 (45·4) | 285 (56·0) |
| Systolic BP (mmHg) | 131 (17) | 132 (18) | 132 (18) |
| Diastolic BP (mmHg) | 80 (11) | 77 (11) | 79 (11) |
| FPG (mmol/l)† | 5·2 (1·3) | 5·3 (1·3) | 5·2 (1·3) |
| 2 h Glucose (mmol/l)† | 6·5 (1·4) | 6·9 (1·4) | 6·7 (1·4) |
| Insulin resistance‡’† | 2·28 (2·79) | 2·57 (2·52) | 2·42 (2·66) |
| Insulin secretion‡’† | 142 (2·52) | 133 (2·26) | 137 (2·39) |
| Triglyceride (mmol/l)† | 1·8 (1·7) | 1·5 (1·7) | 1·6 (1·7) |
| HDL cholesterol (mmol/l) | 0·92 (0·22) | 1·01 (0·24) | 0·96 (0·23) |
| Total cholesterol (mmol/l) | 5·0 (1·0) | 4·9 (0·9) | 4·9 (1·0) |
| Type 2 diabetes (n,%) | 34 (13·2) | 40 (15·9) | 74 (14·5) |
| Metabolic syndrome (n,%) | 17 (6·6) | 27 (10·8) | 44 (8·6) |
| Ischaemic heart disease (n,%) | 24 (9·3) | 27 (10·8) | 51 (10·0) |
| Cortisol (nmol/l) | 312 (107) | 309 (115) | 311 (111) |
| CBG (umol/l) | 0·65 (0·16) | 0·73 (0·19) | 0·69 (0·18) |
| Free cortisol index | 0·50 (0·20) | 0·45 (0·19) | 0·48 (0·20) |
Data are mean (SD) unless otherwise indicated. BMI, body mass index; WHR, waist–hip ratio; BP, blood pressure; FPG, fasting plasma glucose.
Generalized obesity: BMI > 25 kg/m2, central obesity: WHR > 0·9 (men), > 0·85 (women) (Ramachandran et al., 2001).
Geometric mean (SD).
Estimated using the homeostasis model assessment (HOMA; Matthews et al., 1985).
Fasting 09·00 h cortisol concentration ranged from 76 to 780 nmol/l (mean 311, SD 111), CBG ranged from 0·25 to 1·90 μmol/l (mean 0·69, SD 0·18) and free cortisol index ranged from 0·11 to 1·77 (mean 0·48, SD 0·20). Fasting cortisol concentration and free cortisol index were inversely related to BMI [cortisol fell by 2·5 (95% CI 0·4-4·6) nmol/l per unit rise in BMI; P = 0·02], but there were no significant relationships with WHR, subscapular-to-triceps skinfold ratio, total body fat (%) or age. Mean cortisol concentration and free cortisol index were higher in those subjects who smoked (cortisol concentration 329 vs. 305 nmol/l, P = 0·04) and consumed alcohol (cortisol concentration 363 vs. 304 nmol/l, P < 0·001), but there were no gender differences and no associations with social class as measured by the Kuppuswamy (1962) score, a standardized questionnaire method for Urban Indian populations.
There were strong associations between fasting cortisol concentration and cardiovascular risk factors. The partial correlation coefficients (corrected for age, sex and BMI) were significant for systolic blood pressure (r = 0·25, P < 0·001), diastolic blood pressure (r = 0·24, P < 0·001), fasting glucose (r = 0·26, P < 0·001), insulin resistance (r = 0·20, P < 0·001) and fasting triglyceride (r = 0·17, P < 0·001). There was a similar, though weaker, trend for fasting insulin concentration (r = 0·12, P = 0·08). High cortisol concentrations were also associated with reduced insulin secretion (r = −0·14, P = 0·03). These associpations were seen in both sexes but were generally stronger in women (data not shown) and were also observed with the free cortisol index. Table 2 shows the mean value of each risk factor in fasting cortisol quintiles. The prevalence of type 2 diabetes and the metabolic syndrome was increased in those subjects with the highest 09·00 h cortisol concentration (Table 3).
Table 2.
Mean values of cardiovascular risk factors according to fasting 09·00 h cortisol concentration in 509 adults from Mysore, India
| Cortisol (mmol /l) |
No. | Systolic BP (mmHg) |
Diastolic BP (mmHg) |
Fasting glucose (mmol/l) |
Insulin resistance† |
Insulin secretion† |
Triglyceride (mmol/l) |
|---|---|---|---|---|---|---|---|
| < 215 | 100 | 126 | 76 | 4·9 | 2·1 | 152 | 1·4 |
| − 270 | 104 | 129 | 76 | 5·0 | 2·3 | 151 | 1·6 |
| − 320 | 100 | 132 | 77 | 5·3 | 2·6 | 151 | 1·7 |
| − 400 | 102 | 136 | 82 | 5·1 | 2·3 | 137 | 1·7 |
| > 400 | 103 | 136 | 82 | 5·8 | 2·8 | 103 | 1·8 |
| All | 509 | 132 | 79 | 5·2 | 2·4 | 137 | 1·6 |
| P-value* | < 0·001 | < 0·001 | < 0·001 | < 0·001 | 0·001 | < 0·001 |
P·value for trend corrected for age, sex, BMI, smoking and alcohol status;
estimated using the homeostasis model assessment (HOMA; Matthews et al., 1985).
Table 3.
Proportion of subjects (%) with type 2 diabetes and the metabolic syndrome in tertiles of fasting 09·00 h cortisol in 509 adults from Mysore, India
| Cortisol (nmol/ l) |
No. | Type 2 diabetes (%) |
Metabolic syndrome (%) |
|---|---|---|---|
| < 250 | 164 | 9·4 | 6·1 |
| −340 | 174 | 12·3 | 6·3 |
| > 340 | 171 | 22·5 | 13·5 |
| All | 509 | 14·5 | 8·6 |
| P-value for trend* | < 0·001 | 0·001 |
P-value corrected for age, sex, BMI.
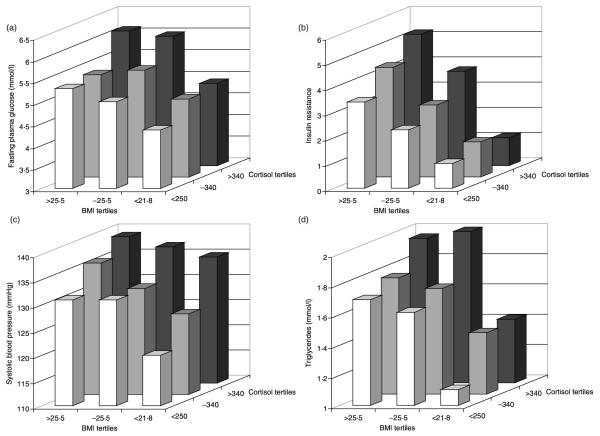
We found that the associations between fasting cortisol concentration and cardiovascular risk factors were particularly evident in men and women with higher BMI (Fig. 1). In multiple regression analyses, the effects of cortisol and BMI on systolic blood pressure and triglycerides were additive. A standard deviation increase in cortisol raised systolic blood pressure by 4·4 mmHg (P < 0·001), while the effect of a standard deviation increase in BMI was 3·9 mmHg (P < 0·001). However, a significant interaction between fasting cortisol concentration and adiposity (whether assessed by BMI or percentage total body fat) was observed for fasting plasma glucose (P = 0·057 and P = 0·045, respectively) and insulin resistance (P = 0·045 and P = 0·006, respectively). Thus, for example, the correlation between fasting cortisol and insulin resistance increased across BMI tertiles: BMI < 21·8 r = 0·07 (P = 0·4), BMI 21·8–25·5 r = 0·22 (P = 0·004) and BMI > 25·5 r = 0·25 (P = 0·001).
Fig. 1.
Histograms showing the combined effects of fasting 09·00 h cortisol concentration (nmol/l) and BMI (kg/m2) on (a) fasting plasma glucose (mmol/l), (b) insulin resistance (HOMA), (c) systolic blood pressure (mmHg) and (d) fasting triglyceride concentration (mmol/l) in 509 adults from Mysore, India.
Discussion
This study has demonstrated that fasting 09·00 h cortisol concentration is strongly associated with cardiovascular risk factors in a South Asian population and that this relationship is amplified by adiposity. As has previously been shown in Asian cohorts, the prevalence of diabetes and the metabolic syndrome is high despite relatively small numbers of people who are obese in standard terms. The cross-sectional design of the study does not allow determination of whether higher cortisol concentration is the forerunner of hypertension, impaired glucose tolerance and dyslipidaemia in these individuals, but the large numbers in the study ensure that the relationship is likely to be robust. Furthermore, as a single cortisol measurement is a crude index of HPA activity, it is likely that we have underestimated the strength of the relationship between HPA activity and disease.
The mean fasting cortisol concentration in this population is similar to, although somewhat lower than, values reported in the UK (Phillips et al., 1998). Likewise, the levels of CBG accord with previously published data (Heyns & Coolens, 1988). Seventeen people had fasting 09·00 h cortisol concentrations above 550 nmol/l (the upper limit of normal in Caucasian populations). Although the cause of these high cortisol concentrations is uncertain, it is likely that the combination of fasting and the novel clinical setting in which our blood samples were obtained will have acted as a significant stressor for some individuals in this population. Omitting these individuals from the analysis did not alter the relationships described, although the interaction between cortisol concentration and BMI was no longer significant.
The inverse association between 09·00 h cortisol concentration and BMI is well documented in Caucasian population studies and is thought to reflect impaired reactivation of cortisol in the liver by 11β-HSD1, together with a possible increase in peripheral metabolism by A-ring reductastes in obese individuals (Andrew et al., 1998; Stewart et al., 1999; Rask et al., 2001, 2002). Despite increased secretion of cortisol by the HPA axis (reflected in enhanced total cortisol metabolite excretion) circulating concentrations often remain low. Future studies in this population should include urine collection to assess tissue steroid metabolism.
The associations between fasting 09·00 h cortisol concentration and risk factors for cardiovascular disease are strong and to our knowledge have not been documented previously in an South Asian population. We found similar relationships in our cohort from Hertfordshire, UK (Phillips et al., 1998), but in Mysore they are even more striking and provide convincing cross-sectional evidence that glucocorticoids may be involved in the development of the metabolic syndrome. As 09·00 h cortisol concentrations were not high on average compared with Western populations, our hypothesis that hyperactivity of the HPA axis may be a determinant of cardiovascular risk in this ethnic group is not supported by the data. However, the strength of the association between circulating cortisol concentration and the metabolic variables suggests that this population may be particularly sensitive to the effects of glucocorticoids, which may in turn explain the ethnic differences in prevalence of the metabolic syndrome. Interestingly, higher concentrations of cortisol were also associated with reduced insulin secretion, which is consistent with in vivo and in vitro experiments showing that glucocorticoids regulate insulin secretion (Delaunay et al., 1997).
Adiposity generally added to the effect of raised 09·00 h cortisol concentration, increasing cardiovascular risk factors, and there was evidence of an interaction between cortisol and adiposity in determining fasting glucose concentration and insulin resistance. The correlation between cortisol and insulin resistance, for example, was strongest in those subjects with the highest BMI or body fat content. This accords with observations that the correlation between cortisol and systolic blood pressure is most marked in obese subjects (Phillips et al., 2000). Although the mechanism of this interaction is unclear, a recent study suggests that glucocorticoid action may be increased in skeletal muscle, an important insulin target tissue, in obese individuals (Whorwood et al., 2002). The interaction could therefore be explained if elevated concentrations of cortisol together with increased tissue sensitivity to glucocorticoids resulted in a marked increase in glucocorticoid hormone action and a high prevalence of the metabolic syndrome.
Individual differences in HPA activity are only partly genetically determined (Kirschbaum et al., 1992). We have previously suggested that intrauterine programming of the HPA axis could provide an alternative explanation for such differences (Phillips et al., 1998). There was no association between fasting cortisol concentration and birthweight or other birth measurements in this cohort (Ward et al., 2001), but some animal models provide evidence for programming of the HPA axis, for example as a result of prenatal immune challenge, without any effect on birthweight (Reul et al., 1994). The Dutch famine of 1944–45 serves as a human model of maternal undernutrition during pregnancy. In this cohort, individuals exposed to famine in mid or late gestation had impaired glucose tolerance as adults, but the effect of famine was greater than that attributable to the reduction in birthweight in these individuals (9% vs. 2%; Ravelli et al., 1998).
In summary, this study has revealed strong relationships between HPA activity, as indicated by a fasting 09·00 h cortisol concentration, and the components of the metabolic syndrome in a South Asian population and has demonstrated that adiposity may amplify these effects. Longitudinal studies are needed to confirm that those individuals with higher circulating cortisol concentrations or increased stress responsiveness in young adulthood go on to develop the metabolic syndrome. Further research into the mechanisms behind these observations may lead to new therapeutic approaches to reduce the prevalence of diabetes and the metabolic syndrome in this population, but controlling obesity is likely to be particularly important.
Acknowledgements
The research was supported by the Wellcome Trust, London, UK and by HOPE, formerly the Wessex Medical Trust. DIWP is the holder of a National Institute of Child Development grant (1 R01 HD41107-01). We are grateful to the men and women who participated in the study and Dr BDR Paul and Dr Prasad Karat, Medical Directors of the Holdsworth Memorial Hospital, Mysore, for their support and advice. We thank Mr Venkatachalam and the staff of HMH medical records department for their assistance in accessing the birth records. We acknowledge the contribution made to the study by MN Jayakumar, I Annamma, Tony Gerald Lawrence, S Geetha and KJ Chachyamma in data collection.
References
- Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. Journal of Clinical Endocrinology and Metabolism. 1998;83:1806–1809. doi: 10.1210/jcem.83.5.4951. [DOI] [PubMed] [Google Scholar]
- Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. Journal of Clinical Endocrinology and Metabolism. 1999;84:137–144. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. Journal of Clinical Investigation. 1997;100:2094–2098. doi: 10.1172/JCI119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. British Journal of Nutrition. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- Fall CHD, Stein CE, Kumaran K, Cox V, Osmond C, Barker DJP, Hales CN. Size at birth, maternal weight, and type 2 diabetes in South India. Diabetic Medicine. 1998;15:220–227. doi: 10.1002/(SICI)1096-9136(199803)15:3<220::AID-DIA544>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Filipovsky J, Ducimetiere P, Eschwege E, Richard JL, Rosselin G, Claude JR. The relationship of blood pressure with glucose, insulin, heart rate, free fatty acids and plasma cortisol levels according to degree of obesity in middle-aged men. Journal of Hypertension. 1996;14:229–235. doi: 10.1097/00004872-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Heyns W, Coolens JL. Physiology of corticosteroid-binding globulin in humans. Annals of the New York Academy of Sciences. 1988;538:122–129. doi: 10.1111/j.1749-6632.1988.tb48857.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Faig H-G, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. Journal of Clinical Endocrinology and Metabolism. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy B. Manual of Socio-Economic Status Scale. Manasayan Publications; Delhi: 1962. [Google Scholar]
- Lee ZS, Chan JC, Yeung VT, Chow CC, Lau MS, Ko GT, Li JK, Cockram CS, Critchley JA. Plasma insulin, growth hormone, cortisol, and central obesity among young Chinese type 2 diabetic patients. Diabetes Care. 1999;22:1450–1457. doi: 10.2337/diacare.22.9.1450. [DOI] [PubMed] [Google Scholar]
- McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Phillips DIW, Barker DJP, Fall CHD, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: a link between low birthweight and the insulin resistance syndrome? Journal of Clinical Endocrinology and Metabolism. 1998;83:757–760. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- Phillips DIW, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJP, Whorwood CB. Low birthweight predicts elevated plasma cortisol concentrations in adults from three populations. Hypertension. 2000;35:1301–1306. doi: 10.1161/01.hyp.35.6.1301. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, Rao PV, Yajnik CS, Prasanna KK, Nair JD. High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001;44:1094–1101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. Journal of Clinical Endocrinology and Metabolism. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- Rask E, Walker BR, Soderberg S, Livingstone DE, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11β-hydroxysteroid dehydrogenase type 1 activity. Journal of Clinical Endocrinology and Metabolism. 2002;87:3330–3336. doi: 10.1210/jcem.87.7.8661. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Reul JM, Stec I, Wiegers GJ, Labeur MS, Linthorst AC, Arzt E, Holsboer F. Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. Journal of Clinical Investigation. 1994;93:2600–2607. doi: 10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DIW. Altered control of cortisol secretion in adult men with low birthweight and cardiovascular risk factors. Journal of Clinical Endocrinology and Metabolism. 2001;86:245–250. doi: 10.1210/jcem.86.1.7145. [DOI] [PubMed] [Google Scholar]
- Shelgikar KM, Hockaday TD, Yajnik CS. Central rather than generalized obesity is related to hyperglycaemia in Asian Indian subjects. Diabetic Medicine. 1991;8:712–717. doi: 10.1111/j.1464-5491.1991.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Stein CE, Fall CHD, Kumaran K, Osmond C, Cox V, Barker DJP. Fetal growth and coronary heart disease in South India. Lancet. 1996;348:1269–1273. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone > cortisol conversion in subjects with central adiposity. Journal of Clinical Endocrinology and Metabolism. 1999;84:1022–1027. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. Journal of Internal Medicine. 2000;247:198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- Ward AMV, Fall CHD, Kumaran K, Phillips DIW, Wood PJ. Programming of the hypothalamic–pituitary–adrenal axis differs in Asian and Caucasian populations. Pediatric Research. 2001;50:38A. Abstract. [Google Scholar]
- Whorwood CB, Donovan SJ, Flanagan D, Phillips DI, Byrne CD. Increased glucocorticoid receptor expression in human skeletal muscle cells may contribute to the pathogenesis of the metabolic syndrome. Diabetes. 2002;51:1066–1075. doi: 10.2337/diabetes.51.4.1066. [DOI] [PubMed] [Google Scholar]