Abstract
Early studies showed that airway cells secrete HCO3− in response to cAMP-mediated agonists and HCO3− secretion was impaired in cystic fibrosis (CF). Studies with Calu-3 cells, an airway serous model with high expression of CFTR, also show the secretion of HCO3− when cells are stimulated with cAMP-mediated agonists. Activation of basolateral membrane hIK-1 K+ channels inhibits HCO3− secretion and stimulates Cl− secretion. CFTR mediates the exit of both HCO3− and Cl− across the apical membrane. Entry of HCO3− on a basolateral membrane NBC or Cl− on the NKCC determines which anion is secreted. Switching between these two secreted anions is determined by the activity of hIK-1 K+ channels.
Cultured human airway epithelial cells (Calu-3 cells), which have high levels of CFTR expression, provide a model for investigating cAMP-stimulated bicarbonate secretion and its impairment in cystic fibrosis.
The recognition that HCO3− secretion is impaired in CF patients dates back to the studies of Hadorn and coworkers in the 1960s (Hadorn et al. 1968). These investigators showed that pancreatic HCO3− and fluid secretion were diminished in CF patients. Moreover, CF patients were refractory to secretin, a cAMP-mediated secretory agonist in the pancreas. These studies were confirmed by Gaskin et al. (1982) and Kopelman et al. (1985, 1988). Extensive transport studies of the exocrine pancreas have shown that HCO3− is the primary anion secreted by the ductal cells, the predominate site of CFTR expression (Marino et al. 1991). The human pancreas can secrete a fluid of 130 mm HCO3− (Schultz 1987). Secreted HCO3− electrically draws Na+ into the lumen and H2O follows osmotically. The secreted fluid and electrolytes serve to flush the digestive enzymes from the acini and ducts of the pancreas. Thus, impaired HCO3− secretion results in poor clearance of the digestive enzymes, and their premature activation eventuates in the destruction of the pancreas in CF. We surmise that a similar sequela follows from impaired HCO3− secretion in the submucosal glands and airways of CF patients. Indeed, several recent studies from Wine and coworkers have shown cAMP-stimulated fluid secretion is impaired from CFTR-deficient submucosal glands (Joo et al. 2006). Verkman and coworkers have also shown impaired fluid secretion from the submucosal glands of CF patients and shown that the secreted fluid is hyperviscous and acidic compared with glands from non-CF patients (Salinas et al. 2005; Song et al. 2006). Analogous to the pancreas, the submucosal glands secrete mucins, protease inhibitors, antibiotic peptides, and enzymes that must be flushed from the glands onto the airway surface epithelium (Basbaum et al. 1990). Moreover, the physical properties of mucus are intrinsically dependent on the composition of the fluid. Most notably, alterations in ionic strength, divalent cation concentration, and pH have profound effects on the viscoelastic properties of mucins (Forstner et al. 1976; List et al. 1978; Tam et al. 1981; Lin et al. 1993). In the pancreas, the pH of the ductal fluid plays a critical role in regulating the activity of the exocytosed digestive enzymes. In contrast, very little is known regarding the electrolyte composition and pH of the submucosal gland fluid and the role it might play in the 1000-fold expansion that a mucin granule undergoes upon release and degranulation (Yeates et al. 1997; Verdugo and Hauser 2012). In addition, the surface epithelium must maintain a periciliary fluid of appropriate volume and composition to ensure proper mucociliary clearance (Randell and Boucher 2006; Boucher 2007). Adversely affected mucus leads to impaired mucociliary clearance from the submucosal glands and airway surface. The uncleared mucus then becomes a sink for bacterial binding, infection, and inflammation, thereby perpetuating a vicious cycle leading to further mucus secretion (Quinton 1999). This sequence of events is not restricted to the 40,000 individuals suffering from CF, but also occurs in more than 10 million patients suffering from COPD (chronic obstructive pulmonary disease) (Celli et al. 1995; O'Byrine et al. 1999). Thus, impaired fluid secretion by the submucosal glands or surface epithelium hinders clearance from the glands and airway surface. Until recently, Cl− was considered to be the secreted anion responsible for fluid secretion in the airways. However, recent studies suggest that HCO3− secretion importantly contributes to the airway surface and submucosal gland microenvironments.
AIRWAY CELLS SECRETE BICARBONATE
Several early studies indicated that the short-circuit current (ISC), a measure of net electrolyte transport, across airway epithelia was not fully accounted for by the net movements of Na+ and Cl−. Instead, 35%–45% of the ISC had to be attributed to an additional ion species. Ion substitution studies revealed that HCO3− but not Cl− was required to observe a cAMP-stimulated increase in ISC in airway monolayers (Al-Bazzaz et al. 1979, 1981; Welsh 1983). These early observations prompted Smith and Welsh (1992) to investigate HCO3− secretion in normal and CF-cultured airway epithelia. The results of this investigation revealed that cAMP-stimulated HCO3− secretion across normal but not CF airway epithelia. These investigators went on to conclude that HCO3− exit at the apical membrane is through the Cl− channel, which is defectively regulated in CF epithelia. In addition, they suggested the possibility that a defect in HCO3− secretion may contribute to the pathophysiology of CF pulmonary disease.
The intracellular microelectrode studies of Willumsen and Boucher (Willumsen et al. 1992), although designed for a different intent, provided important insight regarding the driving forces acting on HCO3− and the question of why normal, but not, CF epithelia can secrete HCO3−. These investigators observed that the intracellular pHs of normal and CF airway cells were equal (normal = 7.15 ± 0.02 vs. CF = 7.11 ± 0.05), but that the apical membrane potentials (Vap) were different and of opposite polarity (normal = −19 ± 2 mV vs. CF = 3 ± 5 mV). This difference has been confirmed in several additional studies by Boucher and coworkers (Boucher et al. 1988; Willumsen et al. 1989a,b). Thus, at an extracellular HCO3− concentration of 25 mm, an extracellular and intracellular pCO2 of 40 mm Hg, and a pH of 7.15, there is an outwardly directed driving force for HCO3− secretion of 6 mV across the apical membrane of normal cells. In contrast, in CF cells, there is an inwardly directed driving force for HCO3− absorption of 16 mV across the apical membrane. Therefore, provided there is a conductive pathway to mediate the movement of HCO3− across the apical membrane, normal cells will secrete HCO3− and CF cells will absorb HCO3−. Although it is often suggested that impaired Cl− secretion must be corrected in CF, it is noteworthy, given the above Vaps and the usual extracellular and intracellular Cl− concentrations, that the net driving force acting on Cl− is in the absorptive direction for both normal (9 mV) and CF (31 mV) cells. Indeed, only after Na+ transport is down-regulated or inhibited with amiloride is it possible to observe Cl− secretion in normal cells. It is generally accepted that the difference in Vap between normal and CF cells is due to the loss of CFTR channels and the higher Na+ permeability in CF cells (Boucher et al. 1986, 1988; Boucher 1994a,b). Besides Cl−, CFTR also conducts HCO3−, as shown in the studies of Gray et al. (1990) and Linsdell and coworkers (Linsdell et al. 1997; Tang et al. 2009; Li et al. 2011) and more recently by Ishiguro et al. (2009). Therefore, normal airway cells can secrete HCO3− through CFTR, whereas CF cells show a HCO3− impermeability. This reasoning leads us to assert that, in addition to abnormal Cl− and Na+ transport, HCO3− transport is also dysfunctional in CF airway epithelia and that HCO3− secretion and not Cl− secretion is critical for normal surface airway epithelial function.
STUDIES WITH Calu-3 CELLS
A second line of evidence that HCO3− secretion in the airways may be more important than previously appreciated comes from the studies of Wine and coworkers (Lee et al. 1998) and the results on Calu-3 cells from my own group (Devor et al. 1999). Calu-3 cells are a human airway serous cell line developed by Wine and Widdicombe and coworkers (Shen et al. 1994). The Calu-3 cells were selected from among 12 lung adenocarcinomas as a cell line consistent with cell biological and electrophysiological characteristics of airway serous cells. Calu-3 cells form confluent monolayers with transepithelial resistances of several hundred ohm-centimeters squared (Ω cm2), express high levels of CFTR, and respond to both cAMP- and Ca2+-mediated agonists with changes in net transepithelial ion transport as measured by ISC (Finkbeiner et al. 1993; Shen et al. 1994). In addition, the Calu-3 cells produce several serous cell-associated proteins including lysozyme, lactoferrin, serine leukoprotease inhibitor (SLP1), secretory component, and mucins (MUC1 and MUC2) (Finkbeiner et al. 1993). The Calu-3 cells are now used by many laboratories as a serous cell model (Kelley et al. 1995; Grygorczyk et al. 1997; Liedtke et al. 1998; Al-Nakkash et al. 1999; Berger et al. 1999; Duszyk et al. 1999; Illek et al. 1999; Ito et al. 1999; Waters et al. 1999). Our studies on Calu-3 cells showed that they secrete HCO3− rather than Cl− in response to cAMP-mediated agonists. In effect, the Calu-3 cells function as one would expect for a pancreatic ductal cell; however, a cell line of the latter is thus far unavailable. Calu-3 cells express high levels of CFTR, as do serous cells of the submucosal glands of the native epithelium (Puchelle et al. 1992; Engelhardt et al. 1994) and pancreatic ductal cells (Marino et al. 1991). In addition, Calu-3 cells express Na:HCO3− cotransporter isoforms pNBC1 (SLC4A4, NBCe1) and NBC4 (SLC4A5, NBCe2) in the basolateral membrane (Kreindler et al. 2006). Studies from Case and coworkers on pancreatic ducts (Ishiguro et al. 1996a,b) and the studies of my laboratory on Calu-3 cells (Devor et al. 1999) suggest that HCO3− entry across the basolateral membrane is mediated by an NBC. If we assume that the transport phenotype expressed by Calu-3 cells accurately reflects native serous cells, then HCO3− secretion must be important in the physiology of submucosal glands. In agreement with this hypothesis, the studies of Ballard and coworkers have shown that inhibitors of HCO3− secretion, acetazolamide, a carbonic anhydrase inhibitor, and DIDS, an inhibitor of Cl−:HCO3− exchangers as well as NBCs, caused mucus obstruction of the submucosal glands in secretagogue-stimulated porcine distal bronchi (Inglis et al. 1997, 1998; Trout et al. 1998). Furthermore, bumetanide, an inhibitor of the Na+:K+:2 Cl− cotransporter (NKCC1) and thereby Cl− secretion failed to inhibit cAMP-induced gland fluid secretion (Corrales et al. 1984). Consistent with these results, the forskolin-stimulated increase in ISC in Calu-3 cells is insensitive to bumetanide but is inhibited by acetazolamide and DNDS, another inhibitor of NBCs (Devor et al. 1999). Collectively, these studies lead us to conclude that HCO3− secretion is important in the physiology of submucosal glands and the airway surface epithelium.
MODEL FOR ANION SECRETION IN AIRWAY CELLS
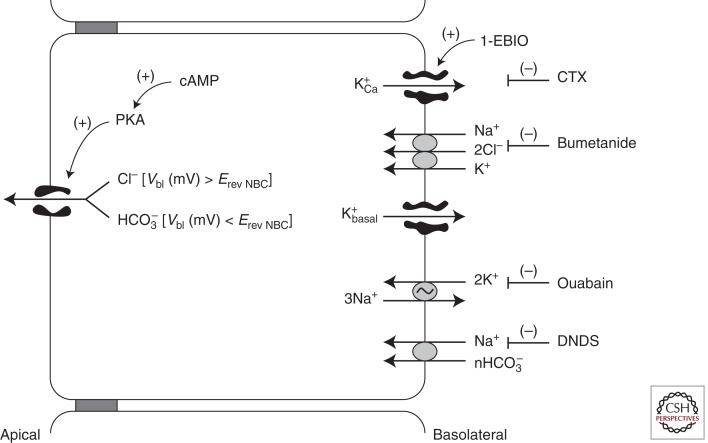
Our model for anion secretion in Calu-3 cells is illustrated in Figure 1. Forskolin-stimulated Calu-3 cells secrete HCO3− by an electrogenic mechanism, that is, Cl−-independent, serosal Na+-dependent, serosal bumetanide-insensitive and inhibited by serosal disulfonic stilbene (DNDS) as judged by transepithelial currents, isotope fluxes, and the results of ion substitution, pharmacology, and pH studies (Devor et al. 1999). However, Calu-3 cells are not limited to the secretion of HCO3−. Instead, when stimulated by 1-EBIO (1-ethyl-2-benzimidazolinone), an activator of the basolateral membrane, Ca+-activated CTX-sensitive, K+ channels (hIK1, KCNN4), Calu-3 cells secrete Cl− by an electrogenic bumetanide-sensitive mechanism, and HCO3− secretion is diminished. Moreover, when stimulated by both forskolin and 1-EBIO, the secretion of HCO3− is diminished and Cl− secretion dominates. To account for these results, we proposed the above model of anion secretion whereby CFTR serves as the cAMP/PKA-activated anion channel for both Cl− and HCO3− exit across the apical membrane. Activation of CFTR alone tends to bring Vap to the equilibrium potential for Cl− (ECl), a value greater than the equilibrium potential for HCO3− (EHCO3), and thereby provides the driving force for HCO3− exit across the apical membrane. Stimulation by cAMP (forskolin) alone leaves the basolateral membrane potential (Vbl) less hyperpolarized than the reversal potential of the DNDS-sensitive NBC (ErevNBC), and HCO3− is secreted. Subsequent activation of hIk1 by 1-EBIO or cholinergic agonists hyperpolarizes Vbl so that Vbl > ErevNBC, which inhibits HCO3− uptake by an NBC but provides a driving force for Cl− secretion as Vap becomes >ECl.
Figure 1.
Model of anion secretion in Calu-3 cells.
The initial results that led to the discovery that Calu-3 cells secrete HCO3− in response to cAMP stimulation and Cl− when hIK1 channels are activated by 1-EBIO are illustrated in Figure 2 (Devor et al. 1999). Calu-3 cells were grown on collagen-coated filters and studied by standard short-circuit current (ISC) methods. In standard bath solutions of NaCl and NaHCO3, the Calu-3 cells display a basal ISC of 13 ± 0.8 µA cm−2 and a transepithelial resistance (Rt) of 353 ± 14 Ω cm2 (n = 216 filters). Stimulation with forskolin (2 µM) induced a damped oscillatory response that became stable and sustained after 5–10 min at a plateau value of 66 ± 4 µA cm−2 and at a reduced RT of 189 ± 7 Ω cm−2 (n = 109). The subsequent addition of 1-EBIO (1 mm) caused a further increase in ISC to 114 ± 5 µA cm−2 without any further significant decrease in Rt (173 ± 9 Ω cm2, n = 36). Unidirectional ion fluxes revealed that forskolin caused a fivefold increase in both the mucosal-to-serosal and serosal-to-mucosal fluxes of Cl−, but no net secretion of Cl− (Fig. 2B). However, when the forskolin-stimulated monolayers were further stimulated with 1-EBIO, there was a further increase in the serosal-to-mucosal flux of Cl−, and the net secretion of Cl− was nearly equal to the ISC. Moreover, whereas the forskolin-stimulated ISC and Cl− fluxes were insensitive to bumetanide, bumetanide completely inhibited the ISC and the net secretion of Cl− in forskolin plus 1-EBIO–treated monolayers (results not shown). Additional ion substitution studies revealed that the forskolin-stimulated ISC was Cl− independent, HCO3− dependent, and serosal Na+ dependent. Pharmacological studies revealed that forskolin-stimulated ISC was DNDS and acetazolamide sensitive. Collectively, these studies led us to conclude that Calu-3 cells secrete HCO3− in response to cAMP stimulation. This conclusion was strongly supported by the observation that forskolin stimulation caused an alkalinization of the mucosal bathing solution of Calu-3 cells studied under open circuit conditions. In agreement with our estimates, Wine and coworkers (Irokawa et al. 2004), using a novel chamber to collect the fluid secreted by Calu-3 cells, showed that forskolin stimulated the secretion of a solution of ∼80 mm HCO3−. Moreover, when forskolin plus 1-EBIO was used to stimulate the cells, the fluid was no longer alkaline, and the HCO3− concentration fell to 17 mm (Irokawa et al. 2004).
Figure 2.
Effect of forskolin (2 µm) and 1-EBIO (1 mm) on Isc and Cl− fluxes across Calu-3 cells. Ion fluxes are given as absolute values. Forskolin caused a fivefold increase in each of the unidirectional fluxes without any net secretion of Cl−. Control data are not shown. (Figure is from Devor et al. 1999; reprinted, with permission, from the author.)
CFTR CL−-DEPENDENT SECRETION
The studies with 1-EBIO reveal that Calu-3 cells are not limited to HCO3− secretion but can also secrete Cl−. We used 1-EBIO in these studies because it causes a sustained activation of the basolateral membrane Ca2+-activated, CTX-sensitive K+ channels (hIK1) (Devor et al. 1996a,b; Syme et al. 2000). Although relatively high concentrations of 1-EBIO are required (1 mm), subsequent studies with newly synthesized derivatives that we have prepared with 100-fold higher affinities produce the same effects (Singh et al. 1999). Wine and coworkers (Lee et al. 1998) also observed similar results using thapsigargin to elevate intracellular Ca2+ and activate hIK channels. These channels would normally be activated by Ca2+-mediated agonists such as acetylcholine or substance-P. However, the former does not cause sustained increases in intracellular Ca2+ and ISC. In addition to causing Cl− secretion, the activation of hIK1 channels diminishes HCO3− secretion. These results combined with the observations that forskolin-stimulated HCO3− secretion required serosal Na+ and was inhibited by DNDS led us to propose that the influx of HCO3− across the basolateral membrane was mediated by an electrogenic NBC that carries two or more HCO3− ions for each Na+ ion. Activation of basolateral membrane K+ channels tends to hyperpolarize Vbl and inhibit NBC-dependent HCO3− influx. The hyperpolarization of Vbl also activates the basolateral membrane NaK2Cl-cotransporter (Haas and Forbush 2000), allowing for the influx of Cl− and its net secretion. Therefore, the activation and inactivation of basolateral membrane K+ channels provides a means of switching between HCO3− and Cl− secretion across airway epithelia as illustrated in Figure 1.
MICROELECTRODE AND IMPEDANCE ANALYSIS STUDIES
To obtain a better understanding of the conductances and driving forces involved in these different modes of anion secretion in Calu-3 cells, we performed microelectrode and impedance analysis experiments (Tamada et al. 2001). The results of these experiments are summarized in cell models shown in Figure 3. We were able to maintain microelectrode impalements for 10–30 min on a routine basis in Calu-3 cells, which allowed us to monitor the same cell under control, forskolin, and forskolin plus 1-EBIO–stimulated conditions. Cells were studied under open circuit conditions, and the transepithelial voltage (Vt) and resistance (Rt) as well as the Vap were measured. The apical fractional resistance (FRap) was calculated from the ΔVt and the ΔVap/ΔVt ratio in response to a 50-µA transepithelial pulse and the Vbl from the Vt and Vap values. Forskolin caused a hyperpolarization of Vt and decreased Rt similar to the changes seen in the short-circuit current experiments yielding equivalent current changes (Ieq) similar to the previously reported ISC values of ∼65 µA/cm2. Forskolin depolarized both Vap and Vbl. Vap decreased from a control value in unstimulated cells of −54.3 mV to −21.3 mV and Vbl from −60.5 mV to −41.2 mV. Consistent with the activation of an apical membrane conductance, the FRap decreased from 0.55 to 0.074 with forskolin stimulation. Double barrel voltage and pH electrodes were used to obtain estimates of the intracellular pH (pHi). Forskolin increased pHi from a control value of 7.02 to 7.16. Assuming a pCO2 of 40 mm Hg, the intracellular HCO3− concentration increased from 11 mm in control cells to 13.5 mm in forskolin-stimulated cells. Thus, forskolin stimulated a driving force of 5.8 mV for HCO3− exit from the cell. Consistent with the dramatic decrease in the FRap, impedance analysis showed that the apical membrane resistance fell to 14 Ω cm2 in forskolin-stimulated cells corresponding to an apical membrane conductance of 71 mS/cm2. With a driving force of 5.8 mV, only 12 mS/cm2 of the apical membrane conductance of 71 mS/cm2 is needed to account for the observed HCO3− current of 65 µA/cm2. The conductance ratio of 12 mS/cm2 to 71 mS/cm2 is 0.17, and this too is consistent with the reported HCO3− conductance compared with Cl− of the apical membrane of Calu-3 cells (Illek et al. 1999) as well as CFTR (Linsdell et al. 1997; Tang et al. 2009; Man-song et al. 2010). It must also be noted that a driving force of 5.8 mV does not explain the reported 80 mm concentration of HCO3− in the thin film experiments (Devor et al. 1999; Irokawa et al. 2004).
Figure 3.
Cell models of unstimulated control cells, forskolin-stimulated cells secreting HCO3−, and forskolin plus 1-EBIO cells secreting Cl−. See text for details.
It is noteworthy that a driving force of 32.4 mV exists for HCO3− exit in unstimulated control cells, and yet not until CFTR is activated is HCO3− secreted, and this secretion is driven by a much lower driving force (5.8 mV). Thus, if alternative HCO3− transporters are present in the apical membrane, they are not active until the cells are stimulated with forskolin. In addition, the intracellular HCO3− concentration is actually increased in forskolin-stimulated cells, indicating that the secreted HCO3− is replenished. We surmise that HCO3− entry via a basolateral membrane NBC is activated by depolarization of the basolateral membrane. Given a reversal potential for an NBC with a stoichiometry of 1 Na+ to 2 HCO3− of nearly −90 mV, we suggested that the NBC that mediates HCO3− entry has a stoichiometry of 1 Na+ to 3 HCO3− with a reversal potential of approximately −60 mV. Depolarization of Vbl from −61.5 mV in unstimulated cells to −44 mV would activate an NBC with a 1:3 stoichiometry. Addition of DNDS to the basolateral side caused a depolarization of 8.5 mV in forskolin-stimulated cells but not in unstimulated cells. Replacement of HCO3− with HEPES also depolarized Vbl by 8.5 mV (T Tamada and RJ Bridges, unpubl.). These results are consistent with the activation of an electrogenic, DNDS-sensitive basolateral membrane NBC in forskolin-stimulated cells. Confirmation of the exact stoichiometry of the NBC will require measurements of the intracellular Na+ activity.
The microelectrode and impedance studies revealed that 1-EBIO hyperpolarized Vbl and Vap when added to forskolin-stimulated cells. Vbl hyperpolarized from −41.2 mV to −61.1 mV and Vap from −21.3 mV to −27.4 mV when 1-EBIO was added to the forskolin-stimulated cells. The hyperpolarization caused by 1-EBIO was reversed by the addition of hIK1 blockers (T Tamada and RJ Bridges, unpubl.). Impedance analysis confirmed that 1-EBIO activated a basolateral membrane conductance, and the reversal of this conductance increased with hIK-1 blockers. 1-EBIO also decreased pHi from 7.16 to 7.01, and intracellular HCO3− from 13.5 to 9.8 mm. Vbl was no longer sensitive to DNDS or to serosal HCO3− replacement with HEPES. However, bumetanide decreased Vt, and as noted above, the current was almost fully accounted for by Cl− secretion in forskolin plus 1-EBIO–stimulated cells. These results are consistent with the activation of basolateral membrane hIK-1 potassium channels. For an NBC with a 1:3 Na+-to-HCO3− stoichiometry, the hyperpolarization of Vbl to −61.1 exceeds the reversal potential of the NBC and thereby would allow HCO3− exit from the cell on the NBC. Consequently, both pHi and intracellular HCO3− decrease. Even though the pHi and intracellular HCO3− have decreased, a driving force of 3.0 mV still exists for HCO3− exit across the apical membrane. If cell metabolism produces sufficient HCO3−, then HCO3− secretion should persist. Indeed, acetazolamide causes a small decrease in the ISC in forskolin plus 1-EBIO-stimulated cells.
1-EBIO also appears to activate the basolateral membrane NKCC cotransporter. Activation of the NKCC may result from the hyperpolarization of Vbl as well as cell shrinkage due to the loss of intracellular Cl− and K+ (Haas and Forbush 2000). Impedance analysis and cell height measurements confirm that Calu-3 cells shrink when 1-EBIO is added to forskolin-stimulated cells (RJ Bridges and W van Driessche, unpubl.). Addition of 1-EBIO also hyperpolarizes Vap in forskolin-stimulated cells. If intracellular Cl− were to remain unchanged in forskolin- and forskolin plus 1-EBIO-stimulated cells, a driving force of 6.1 mV would exist for Cl− exit across the apical membrane. A 6.1-mV driving force across an apical membrane conductance of 71 mS/cm2 should result in a current of 433 µA/cm2. Because we observe a Cl− current of only ∼140 µA/cm2, we predict that the intracellular Cl− concentration must decrease by 8 mm when 1-EBIO is added to the forskolin-stimulated cells. Unfortunately, we were unsuccessful in our attempts to fabricate a double barrel voltage and chloride-sensitive microelectrode to test this hypothesis.
SUMMARY
Calu-3 cells, an airway serous cell model with high levels of CFTR expression, secrete HCO3− in response to a cAMP-mediated agonist but can be stimulated to secrete Cl− with a basolateral membrane K+ channel-activating agonist such as 1-EBIO. Activation of CFTR by forskolin results in a very high apical membrane conductance and a depolarization of Vap to a value that approaches ECl . With Vap at ECl, there is a net driving force for HCO3− exit from the cell across the apical membrane. Because of this driving force and the very high apical membrane conductance, HCO3− can be secreted from the cells despite the lower conductance of CFTR for HCO3− compared with Cl−. The very high apical membrane anion conductance also results in a depolarization of Vbl and the activation of a basolateral membrane NBC. Secreted HCO3− is supplied by the basolateral entry of HCO3− by this NBC. Thus, the activation of CFTR sets the Vap at ECl, provides the driving force for HCO3− exit, and results in the activation of a basolateral membrane NBC. Activation of basolateral membrane K+ channels hyperpolarizes Vbl and Vap. The hyperpolarization of Vap provides a driving force for Cl− secretion. The hyperpolarization of Vbl results in an inhibition of the NBC and the activation of the NKCC. Therefore, the switch from HCO3− secretion to Cl− secretion is mediated by the activity of the basolateral K+ channel and which of the basolateral membrane cotransporters is active, NBC or the NKCC. Depending on which of these cotransporters is active, CFTR will secret either HCO3− or Cl−.
ACKNOWLEDGMENTS
I thank my collaborators Dr. Dan Devor, Dr. Tsutomu Tamada, Dr. Martin Hug, Dr. Jim Kreindler, Dr. Willy van Driessche, and Dr. Raymond Frizzell for their many helpful discussions and contributions during the course of these studies. The skillful technical assistance of Mathew Green and Ashwini Mokashi as well as the secretarial assistance of Kim Hankin are acknowledged. Special thanks go to Dr. Paul Quinton, who encouraged me to prepare this article and waited very patiently for its completion.
Footnotes
Editors: John R. Riordan, Richard C. Boucher, and Paul M. Quinton
Additional Perspectives on Cystic Fibrosis available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Al-Bazzaz FJ 1981. Role of cyclic AMP in regulation of chloride secretion by canine tracheal mucosa. Am Rev Respir Dis 123: 295–298 [DOI] [PubMed] [Google Scholar]
- Al-Bazzaz FJ, Al Awqati Q 1979. Interaction between sodium and chloride transport in canine tracheal mucosa. J App Physiol 46: 111–119 [DOI] [PubMed] [Google Scholar]
- Al-Nakkash L, Hwang TC 1999. Activation of wild-type and ΔF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pflugers Arch 437: 553–561 [DOI] [PubMed] [Google Scholar]
- Basbaum CB, Jany B, Finkbeiner WE 1990. The serous cell. Annu Rev Physiol 52: 97–113 [DOI] [PubMed] [Google Scholar]
- Berger JT, Voynow JA, Peters KW, Rose MC 1999. Respiratory carcinoma cell lines. MUCgenes and glycoconjugates. Am J Respir Cell Mol Biol 20: 500–510 [DOI] [PubMed] [Google Scholar]
- Boucher RC 1994a. Human airway ion transport. Part one. Am J Respir Crit Care Med 150: 271–281 [DOI] [PubMed] [Google Scholar]
- Boucher RC 1994b. Human airway ion transport. Part two. Am J Respir Crit Care Med 150: 581–593 [DOI] [PubMed] [Google Scholar]
- Boucher RC 2007. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu Rev Med 58: 157–170 [DOI] [PubMed] [Google Scholar]
- Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT 1986. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest 78: 1245–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR 1988. Evidence for reduced Cl− and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol 405: 77–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli BR, Snider GL, Heffner J, Tiep B, Ziment I, Make B, Braman S, Olsen G, Phillips Y 1995. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 152: S77–S120 [PubMed] [Google Scholar]
- Corrales RJ, Nadel JA, Widdicombe JH 1984. Source of the fluid component of secretions from tracheal submucosal glands in cats. J Appl Physiol 56: 1076–1082 [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Frizzell RA, Bridges RJ 1996a. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am J Physiol 271: L775–L784 [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Bridges RJ, Frizzell RA 1996b. Modulation of Cl− secretion by benzimidazolones. II. Coordinate regulation of apical GCl and basolateral GK. Am J Physiol 271: L785–L795 [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ 1999. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol 113: 743–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duszyk M, Shu Y, Sawicki G, Radomski A, Man SF, Radomski MW 1999. Inhibition of matrix metalloproteinase MMP-2 activates chloride current in human airway epithelial cells. Can J Physiol Pharmacol 77: 529–535 [PubMed] [Google Scholar]
- Engelhardt JF, Zepeda M, Cohn JA, Yankaskas JR, Wilson JM 1994. Expression of the cystic fibrosis gene in adult human lung. J Clin Invest 93: 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner WE, Carrier SD, Teresi CE 1993. Reverse transcription-polymerase chain reaction (RT-PCR) phenotypic analysis of cell cultures of human tracheal epithelium, tracheobronchial glands, and lung carcinomas. Am J Respir Cell Mol Biol 9: 547–556 [DOI] [PubMed] [Google Scholar]
- Forstner JF, Jabbal I, Findlay BP, Forstner GG 1976. Interaction of mucins with calcium, H+ ion and albumin. Mod Probl Paediatr 19: 54–65 [PubMed] [Google Scholar]
- Gaskin KJ, Durie PR, Corey M, Wei P, Forstner GG 1982. Evidence for a primary defect of pancreatic HCO3− secretion in cystic fibrosis. Pediatr Res 16: 554–557 [DOI] [PubMed] [Google Scholar]
- Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE 1990. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol 259: C752–C761 [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan JW 1997. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol 272: C1058–C1066 [DOI] [PubMed] [Google Scholar]
- Haas M, Forbush B III 2000. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol 62: 515–534 [DOI] [PubMed] [Google Scholar]
- Hadorn B, Johansen PG, Anderson CM 1968. Pancreozymin secretin test of exocrine pancreatic function in cystic fibrosis and the significance of the result for the pathogenesis of the disease. Can Med Assoc J 98: 377–385 [PMC free article] [PubMed] [Google Scholar]
- Illek B, Tam AW, Fischer H, Machen TE 1999. Anion selectivity of apical membrane conductance of Calu-3 human airway epithelium. Pflugers Arch 437: 812–822 [DOI] [PubMed] [Google Scholar]
- Inglis SK, Corboz MR, Taylor AE, Ballard ST 1997. Effect of anion transport inhibition on mucus secretion by airway submucosal glands. Am J Physiol 272: L372–L377 [DOI] [PubMed] [Google Scholar]
- Inglis SK, Corboz MR, Ballard ST 1998. Effect of anion secretion inhibitors on mucin content of airway submucosal gland ducts. Am J Physiol 274: L762–L766 [DOI] [PubMed] [Google Scholar]
- Irokawa T, Krouse ME, Joo NS, Wu JV, Wine JJ 2004. A “virtual gland” method for quantifying epithelial fluid secretion. Am J Physiol Lung Cell Mol Physiol 287: L784–L793 [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Wilson RW, Case RM 1996a. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol 495: 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Lindsay AR, Case RM 1996b. Accumulation of intracellular HCO3− by Na+–HCO3− cotransport in interlobular ducts from guinea-pig pancreas. J Physiol 495: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Naruse S, Ko SBH, Goto H, Case RM, Kondo T, Yamamoto A 2009. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol 133: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Kume H, Yamaki K, Takagi K 1999. Tetracyclines reduce Na+/K+ pump capacity in Calu-3 human airway cells. Biochem Biophys Res Commun 260: 13–16 [DOI] [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Robbins RC, Wine JJ 2006. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem 281: 7392–7398 [DOI] [PubMed] [Google Scholar]
- Kelley TJ, al-Nakkash L, Drumm ML 1995. CFTR-mediated chloride permeability is regulated by type III phosphodiesterases in airway epithelial cells. Am J Respir Cell Mol Biol 13: 657–664 [DOI] [PubMed] [Google Scholar]
- Kopelman H, Durie P, Gaskin K, Weizman Z, Forstner G 1985. Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. N Engl J Med 312: 329–334 [DOI] [PubMed] [Google Scholar]
- Kopelman H, Corey M, Gaskin K, Durie P, Weizman Z, Forstner G 1988. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology 95: 349–355 [DOI] [PubMed] [Google Scholar]
- Kreindler JL, Peters KW, Frizzell RA, Bridges RJ 2006. Identification and membrane localization of electrogenic sodium bicarbonate cotransporters in Calu-3 cells. Biochim Biophys Acta 1762: 704–710 [DOI] [PubMed] [Google Scholar]
- Lee MC, Penland CM, Widdicombe JH, Wine JJ 1998. Evidence that Calu-3 human airway cells secrete bicarbonate. Am J Physiol 274: L450–L453 [DOI] [PubMed] [Google Scholar]
- Li M-S, Holstead RG, Wang W, Linsdell P 2011. Regulation of CFTR chloride channel macroscopic conductance by extracellular bicarbonate. Am J Physiol Cell Physiol 300: C65–C74 [DOI] [PubMed] [Google Scholar]
- Liedtke CM, Cole TS 1998. Antisense oligonucleotide to PKC-ε alters cAMP-dependent stimulation of CFTR in Calu-3 cells. Am J Physiol 275: C1357–C1364 [DOI] [PubMed] [Google Scholar]
- Lin SY, Amidon GL, Weiner ND, Goldberg AH 1993. Viscoelasticity of anionic polymers and their mucociliary transport on the frog palate. Pharm Res 10: 411–417 [DOI] [PubMed] [Google Scholar]
- Linsdell P, Tabcharani JA, Hanrahan JW 1997. Multi-ion mechanism for ion permeation and block in the cystic fibrosis transmembrane conductance regulator chloride channel. J Gen Physiol 110: 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- List SJ, Findlay BP, Forstner GG, Forstner JF 1978. Enhancement of the viscosity of mucin by serum albumin. Biochem J 175: 565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino CR, Matovcik LM, Gorelick FS, Cohn JA 1991. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest 88: 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Byrine PM, Postma DS 1999. The many faces of airway inflammation: Asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: S41–S66 [PubMed] [Google Scholar]
- Puchelle E, Gaillard D, Ploton D, Hinnrasky J, Fuchey C, Boutterin MC, Jacquot J, Dreyer D, Pavirani A, Dalemans W 1992. Differential localization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis airway epithelium. Am J Respir Cell Mol Biol 7: 485–491 [DOI] [PubMed] [Google Scholar]
- Quinton PM 1999. Physiological basis of cystic fibrosis: A historical perspective. Physiol Rev 79: S3–S22 [DOI] [PubMed] [Google Scholar]
- Randell SH, Boucher RC 2006. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 35: 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, Nielson DW, Verkman AS 2005. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J 19: 431–433 [DOI] [PubMed] [Google Scholar]
- Schultz I 1987. Electrolyte and fluid secretion in the exocrine pancreas. In Physiology of the gastrointestinal tract (ed. Johnson LR), Vol. 2, pp. 1147–1172 Raven, New York [Google Scholar]
- Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH 1994. Calu-3: A human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am J Physiol 266: L493–L501 [DOI] [PubMed] [Google Scholar]
- Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ 1999. Development of benzimidazolones as chloride secretory agonists. Ped Pulmonl Suppl 19: 190 [Google Scholar]
- Smith JJ, Welsh MJ 1992. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest 89: 1148–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Salinas D, Nielson DW, Verkman AS 2006. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol 290: C741–C749 [DOI] [PubMed] [Google Scholar]
- Syme CA, Gerlach AC, Singh AK, Devor DC 2000. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 278: C570–C581 [DOI] [PubMed] [Google Scholar]
- Tam PY, Verdugo P 1981. Control of mucus hydration as a Donnan equilibrium process. Nature 292: 340–342 [DOI] [PubMed] [Google Scholar]
- Tamada T, Hug MJ, Frizzell RA, Bridges RJ 2001. Microelectrode and impedance analysis of anion secretion in Calu-3 cells. JOP 2: 219–228 [PubMed] [Google Scholar]
- Tang L, Fatehi M, Linsdell P 2009. Mechanism of direct bicarbonate transport by the CFTR anion channel. J Cyst Fibros 8: 115–121 [DOI] [PubMed] [Google Scholar]
- Trout L, King M, Feng W, Inglis SK, Ballard ST 1998. Inhibition of airway liquid secretion and its effect on the physical properties of airway mucus. Am J Physiol 274: L258–L263 [DOI] [PubMed] [Google Scholar]
- *.Verdugo P 2012. Supramolecular dynamics of mucus. Cold Spring Harb Perspect Med 10.1101/cshperspect.a009597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Savla U 1999. Keratinocyte growth factor accelerates wound closure in airway epithelium during cyclic mechanical strain. J Cell Physiol 181: 424–432 [DOI] [PubMed] [Google Scholar]
- Welsh MJ 1983. Inhibition of chloride secretion by furosemide in canine tracheal epithelium. J Membr Biol 71: 219–226 [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RC 1992. Intracellular pH and its relationship to regulation of ion transport in normal and cystic fibrosis human nasal epithelia. J Physiol 455: 247–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen NJ, Davis CW, Boucher RC 1989a. Intracellular Cl− activity and cellular Cl− pathways in cultured human airway epithelium. Am J Physiol 256: C1033–C1044 [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Davis CW, Boucher RC 1989b. Cellular Cl− transport in cultured cystic fibrosis airway epithelium. Am J Physiol 256: C1045–C1053 [DOI] [PubMed] [Google Scholar]
- Yeates DB, Besseris GJ, Wong LB 1997. Physiochemical properties of mucus and its propulsion. In The lung, scientific foundations, 2nd ed (ed. Crystal RG), pp. 487–515 Lippincott Williams & Wilkins, New York [Google Scholar]