Abstract
Hemoglobin E (HbE) is an extremely common structural hemoglobin variant that occurs at high frequencies throughout many Asian countries. It is a β-hemoglobin variant, which is produced at a slightly reduced rate and hence has the phenotype of a mild form of β thalassemia. Its interactions with different forms of α thalassemia result in a wide variety of clinical disorders, whereas its coinheritance with β thalassemia, a condition called hemoglobin E β thalassemia, is by far the most common severe form of β thalassemia in Asia and, globally, comprises approximately 50% of the clinically severe β-thalassemia disorders.
Hemoglobin E (HbE) is a common structural β-hemoglobin variant. Although HbE alone does not cause significant clinical problems, its interactions with other thalassemias produce syndromes of varying severity.
As discussed by Williams and Weatherall (2012), HbE occurs at an extremely high frequency in many countries in Asia. Because there is also a high frequency of different β-thalassemia alleles in these populations, the coinheritance of HbE and β thalassemia, HbE β thalassemia, occurs very frequently. Similarly, because different forms of α thalassemia are also very common in these countries, HbE also occurs together with them, producing a complex series of phenotypes.
The first description of HbE β thalassemia appeared in a paper by Minnich and her colleagues in 1954 under what, at the time, was the rather surprising title “Mediterranean Anaemia: A study of 32 cases in Thailand” (Minnich et al. 1954). In the same year, the first electrophoretic identification of HbE was reported independently (Itano et al. 1954). The first detailed clinical description of HbE β thalassemia was reported in 1956 by Chernoff and colleagues (1956). Much later, groups in Thailand began a detailed analysis of the interaction of the various forms of α thalassemia with HbE, which result in a complex series of phenotypes, most of which are much milder than HbE β thalassemia (Wasi et al. 1969).
MOLECULAR PATHOLOGY AND PROPERTIES OF HEMOGLOBIN E
At least in vitro, HbE appears to be mildly unstable and shows increased sensitivity to oxidants (Frischer and Bowman 1975). However, in vitro studies of hemoglobin synthesis do not show evidence of instability similar to that found in other unstable hemoglobin variants, although HbE is unstable at increased temperatures, similar to those that would occur in a wide range of infective diseases (Rees et al. 1998). The whole-blood oxygen dissociation curves of homozygotes for HbE appear to be normal or very slightly right-shifted. HbE is synthesized at a slightly reduced rate and homozygotes show mild globin-chain imbalance, similar to that observed in β-thalassemia heterozygotes. It is caused by a base substitution at codon 26 of the β-globin gene, GAG-AAG, which results in the substitution of lysine for glutamic acid. This mutation also activates a cryptic splice site that causes abnormal messenger RNA processing (Orkin et al. 1982). Because the normal donor site has to compete with this new site, the level of normally spliced βE messenger RNA is reduced (Traeger et al. 1980), resulting in the clinical phenotype of a mild form of β thalassemia.
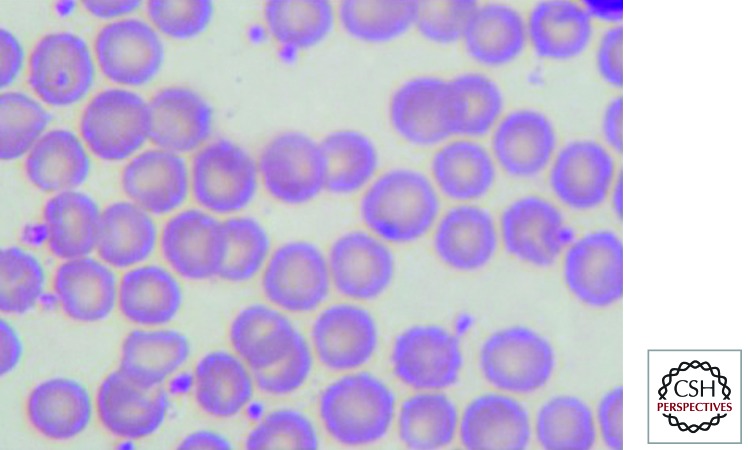
The heterozygous state for HbE is characterized by minimal morphological abnormalities of the red cells and normal red cell indices; HbE constitutes 25%–30% of the hemoglobin (Fig. 1). Homozygotes for HbE have hypochromic microcytic red cells with significant morphological abnormalities including increased numbers of target cells (Fig. 2). They are mildly anemic and the overall hematological findings are very similar to those of heterozygous β thalassemia.
Figure 1.
The peripheral blood film in hemoglobin E trait showing normal red cell morphology.
Figure 2.
The peripheral blood film in the homozygous state for hemoglobin E showing large numbers of target cells.
THE INTERACTIONS OF HEMOGLOBIN E WITH DIFFERENT FORMS OF THALASSEMIA
Although HbE alone does not cause any significant clinical problems, its interactions with various forms of α and β thalassemia produce a very wide range of clinical syndromes of varying severity. The molecular basis for the different forms of α and β thalassemia, which are coinherited with HbE, are described by Thein (2012) and Higgs (2012).
The various interactions of HbE and α thalassemia, which have been defined in the Thai population, and occur elsewhere in SE Asia, are described by their particular hemoglobin constitutions (Table 1). Heterozygotes for HbE, which are also heterozygous for α+ thalassemia (−α/αα), have similar levels of HbE to HbE heterozygotes, whereas those that are heterozygous for α0 thalassemia (−/αα) have mild thalassemic red cell changes and the level of HbE ranges between 19% and 21%. HbE heterozygotes who also inherit the genotype of HbH disease (−/−α) have a marked decrease of HbE in the 13%–15% range and a thalassemic disorder of intermediate severity, which is called HbAE Bart’s disease.
Table 1.
Hematological data and clinical picture of subjects with HbE with different kinds of α-globin gene interactions
| Hb E | α-Globin gene | Hb (g/dl) | MCV (fl) | Hb typing | Hb E (%) | HbBart’s (%) | Hb F (%) | Clinical |
|---|---|---|---|---|---|---|---|---|
| Hb E heterozygote | αα/αα | 12.8 ± 1.5 | 84 ± 5 | EA | 29 ± 2.3 | - | 0.9 ± 0.7 | Normal |
| −α/αα | 13.1 ± 1.4 | 88 ± 4 | EA | 28 ± 1.5 | - | 0.7 ± 0.6 | Normal | |
| −/αα | 12.5 ± 1.4 | 77 ± 5 | EA | 21 ± 1.2 | - | 0.9 ± 0.4 | Normal | |
| −/−α | 9.1 ± 1.1 | 60 ± 3 | EFA Bart’s | 13 ± 2.1 | 4.5 ± 1.9 | 2.2 ± 1.9 | Thal intermedia (AEBart’s disease) | |
| Hb E homozygote | αα/αα | 10.6 ± 1.2 | 65 ± 3 | EF | 88 ± 2.6 | - | 3.6 ± 1.6 | Normal |
| −α/αα | 11.0 ± 1.6 | 65 ± 4 | EF | 87 ± 3.3 | - | 4.8 ± 3.7 | Normal | |
| −/αα | 10.5 ± 2.4 | 64 ± 7 | EF | 88 ± 5.7 | - | 3.8 ± 2.1 | Normal | |
| −/−α | 7.5 ± 0.8 | 60 ± 2 | EF Bart’s | 81 ± 1.5 | 4.2 ± 1.1 | 6.4 ± 1.2 | Thal intermedia (EFBart’s disease) | |
| Hb E β thalassemia | αα/αα | 7.1 ± 1.4 | 59 ± 3 | EF | 58 ± 9.5 | - | 38 ± 11.7 | Mild to severe disease |
| −α/αα | 8.5 ± 1.1 | 55 ± 3 | EF | 71 ± 7.5 | - | 24 ± 8.7 | Mild disease | |
| −/αα | 9.3 ± 0.5 | 52 ± 1 | EF | 84 ± 3.8 | - | 12 ± 2.5 | Mild disease | |
| −/−α | 7.6 ± 1.2 | 61 ± 2 | EF Bart’s | 82 ± 2.5 | 1.5 ± 0.3 | 5.5 ± 0.7 | Thal intermedia (EFBart’s disease) |
Hemoglobin E homozygotes who coinherit the heterozygous state for α+ or α0 thalassemia have a mild hypochromic microcytic anemia with slightly elevated levels of HbF. Those who coinherit the genotype of HbH disease have a thalassemic disorder of intermediate severity with moderate anemia and elevated levels of HbF and Bart’s, a condition called EF Bart’s disease.
The compound heterozygous state for HbE and β thalassemia, HbE β thalassemia, is a remarkably heterogenous disease with a phenotype ranging from mild anemia to the most severe forms of β-thalassemia major (Weatherall and Clegg 2001). This condition may also be coinherited with a variety of different forms of α thalassemia. The coinheritance of the heterozygous states for α+ and α0 thalassemia have an ameliorating effect on the disease, whereas those who also inherit the genotype of HbH disease have a form of HbEF Bart’s disease, as described above.
These complex interactions, which are summarized in Table 1, produce three significant clinical disorders; HbAE Bart’s disease, HbEF Bart’s disease, and HbE β thalassemia.
HbAE BART’S DISEASE
This thalassemia syndrome is characterized by the presence of HbA, HbE, and Hb Bart’s, and results from the interaction of the genotype of HbH disease (see Higgs 2012; Vichinsky 2012) with heterozygous HbE (Wasi et al. 1967; Thonglairuam et al. 1989). Two common subtypes of HbAE Bart’s disease have been observed: α+ thalassemia/α0 thalassemia—A/E and α0 thalassemia/Hb Constant Spring—A/E. Clinically, HbAE Bart’s disease is similar to HbH disease, and like the latter, the α0 thalassemia/Hb Constant Spring interaction is more severe (see Vichinsky 2012). However, in this syndrome there are no hemolytic crises during stress similar to those seen in HbH disease. The amount of HbE is decreased to 13%–15%. This is because α-globin chains have a lower affinity for βE than βA-globin chains. Small amounts of Hb Bart’s are always present in this genotype and intraerythrocytic inclusion bodies (HbH inclusions) can be demonstrated in ∼5% of the erythrocytes, indicating the presence of small amounts of HbH that is insufficient to be detected by electrophoresis.
The diagnosis of this disorder requires detailed family studies together with DNA analysis to define the type of α thalassemia. Management is similar to HbH disease (see Vichinsky 2012).
HbEF BART’S DISEASE
HbEF Bart’s disease is characterized by the presence of HbE, HbF, and Hb Bart’s (Fucharoen et al. 1988b). HbE constitutes ∼80% and HbF 10% of the hemoglobin; the remainder is Hb Bart’s. The presence of Hb Bart’s indicates that there are excess γ-globin chains. However, no inclusion bodies or HbH are present, probably because the abnormal βE-globin chains do not form tetramers. Four genotypes of HbEF Bart’s disease have been identified. They result from interactions between the genotype for HbH disease, either α0 thalassemia/α+ thalassemia or α0 thalassemia/Hb Constant Spring, with either homozygous HbE or HbE β thalassemia. Hb Constant Spring and small amounts of HbA may be observed in patients with the α0 thalassemia/Hb Constant Spring and HbE β+ thalassemia genotype. To differentiate among these genotypes, family studies and further investigation by DNA analysis are required.
Overall, the interactions of the different genotypes for HbH disease with the homozygous state for HbE produce relatively mild forms of thalassemia intermedia, not dissimilar to HbH disease (see Vichinsky 2012). Their interactions with HbE β thalassemia are more complex and clinically variable, and are discussed in more detail in the next section.
HEMOGLOBIN E β THALASSEMIA
In general, HbE β thalassemia is a thalassemia syndrome of intermediate severity with a very heterogeneous clinical spectrum. Two types have been described, depending on the presence or absence of HbA. In HbE, β0 thalassemia, βA-globin chains are not synthesized and the condition is characterized by the production of HbE and HbF without detectable HbA; HbE constitutes between 30% and 70% of the hemoglobin with the remainder HbF. Variable amounts of HbA are detected, in addition to HbE and HbF, in HbE β+ thalassemia. Different β+ thalassemia mutations result in variable severity of the disease, reflecting different levels of HbA.
Pathophysiology
The pathophysiology of HbE β thalassemia reflects both the reduced output of HbE together with the added globin-chain imbalance consequent on the coinheritance of β thalassemia. Although early studies suggested that HbE is slightly unstable and may precipitate under conditions of oxidative stress, later biosynthetic analyses showed little evidence of its instability in the red cells of patients with HbE β thalassemia. The one exception was if the cells were exposed to increased temperatures at the level that might be encountered in severe forms of infection at which there was evidence of instability (Rees et al. 1998). However, the remarkable ameliorating effect on the phenotype that results from the coinheritance of α thalassemia or other modifiers of the degree of globin chain imbalance (see later section) suggests that defective β chain synthesis is the major factor in the pathophysiology of this condition (reviewed by Weatherall and Clegg 2001). Like other forms of severe β thalassemia, there is marked expansion of the erythroid bone marrow with ineffective erythropoiesis. Interestingly, using a combination of electron-microscopic and immunocytochemical studies, Wickramasinghe and Lee (1997) demonstrated that the erythroblast inclusions in the bone marrow of patients with this condition consist entirely of precipitated α chains; there is no evidence of coprecipitation of βE chains. Therefore the mechanisms of damage to red cells and their precursors are similar to those described in other forms of β thalassemia (see Nienhuis and Nathan 2012).
Recent studies have suggested that there are important differences in the compensatory mechanisms between HbE β thalassemia and other forms of severe thalassemia intermedia. In particular, patients with HbE β thalassemia appear to be able to compensate for anemia by a right shift in their oxygen dissociation curves, unlike those with many other forms of β-thalassemia intermedia (Allen et al. 2010). This probably reflects both the properties of HbE and the lower mean levels of HbF that occur in HbE β thalassemia compared with other forms of β-thalassemia intermedia. Studies of the erythropoietin response to anemia in this condition have shown that hemoglobin level and age are independent variables with respect to the erythropoietin level and that there is a relative reduction in response to a particular hemoglobin level with aging (O’Donnell et al. 2007). This may be at least partly responsible for some of the remarkable phenotypic heterogeneity observed during the early years of development in babies with HbE β thalassemia.
Clinical Features
One of the most striking features of HbE β thalassemia is its remarkable clinical heterogeneity. At one end of the spectrum, there are patients whose clinical course is almost indistinguishable from that of severe β-thalassemia major; whereas at the other end, there are patients who grow and develop normally without the need for blood transfusion, albeit often at a relatively low hemoglobin level.
At birth, infants with severe HbE β thalassemia are asymptomatic because HbF levels are high. As HbF production decreases and is replaced by HbE at 6–12 months of age, anemia with splenomegaly develops. Signs of impaired growth appear during the first decade of life. The initial complaints vary from patient to patient, and several symptoms usually appear simultaneously (Table 2). Most common are the development of a mass in the left upper quadrant and pallor. With time and without transfusions, anemia, jaundice, hepatosplenomegaly, growth retardation, and thalassemic facies evolve (Table 3). Absence of secondary sexual development is common and chronic leg ulcers are sometimes observed. These manifestations, which are secondary to decreased oxygen delivery to tissue, ineffective erythropoiesis, and iron overload, resemble those of β-thalassemia major.
Table 2.
Presenting symptoms in 378 HbE β-thalassemia patients in Thailand
| Symptoms | Number | % |
|---|---|---|
| 1. Pallor | 150 | 39.7 |
| 2. Fever | 72 | 19.1 |
| 3. Abdominal mass | 36 | 9.5 |
| 4. Abdominal pain | 23 | 6.1 |
| 5. Combined: Abdominal mass and pain | 21 | 5.6 |
| 6. Yellow eyes (jaundice) | 16 | 4.2 |
| 7. Edema | 9 | 2.4 |
| 8. Pregnancy with anemia | 3 | 0.8 |
| 9. Bone pain | 3 | 0.8 |
| 10. Paraplegia | 2 | 0.5 |
| 11. Headache and dizziness | 2 | 0.5 |
| 12. Miscellaneousa | 41 | 10.8 |
| Total | 378 | 100 |
aMiscellaneous includes lymphadenopathy, skin rashes, chest pain, request for plastic surgery, joint pain, and cases with multiple symptoms.
Table 3.
Clinical signs in 378 adult HbE β-thalassemia patients in Thailand
| Signa | Number | % |
|---|---|---|
| 1. Splenomegaly | 369 | 97.6 |
| 2. Jaundice | 350 | 92.6 |
| 3. Hepatomegaly | 337 | 89.1 |
| 4. Thalassemic facies | 313 | 82.8 |
| 5. Growth retardation | 284 | 75.1 |
| 6. Anemia | 152 | 40.2 |
| 7. Abnormal cardiovascular systemb | 70 | 18.5 |
| 8. Respiratory tract infection | 44 | 11.6 |
| 9. Arthritis and bone pain | 40 | 10.6 |
| 10. Abnormal neurological systemc | 32 | 8.5 |
| 11. Chronic leg ulcer | 31 | 8.2 |
| 12. Soft tissue infection | 7 | 1.8 |
aMost patients have more than one clinical sign.
bAbnormal cardiovascular manifestations are mainly related to congestive heart failure (50 cases), deep vein thrombosis (five cases), pericarditis (four cases), and others.
cAbnormal neurological features are mainly weakness of both hands (14 cases), headache (11 cases), and paraplegia (two cases).
Patients with the milder forms of HbE β thalassemia tend to grow and develop reasonably well during early childhood and are fully active. There may be some delay in the pubertal growth spurt and in the appearance of secondary sexual characteristics but they usually attain a reasonable height and sexual maturation. Further work is required to determine whether they develop the later complications that have been described in older patients with other forms of thalassemia intermedia, such as renal disease, pulmonary hypertension, cerebral infarcts, and others (see Musallam et al. 2012). Certainly some of them accumulate iron through increased absorption and may develop associated endocrine complications including diabetes. Furthermore, there is undoubtedly phenotypic instability in the early years of life; in a series of children observed over 15 years with HbE β thalassemia in Sri Lanka, at least 20 cases changed from a mild to a more severe phenotype during the first 15 years of life.
Laboratory Findings
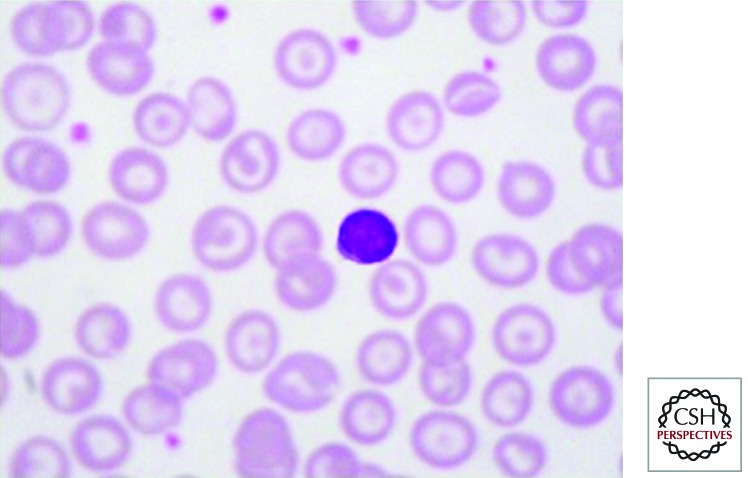
The steady-state hemoglobin levels in HbE β thalassemia range widely between the different phenotypes, from 3 g/dl or less to as high as 11 g/dl. The red-cell indices and morphological changes are similar to those described in other forms of severe β thalassemia (Fig. 3). Hemoglobin analysis reveals a preponderance of HbE with low levels of HbA in those who have inherited a β+-thalassemia mutation. The mean level of HbF in 200 patients from Sri Lanka was approximately 28%, ranging from less than 10% to 50%; even higher levels of HbF have been reported from Thailand. Family studies reveal the carrier state for HbE in one parent and for β-thalassemia trait in the other. The laboratory findings associated with different complications are discussed in the following sections.
Figure 3.
The peripheral blood film in hemoglobin E β thalassemia after splenectomy showing numerous nucleated red cells and a high platelet count.
Complications
Hypersplenism
Splenomegaly, together with pooling of red cells and their increased rate of destruction, is extremely common. In the more severe phenotypes, it often progresses rapidly from the first few years of life, whereas in the milder phenotypes, although the spleen is palpable, it usually does not attain a size greater than 5–6 cm below the costal margin. Much less common, and usually in the milder phenotypes, splenomegaly may slowly increase over 10–20 years and only become a problem later in life. As well as resulting in increasing anemia, progressive enlargement of the spleen is quite often associated with pain and discomfort in the left upper quadrant.
Infection
Patients with HbE β thalassemia are susceptible to viral, bacterial, and fungal infection, which are common causes of mortality (Aswapokee et al. 1988a,b). In splenectomized patients, septicemia can be very acute and overwhelming, leading to death in a short period. Gram-negative and gram-positive bacteria are frequent causes of septicemia. Fungal infection with Pythium can lead to arterial occlusion and gangrene of the legs (Sathapatayavongs et al. 1989; Wanachiwanawin et al. 1993). The mechanism that causes increased susceptibility to infections is not known. Recent studies have suggested that patients with HbE β thalassemia may be more prone to infection by both P. falciparum and P. vivax malaria, particularly the latter, and that those who have undergone splenectomy may be even more susceptible (O’Donnell et al. 2009).
Cardiac Disease
Approximately half of the patients with HbE β thalassemia in Thailand die of heart failure. This is associated with failure of other organs, delayed growth and sexual maturation, hepatomegaly, and endocrinopathies. Organ failure results from iron deposition in the heart and other tissues (Vannasaeng et al. 1981; Sonakul et al. 1988; Thakerngpol et al. 1988) resulting from increased absorption and blood transfusion. Myocardial iron deposition is usually limited, occurring primarily as small granules in perinuclear areas, with later accumulation throughout the muscle fibers, predominantly subepicardial and occasionally subendocardial (Sonakul et al. 1984). The small amount of iron deposited in the heart is in marked contrast to enormous iron deposition in the liver and pancreas. Cardiomegaly is proportional to the severity of anemia and systolic murmurs are frequently present (Jootar and Fucharoen 1990). Chronic pericarditis following upper respiratory tract infection is frequently encountered, more commonly in splenectomized patients. A pericardial rub may be detected, often transiently. Intractable pericardial effusion may follow, causing cardiac tamponade and failure, and requires aspiration. In a very few cases, chronic constrictive pericarditis develops, requiring surgical intervention.
Heart rate variability (HRV) has been developed to determine cardiac autonomic function and is applied to investigate patients with thalassemia major (De Chiara et al. 2005). Studies of the HRV in HbE β thalassemia have obtained similar results to those in thalassemia major (Rutjanaprom et al. 2009). The depressed HRV compared to normal suggests that HRV may be a marker of cardiac sympatho-vagal imbalance as well as an early indicator of cardiac involvement in both thalassemia major and HbE β thalassemia.
Pulmonary hypertension and right heart failure are discussed in the following sections.
Hypoxemia
The majority of splenectomized HbE β-thalassemia patients in Thailand develop hypoxemia with low arterial pO2 (Wasi et al. 1982). Platelet counts in these patients are double those of nonsplenectomized patients; young and larger platelets are also observed in the absence of the spleen. Platelet microaggregates have been detected in the circulation of these splenectomized patients. One hypothesis for the pathogenesis of hypoxemia in HbE β thalassemia is that platelets increase in number, are younger and more active after splenectomy, and aggregate in the circulation and in the pulmonary vasculature. Substances released during platelet aggregation may cause constriction of the terminal bronchioles leading to decreased oxygenation and hypoxemia. Administration of aspirin to inhibit platelet aggregation reduces the degree of hypoxemia in the majority of cases (Fucharoen et al. 1981), suggesting that aspirin should be routinely given to splenectomized patients with HbE β thalassemia. Interestingly, this combination of pulmonary hypertension and hypoxemia, together with right ventricular failure, has not been observed so frequently in other populations, suggesting that other factors may be involved in the Thai population.
Thromboembolism
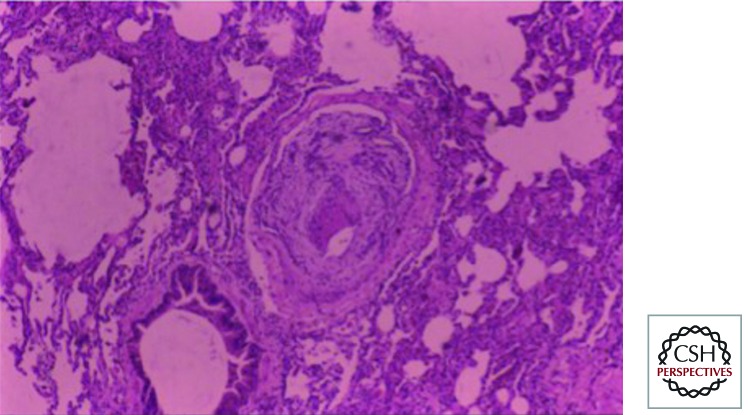
Autopsy findings in a large number of Thai patients with HbE β thalassemia revealed striking pulmonary artery occlusion (Fig. 4) (Sonakul et al. 1980). Thromboembolism in HbE β thalassemia seems to involve platelets, a reactive thalassemic red cell surface, coagulation factors, and abnormal endothelium (Butthep et al. 2002; Pattanapanyasat et al. 2004).
Figure 4.
Pulmonary vascular occlusion in hemoglobin E β thalassemia after splenectomy.
Hypertension, Convulsions, and Cerebral Hemorrhage
Some patients in Thailand develop hypertension, convulsions, and cerebral hemorrhage after transfusion of 2 units or more of blood (Wasi et al. 1978). This complication may develop as late as 2 weeks after multiple transfusions, suggesting that blood volume overload is not the cause of hypertension. Monitoring blood pressure during and after blood transfusions with prompt antihypertensive intervention has reduced deaths from this complication. This syndrome has not been reported in other populations.
Extramedullary Hemopoiesis
Erythropoiesis is massively increased to 10–15 times normal because anemia stimulates erythropoietin production. Extensive erythropoiesis can be found in the liver, spleen, bone marrow, and in extramedullary sites. Erythropoietic masses in the spinal canal can cause spinal cord compression and paraplegia, and when they occur intracranially, convulsions may result (Issaragrisil et al. 1981). Massive erythropoiesis leads to fragility and distortion of the bones and decreases bone density because of osteoporosis and osteomalacia, as observed in irregularly transfused patients (Pootrakul et al. 1980).
Jaundice, Gallstones, and Cholecystitis
Some HbE β-thalassemia patients have severe and persistent jaundice in the absence of definable liver disease. It turns out that this is a result of the homozygous inheritance of the TA(7) allele of the promoter of the glucuronyltransferase 1 gene, a polymorphism that is particularly common in Sri Lanka (Premawardhena et al. 2001). These patients have a highly significant increase in the incidence of gallstones. Homozygosity for the TA(7) allele occurs in 10%–25% of some populations of Africa and the Indian subcontinent but at a much lower frequency in Southeast Asia (Premawardhena et al. 2003). Stones are found in approximately 50% of HbE β-thalassemia patients in Thailand (Chandcharoensin-Wilde et al. 1988). For the detection of biliary calculi, ultrasonography is more sensitive than oral cholecystography and plain abdominal films. Cholecystitis and ascending cholangitis may occur with abdominal pain, fever, and increasing jaundice (Vathanopas et al. 1988).
Iron Overload
Iron overload occurs commonly (Pootrakul et al. 1981). Excessive iron accumulates because of blood transfusions and enhanced gastrointestinal absorption. The skin is darkened and iron deposition occurs in the bone marrow, liver, spleen, heart, pancreas, and elsewhere (Sonakul et al. 1988; Thakerngpol et al. 1988). The related cardiac complications were discussed earlier. Although liver fibrosis from iron overload is common, ascites and other signs of cirrhosis are very rare. Diabetes mellitus, secondary to iron deposition in the pancreas, frequently develops in untreated adult patients (Vannasaeng et al. 1981). A terminal wasting stage occurs in some patients who survive to their fourth and fifth decades. They develop severe skin pigmentation, poor appetite, weight loss, and increasing anemia, and eventually die. This is believed to result from organ failure caused by uncontrolled tissue oxidation from chronic, severe iron overload.
Recently, it has been possible to obtain more direct data about the liver iron concentration in HbE β thalassemia using spin density projection assisted R2-MRI technology (Olivieri et al. 2011). These studies showed a marked variation in hepatic iron levels even in patients who had received only minimal transfusion. They also underlined the rather poor agreement between hepatic iron levels as measured in this way and serum ferritin values. Clearly, a great deal more work is required to try to determine the reasons for the variable rate of iron accumulation by increased intestinal absorption and, in particular, whether this reflects polymorphisms in one or more of the genes that are involved in the regulation of iron absorption.
Other Endocrine Disorders
As well as diabetes there are other important endocrine disorders that occur as a result of iron loading. In particular hypothyroidism and hypoparathyroidism are quite common and the former is frequently associated with growth retardation. It is vital, therefore, to carry out regular assessments of thyroid and parathyroid function in patients with HbE β thalassemia, regardless of the severity of their phenotype.
Genotype–Phenotype Interaction
Definition of Severity
Despite seemingly identical genotypes, compound heterozygotes for β thalassemia and HbE have remarkably variable phenotypes. Notable are variations in the degree of anemia, growth, development, hepatosplenomegaly, and transfusion requirements. A novel scoring system based on six independent parameters—hemoglobin level, age at disease presentation, age at receiving first blood transfusion, requirement for transfusion, spleen size, growth and development—was able to separate patients into three distinctive severity categories: mild, moderate, and severe. The scoring system consisting of six clinical criteria scored as 0, 0.5, 1, or 2, according to clinical presentation. HbE β-thalassemia patients with total scores ranging from 0 to 3.5, 4 to 7, and 7.5 to 10 are grouped as mild, moderate, and severe cases, respectively. The severe patients are very anemic and are usually transfusion dependent; some may have marked growth retardation, whereas the mild cases have mild anemia and usually have normal growth and development (Sripichai et al. 2008).
As indicated by recent studies in Sri Lanka, the application of a clearly defined scoring system for severity combined with a long period of observation and genetic and environmental analysis (Premawardhena et al. 2005; Olivieri et al. 2010) should help us to understand the factors that determine the broad range of severity of HbE β thalassemia.
β-Thalassemia Mutations
Although β0 thalassemia is caused by many mutations, all result in absence of β-globin chain production by the abnormal gene. β0 thalassemia is more severe than β+ thalassemia, in which a wide range of β-globin chain production is observed. In most countries with a high frequency of HbE β thalassemia, the common β-thalassemia mutations are either β0 thalassemia or β+ thalassemia associated with very small amounts of β-globin chain synthesis. The fact that there is still considerable clinical heterogeneity in these patients is clearly not a result of variation in β-globin chain synthesis directed by the chromosome containing the β-thalassemia mutation. There are, however, certain milder forms of β+ thalassemia associated with much higher levels of β-chain production and, when inherited together with HbE, produce a much milder form of HbE β thalassemia. The phenotypes and hemoglobin findings in patients of this type are summarized by Weatherall and Clegg (2001).
Coinheritance of α Thalassemia
The concomitant inheritance of α thalassemia or Hb Constant Spring may be responsible for less severe anemia and a milder phenotype in HbE β0 thalassemia (Winichagoon et al. 1985, 1993). Coinheritance of α0 thalassemia with HbE β0 thalassemia may lead to so mild a condition that the individuals do not have a clinical abnormality that requires medical attention. Similar findings have been observed in Sri Lankan populations, in which the coinheritance of the heterozygous state for α+ thalassemia has been found to result in a remarkable degree of amelioration of the clinical severity of HbE β thalassemia (Premawardhena et al. 2005).
Association with Increased HbF
Coinheritance of determinants that increase HbF expression can ameliorate the severity of HbE β thalassemia. Inheritance of a chromosome with the C → T polymorphism that results in an Xmn-1 cleavage site at position −158 to the Gγ-globin gene is associated with increased HbF and milder anemia (Winichagoon et al. 1987). Two copies of this allele (Xmn-1 +/+) are necessary to produce a significant clinical effect. Increased expression of the Gγ-globin gene was also detected in the Xmn-1 +/+ patients. This increase of γ-globin gene activity reduces the overall globin chain imbalance and thus ameliorates the anemia. The association between the Xmn-1 +/+ genotype and a highly significant increase in the absolute level of HbF and a milder phenotype has also been observed in patients with HbE β thalassemia in Sri Lanka (Premawardhena et al. 2005). It is likely that several other polymorphisms will have this effect (see later section and Sankaran and Orkin 2012).
Amount of Alternatively Spliced βE-Globin mRNA
An underproduction of β-globin chains from the βE-globin gene strongly suggests that alternative RNA splicing is of functional significance. The percentage of alternative spliced βE-globin mRNA was determined by the reverse transcriptase polymerase chain reaction technique in 14 patients with the same thalassemia mutation (Winichagoon et al. 1995). Preliminary results showed abnormally spliced βE-globin mRNA in patients with severe symptoms and low hemoglobin levels between 2.9% and 6.1%, whereas those with higher hemoglobin levels had values from 1.6% to 2.6%. The majority of patients with the Xmnl-negative genotype had more severe anemia and a higher percentage of abnormally spliced βE-globin mRNA. This indicated that the amount of alternatively spliced E-globin mRNA was a more important factor in determining severity of anemia than the pattern of Xmnl polymorphism or the level of HbF. Recently, Tubsuwan et al. used the allele-specific RT-qPCR to study βE-globin gene expression and found that the correctly to aberrantly spliced βE-globin mRNA ratio in 30% of mild HbE β-thalassemia patients was higher than that of the severe patients. It appears therefore that the splicing process of βE-globin pre-mRNA differs among HbE β-thalassemia patients and serves as one of the modifying factors for disease severity (Tubsuwan et al. 2011). It will be important to determine whether this phenomenon occurs in other ethnic groups.
Pyrimidine 5′ Nucleotidase Deficiency
In a Bangladeshi family, an individual homozygous for both HbE and pyrimidine 5′ nucleotidase deficiency was found. The patient had a severe hemolytic anemia in contrast to HbE homozygotes. Globin chain synthesis experiments showed that the mechanism underlying the interaction between these two genotypes was a marked decrease in the stability of HbE in pyrimidine 5′ nucleotidase-deficient red blood cells. In these cells, free α-globin chains but not βE-globin chains accumulated on the membrane. It was hypothesized that the marked instability of HbE in the enzyme-deficient cells resulted from oxidant damage to mildly unstable HbE (Rees et al. 1996). Clearly this interaction also has the potential to modify the phenotype of HbE β thalassemia.
Genome-Wide Association Study
Recently, a genome-wide association study (GWAS) was performed in 618 Thai HbE β0-thalassemia patients using the Illumina Human 610-Quad BeadChips array (Nuinoon et al. 2010). DNAs were extracted from 383 severe and 235 mild phenotypes, by a validated scoring system, after the exclusion of α thalassemia. Twenty-three single nucleotide polymorphisms (SNPs) in three independent genes/regions were identified as being significantly associated with the disease severity. The highest association was observed with SNPs in the β-globin gene cluster (chr.11p15). The second was identified in the intergenic region between the HBS1L and MYB genes (chr.6q23), and the third region was located in the BCL11A gene (chr.2p16.1). An association to HbF levels with SNPs in these three regions was observed. This result suggests that several genetic loci act in concert to influence HbF levels of HbE β0-thalassemia patients (see Sankaran and Orkin 2012).
Variation in Adaptation to Anemia and Environmental Factors
As already mentioned, there appears to be a reduction in erythropoietin production in relationship to a similar hemoglobin level with aging, a finding which may explain some of the phenotypic instability during the early years of life. Whether there is a genetic component to the magnitude of erythropoietin response remains to be determined. Undoubtedly, patients with HbE β thalassemia adapt more readily to low hemoglobin levels than those with other forms of β-thalassemia intermedia (Allen et al. 2010). Although as is the case for most inherited hemoglobin disorders, the role of the environment in modifying the phenotype has been neglected; recent studies suggest that patients with HbE β thalassemia are more susceptible to malaria infection, particularly that caused by P. vivax, than age-matched controls in the population (O’Donnell et al. 2009). There is also reasonable evidence that those who have been exposed to malaria tend to have larger spleens and fall into the more severe phenotypic categories. Much more work is required to further elucidate environmental factors that may modify the phenotype.
Conclusion
The genotypic factors that can be used to predict a mild phenotype in HbE β thalassemia are mild β+-thalassemia mutations, the coinheritance of α thalassemia, the polymorphisms associated with HbF production such as homozygosity for Xmn-l restriction site 5′ to the Gγ-globin gene and the BCL11A gene. Some complications of the disease such as severe jaundice are also affected by genetic modifiers. And it is also clear that at least some factors that modify the response to anemia or the environment of patients with this disease are also responsible for phenotypic diversity. But although progress has been made, it is still only possible to explain part of its wide phenotypic diversity.
Treatment
Because HbE β thalassemia has such a variable phenotype and patients with this disorder—probably because they have relatively lower levels of HbF and reflecting the oxygen affinity of HbE—are able to adapt to anemia better than patients with other forms of thalassemia intermedia, it is vital to observe babies and young children with this condition after presentation for a reasonable period before deciding on the best approach to management. It is important to remember that they may present with a particularly low hemoglobin level consequent to a recent infection, and it is particularly important therefore not to establish them on a regular transfusion until their steady-state hemoglobin level and level of growth and degree of splenomegaly has been assessed. Particularly in areas where malaria is endemic it is also important to exclude chronic P. vivax infection as a possible cause of rapidly progressive splenomegaly.
The hemoglobin level alone should not be the major factor in initiating transfusion. Rather, the broader picture should be considered with particular attention to growth failure, lack of activity, and the earlier appearance of skeletal change. If it is clear that the patient requires regular transfusion the regimen to be followed, including chelation, is similar to that for the management of thalassemia major (see Brittenham and Olivieri 2012). Those who do not require transfusion should be maintained on folic acid supplements and advised about the early treatment of infective episodes. Although some patients with increasing splenomegaly and evidence of hypersplenism may benefit from splenectomy, this should be avoided where possible because of the particularly high risk of infection.
Patients who do not require regular transfusion should have serum ferritin or hepatic MRI estimations at least twice per year. Increased iron levels should be controlled by intermittent courses of chelating agents.
Hydroxyurea therapy may increase HbF levels (Fucharoen et al. 1996), although recent studies in other populations have shown that this effect is not great, even when combined with erythropoietin. For those who present early with severe disease, bone marrow transplantation remains an important option (Issaragrisil 1994; Leelahavarong et al. 2010).
Rapidly expanding extramedullary hemopoietic masses, particularly involving the brain or spinal cord, require urgent treatment by blood transfusion, hydroxyurea, or possibly, radiotherapy. Limited experience in those with profound jaundice as a result of genetic inability to conjugate bilirubin suggest that, at least in some cases, very low doses of phenobarbitone may be helpful.
The first successful report of gene therapy in thalassemia involved a patient with HbE β thalassemia (Cavazzana-Calvo et al. 2010). Although the patient showed clinical improvement and did not need further blood transfusion, it is not yet clear whether this is mainly because of the action of the inserted “normal” β-globin gene. The improvement also seems to be at least partly a result of an increase in HbF levels by an unknown mechanism.
Prevention of HbE β Thalassemia
Effective prevention programs for thalassemia have been demonstrated in many countries in which carrier rates for different types of thalassemia are very high (see Brittenham and Olivieri 2012). In Thailand, screening has improved by using an automatic high-performance liquid chromatography (HPLC) system (Fucharoen et al. 1998). In recent years, a nationwide program in Thailand has been developed to prevent homozygous β thalassemia, HbE β thalassemia, and Hb Bart’s hydrops fetalis, with encouraging results (Tongsong et al. 2000). The prospective screening consisted of osmotic fragility (OF) and HbE screening tests in pregnant women, followed by testing the husbands of the women with a positive result. Subsequently, the OF test was replaced by mean corpuscular volume (MCV) when automated cell counters became available nationwide. If both partners of the couple have a positive result, further diagnostic tests by HPLC and genotyping of the carrier are carried out. A pregnancy in which both partners of the couple are carriers is considered as a couple at risk, and further detailed counseling and prenatal diagnosis is offered for the severe thalassemia syndromes (Fucharoen and Winichagoon 2007).
Since the program began, the number of new cases of thalassemia in Thailand has gradually decreased. In the first few years of the program, its cost-effectiveness was evaluated and it was found that among a total pregnant population of 21,000 individuals that were screened, 80 affected fetuses had been identified and the pregnancy terminated. The total cost of the prevention program was about U.S. $257,140, and the cost of management of these affected cases, if they had been born, would have been U.S. $7,200,000. The cost-benefit ratio was 1:28, which indicates a highly cost-effective project.
CONCLUSIONS
HbE β thalassemia is a major public health problem in Southeast Asia and in other Asian countries. Although some progress has been made toward a better understanding of its pathophysiology and clinical management a great deal remains to be learned. Recent work has made it absolutely clear that there must be other genetic modifiers to be discovered that are responsible for the variable phenotype. A better approach to predicting the phenotype is urgently required, particularly if prenatal diagnosis is to be widely used for the control of this condition and, even more so, if experimental forms of gene therapy become available in the future. Because it may be some time before there are more definitive forms of treatment, it is important to utilize the information that we already have more effectively. For example, in malarious areas it will be very important to conduct trials of malaria prophylaxis. With particular respect to the phenotype of patients with this condition early in life, and because recent evidence suggests that the erythropoietin response to anemia tends to decline with age, the possibility of transient periods of transfusion during maximum erythroid expansion should be seriously considered. And because genetic evidence indicates that the phenotype in this condition may be improved quite dramatically with only a modest increase in steady-state hemoglobin level, more efforts should be directed at trying to raise the HbF level in these patients.
Other Interactions of HbE
This article has considered the most common and important clinical interactions of HbE. Because it is so common it is not surprising that many rare interactions between this variant and a wide variety of structural hemoglobin variants and related conditions have been reported. The resulting disorders are described briefly in a recent review (Fucharoen and Weatherall 2009).
ACKNOWLEDGMENTS
This work is supported by the Office of the Higher Education Commission and Mahidol University under the National Research University Initiative and the National Center for Genetic Engineering, Thailand and The Wellcome Trust, UK. We thank Liz Rose for her help in preparing this work.
Footnotes
Editors: David Weatherall, Alan N. Schechter, and David G. Nathan
Additional Perspectives on Hemoglobin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Allen A, Fisher C, Premawardena A, Peto T, Allen S, Arambepola K, Thayalsuthan V, Olivieri N, Weatherall D 2010. Adaptation to anemia in hemoglobin E-β thalassemia. Blood 116: 5368–5370 [DOI] [PubMed] [Google Scholar]
- Aswapokee N, Aswapokee P, Fucharoen S, Wasi P 1988a. A study of infective episodes in patients with β-thalassemia/Hb E disease in Thailand. Birth Defects 23: 513–520 [PubMed] [Google Scholar]
- Aswapokee P, Aswapokee N, Fucharoen S, Sukroongreung S, Wasi P 1988b. Severe infection in thalassemia: A prospective study. Birth Defects 23: 521–526 [PubMed] [Google Scholar]
- *.Brittenham G, Olivieri N 2012. Cold Spring Harb Perspect Med (to be published) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butthep P, Rummavas S, Wisedpanichkij R, Jindadamrongwech S, Fucharoen S, Bunyaratvej A 2002. Increased circulating activated endothelial cells, vascular endothelial growth factor, and tumor necrosis factor in thalassemia. Am J Hematol 70: 100–106 [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, et al. 2010. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 467: 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandcharoensin-Wilde C, Chairoongruang S, Jitnuson P, Fucharoen S, Vathanopas V 1988. Gallstones in thalassemia. Birth Defects 23: 263–267 [PubMed] [Google Scholar]
- Chernoff AI, Minnich V, Na-Nakorn S, Tuchinda S, Kashamsant C, Chernoff RR 1956. Studies on hemoglobin E: I. The clinical, hematologic, and genetic characteristics of the hemoglobin E syndromes. J Lab Clin Med 47: 490–498 [PubMed] [Google Scholar]
- De Chiara B, Crivellaro W, Sara R, Ruffini L, Parolini M, Fesslova V, Carnelli V, Fiorentini C, Parodi O 2005. Early detection of cardiac dysfunction in thalassemic patients by radionuclide angiography and heart rate variability analysis. Eur J Haematol 74: 517–522 [DOI] [PubMed] [Google Scholar]
- Frischer H, Bowman J 1975. Hemoglobin E, an oxidatively unstable mutation. J Lab Clin Med 85: 531–539 [PubMed] [Google Scholar]
- Fucharoen S, Weatherall DJ 2009. Hemoglobin E disorders. In Disorders of hemoglobin (ed. Steinberg MH, Forget BG, Higgs DR, Weatherall DJ), pp. 417–433 Cambridge University Press, Cambridge [Google Scholar]
- Fucharoen S, Winichagoon P 2007. Prevention and control of thalassemia in Asia. Asian Biomed 1: 1–6 [Google Scholar]
- Fucharoen S, Youngchaiyud P, Wasi P 1981. Hypoxaemia and the effect of aspirin in thalassaemia. Southeast Asian J Trop Med Public Health 12: 90–93 [PubMed] [Google Scholar]
- Fucharoen S, Winichagoon P, Thonglairuam V 1988a. Beta-thalassemia associated with α-thalassemia in Thailand. Hemoglobin 12: 581–592 [DOI] [PubMed] [Google Scholar]
- Fucharoen S, Winichagoon P, Thonglairuam V, Wasi P 1988b. EF Bart’s disease: Interaction of the abnormal α- and β-globin genes. Eur J Haematol 40: 75–78 [DOI] [PubMed] [Google Scholar]
- Fucharoen S, Siritanaratkul N, Winichagoon P, Chowthaworn J, Siriboon W, Muangsup W, Chaicharoen S, Poolsup N, Chindavijak B, Pootrakul P, et al. 1996. Hydroxyurea increases hemoglobin F levels and improves the effectiveness of erythropoiesis in β-thalassemia/hemoglobin E disease. Blood 87: 887–892 [PubMed] [Google Scholar]
- Fucharoen S, Winichagoon P, Wisedpanichkij R, Sae-Ngow B, Sriphanich R, Oncoung W, Muangsapaya W, Chowthaworn J, Kanokpongsakdi S, Bunyaratvej A, et al. 1998. Prenatal and postnatal diagnoses of thalassemias and hemoglobinopathies by HPLC. Clin Chem 44: 740–748 [PubMed] [Google Scholar]
- *.Higgs DR 2012. The molecular basis of α thalassemia. Cold Spring Harb Perspect Med 10.1101/cshperspect a011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issaragrisil S 1994. Bone marrow transplantation in Thailand. Bone Marrow Transplant 13: 721–723 [PubMed] [Google Scholar]
- Issaragrisil S, Piankigagum A, Wasi P 1981. Spinal cord compression in thalassemia. Report of 12 cases and recommendations for treatment. Arch Intern Med 141: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Itano HA, Bergren WR, Sturgeon P 1954. Identification of a fourth abnormal human hemoglobin. J Am Chem Soc 76: 2278 [Google Scholar]
- Jootar P, Fucharoen S 1990. Cardiac involvement in β-thalassemia/hemoglobin E disease: Clinical and hemodynamic findings. Southeast Asian J Trop Med Public Health 21: 269–273 [PubMed] [Google Scholar]
- Leelahavarong P, Chaikledkaew U, Hongeng S, Kasemsup V, Lubell Y, Teerawattananon Y 2010. A cost-utility and budget impact analysis of allogeneic hematopoietic stem cell transplantation for severe thalassemic patients in Thailand. BMC Health Serv Res 10: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich V, Na-Nakorn S, Chongchareonsuk S, Kochaseni S 1954. Mediterranean anemia: A study of 32 cases in Thailand. Blood 9: 1–23 [PubMed] [Google Scholar]
- *.Musallam KM, Taher AT, Rachmilewitz EA 2012. β-Thalassemia intermedia: A clinical perspective. Cold Spring Harb Perspect Med 10.1101/cshperspect.a013482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Nienhuis AW, Nathan DG 2012. Pathophysiology and clinical manifestations of the β thalassemias. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuinoon M, Makarasara W, Mushiroda T, Setianingsih I, Wahidiyat PA, Sripichai O, Kumasaka N, Takahashi A, Svasti S, Munkongdee T, et al. 2010. A genome-wide association identified the common genetic variants influence disease severity in β0-thalassemia/hemoglobin E. Hum Genet 127: 303–314 [DOI] [PubMed] [Google Scholar]
- O’Donnell A, Premawardhena A, Arambepola M, Allen SJ, Peto TE, Fisher CA, Rees DC, Olivieri NF, Weatherall DJ 2007. Age-related changes in adaptation to severe anemia in childhood in developing countries. Proc Natl Acad Sci 104: 9440–9444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell A, Premawardhena A, Arambepola M, Samaranayake R, Allen SJ, Peto TE, Fisher CA, Cook J, Corran PH, Olivieri NF, et al. 2009. Interaction of malaria with a common form of severe thalassemia in an Asian population. Proc Natl Acad Sci 106: 18716–18721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri NF, Thayalsuthan V, O’Donnell A, Premawardena A, Rigobon C, Muraca G, Fisher C, Weatherall DJ 2010. Emerging insights in the management of hemoglobin E β thalassemia. Ann NY Acad Sci 1202: 155–157 [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Thayalasuthan V, Muraca G, Weatherall D, Kim C, Premawardhena A, Perera A, O’Donnell A, St Pierre T 2011. Relationship between serum ferritin and liver iron concentration in hemoglobin E thalassemia. 16th Congress of European Hematology Association, London, June 9–12 [Google Scholar]
- Orkin SH, Kazazian HH, Antonarakis SE, Ostrer H, Goff SC, Sexton JP 1982. Abnormal RNA processing due to the exon mutation of βE-globin gene. Nature 300: 768–769 [DOI] [PubMed] [Google Scholar]
- Pattanapanyasat K, Noulsri E, Fucharoen S, Lerdwana S, Lamchiagdhase P, Siritanaratkul N, Webster HK 2004. Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin Cytom 57: 23–31 [DOI] [PubMed] [Google Scholar]
- Pootrakul P, Rugkiatsakul R, Wasi P 1980. Increased transferrin iron saturation in splenectomized thalassaemia patients. Brit J Haematol 46: 143–145 [DOI] [PubMed] [Google Scholar]
- Pootrakul P, Vougsmasa V, Laongpanich P, Wasi P 1981. Serum ferritin levels in thalassemias and the effect of splenectomy. Acta Haematol 66: 244–250 [DOI] [PubMed] [Google Scholar]
- Premawardhena A, Fisher CA, Fathiu F, de Silva S, Perera W, Peto TE, Olivieri NF, Weatherall DJ 2001. Genetic determinants of jaundice and gallstones in haemoglobin E β thalassaemia. Lancet 357: 1945–1946 [DOI] [PubMed] [Google Scholar]
- Premawardhena A, Fisher CA, Liu YT, Verma IC, de Silva S, Arambepola M, Clegg JB, Weatherall DJ 2003. The global distribution of length polymorphisms of the promoters of the glucuronosyltransferase 1 gene (UGT1A1): Hematologic and evolutionary implications. Blood Cells Mol Dis 31: 98–101 [DOI] [PubMed] [Google Scholar]
- Premawardhena A, Fisher CA, Olivieri NF, de Silva S, Arambepola M, Perera W, O’Donnell A, Peto TEA, Viprakasit V, Merson L, et al. 2005. Haemoglobin E β thalassaemia in Sri Lanka. Lancet 366: 1467–1470 [DOI] [PubMed] [Google Scholar]
- Rees DC, Duley J, Simmonds HA, Wonke B, Thein SL, Clegg JB, Weatherall DJ 1996. Interaction of hemoglobin E and pyrimidine 5′ nucleotidase deficiency. Blood 88: 2761–2767 [PubMed] [Google Scholar]
- Rees DC, Clegg JB, Weatherall DJ 1998. Is hemoglobin instability important in the interaction between hemoglobin E and β thalassemia? Blood 92: 2141–2146 [PubMed] [Google Scholar]
- Rutjanaprom W, Kanlop N, Charoenkwan P, Sittiwangkul R, Srichairatanakool S, Tantiworawit A, Phrommintikul A, Chattipakorn S, Fucharoen S, Chattipakorn N 2009. Heart rate variability in β-thalassemia patients. Eur J Haematol 83: 483–489 [DOI] [PubMed] [Google Scholar]
- *.Sankaran VG, Orkin SH 2012. The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathapatayavongs B, Leelachaikul P, Prachaktam R, Atichartakarn V, Sriphojanart S, Trairatvorakul P, Jirasiritham S, Nontasut S, Eurvilaichit C, Flegel T 1989. Human pythiosis associated with thalassemia hemoglobinopathy syndrome. J Infect Dis 159: 274–280 [DOI] [PubMed] [Google Scholar]
- Sonakul D, Pacharee P, Laohapand T, Fucharoen S, Wasi P 1980. Pulmonary artery obstruction in thalassaemia. Southeast Asian J Trop Med Public Health 11: 516–523 [PubMed] [Google Scholar]
- Sonakul D, Pacharee P, Wasi P, Fucharoen S 1984. Cardiac pathology in 47 patients with β thalassaemia/haemoglobin E. Southeast Asian J Trop Med Public Health 15: 554–563 [PubMed] [Google Scholar]
- Sonakul D, Pacharee P, Thakerngpol K 1988. Pathologic findings in 76 autopsy cases of thalassemia. Birth Defects 23: 157–176 [PubMed] [Google Scholar]
- Sripichai O, Makarasara W, Munkongdee T, Kumkhaek C, Nuchprayoon I, Chuansumrit A, Chuncharunee S, Chantrakoon N, Boonmongkol P, Winichagoon P, et al. 2008. A scoring system for the classification of β-thalassemia/Hb E disease severity. Am J Hematol 83: 482–484 [DOI] [PubMed] [Google Scholar]
- Thakerngpol K, Sonakul D, Fucharoen S, Vathanopas V, Stitnimankarn T 1988. Histochemical study of liver tissue from thalassemic patients. Birth Defects 23: 193–198 [PubMed] [Google Scholar]
- *.Thein SL 2012. Molecular basis of β thalassemia. Cold Spring Harb Perspect Med 10.1101/cshperspect a011700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonglairuam V, Winichagoon P, Fucharoen S, Wasi P 1989. The molecular basis of AE-Bart’s disease. Hemoglobin 13: 117–124 [DOI] [PubMed] [Google Scholar]
- Tongsong T, Wanapirak C, Sirivatanapa P, Sanguansermsri T, Sirichotiyakul S, Piyamongkol W, Chanprapaph P 2000. Prenatal control of severe thalassaemia: Chiang Mai strategy. Prenat Diag 20: 229–234 [PubMed] [Google Scholar]
- Traeger J, Wood WG, Clegg JB, Weatherall DJ, Wasi P 1980. Defective synthesis of Hb E is due to reduced levels of βE mRNA. Nature 288: 497–499 [DOI] [PubMed] [Google Scholar]
- Tubsuwan A, Munkongdee T, Jearawiriyapaisarn N, Boonchoy C, Winichagoon P, Fucharoen S, Svasti S 2011. Molecular analysis of globin gene expression in different thalassaemia disorders: Individual variation of β(E) pre-mRNA splicing determine disease severity. Brit J Haematol 154: 635–643 [DOI] [PubMed] [Google Scholar]
- Vannasaeng S, Ploybutr S, Visutkul P, Tandhanand S, Suwanik R, Wasi P 1981. Endocrine function in thalassaemia. Clin Endocrinol (Oxf) 14: 165–173 [DOI] [PubMed] [Google Scholar]
- Vathanopas V, Fucharoen S, Chandrcharoensin-Wilde C, Sukroongreung S, Nilakul C 1988. Cholecystectomy in thalassemia. Birth Defects 23: 269–273 [PubMed] [Google Scholar]
- *.Vichinsky E 2012. Natural history and clinical manifestations of the α thalassemias. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanachiwanawin W, Thianprasit M, Fucharoen S, Chaiprasert A, Sudasna N, Ayudhya N, Sirithanaratkul N, Piankijagum A 1993. Fatal arteritis due to Pythium insidiosum infection in patients with thalassaemia. Trans R Soc Trop Med Hyg 87: 296–298 [DOI] [PubMed] [Google Scholar]
- Wasi P, Sookanek M, Pootrakul S, Na-Nakorn S, Suingdumrong A 1967. Haemoglobin E and α-thalassaemia. Br Med J 4: 29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasi P, Na-Nakorn S, Pootrakul S, Sookanek M, Disthasongcham P, Pornpatkul M, Manich V 1969. Alpha- and β-thalassemia in Thailand. Ann NY Acad Sci 165: 60–82 [DOI] [PubMed] [Google Scholar]
- Wasi P, Na-Nakorn S, Pootrakul P, Sonakul D, Piankijagum A, Pacharee P 1978. A syndrome of hypertension, convulsions, and cerebral haemorrhage in thalassaemic patients after multiple blood transfusions. Lancet ii: 602–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasi P, Fucharoen S, Younghchaiyud P, Sonakul D 1982. Hypoxemia in thalassemia. Birth Defects 18: 213–217 [PubMed] [Google Scholar]
- Weatherall DJ, Clegg JB 2001. The thalassaemia syndromes. Blackwell Science, Oxford [Google Scholar]
- Wickramasinghe SN, Lee MJ 1997. Observations on the relationship between g-globin chain content and globin chain precipitation in thalassaemic erythroblasts and on the composition of erythroblastic inclusions in Hb E/β-thalassaemia. Eur J Haematol 59: 305–309 [DOI] [PubMed] [Google Scholar]
- *.Williams TN, Weatherall DJ 2012. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med 10.1101/cshperspecta011692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winichagoon P, Fucharoen S, Weatherall DJ, Wasi P 1985. Concomitant inheritance of α-thalassemia in β0-thalassemia/Hb E disease. Am J Hematol 20: 217–222 [DOI] [PubMed] [Google Scholar]
- Winichagoon P, Fucharoen S, Thonglairoam V, Wasi P 1987. Different severity of homozygous β-thalassemia among siblings. Hum Genet 76: 296–297 [DOI] [PubMed] [Google Scholar]
- Winichagoon P, Thonglairoam V, Fucharoen S, Wilairat P, Fukimaki Y, Wasi P 1993. Severity differences in β-thalassaemia haemoglobin E syndromes: Implication of genetic factors. Brit J Haematol 83: 633–639 [DOI] [PubMed] [Google Scholar]
- Winichagoon P, Fucharown S, Wilairat P, Chihara K, Fukumaki Y 1995. The role of alternatively spliced βE-globin mRNA on clinical severity of β-thalassemia/hemoglobin E disease. Southeast Asian J Trop Med Public Health 26: 241–245 [PubMed] [Google Scholar]