Abstract
Parkinsonism, the clinical term for a disorder with prominent bradykinesia and variable associated extrapyramidal signs and symptoms, is accompanied by degeneration of the nigrostriatal dopaminergic system, with neuronal loss and reactive gliosis in the substantia nigra found at autopsy. Parkinsonism is pathologically heterogeneous, with the most common pathologic substrates related to abnormalities in the presynaptic protein α-synuclein or the microtubule binding protein tau. In idiopathic Parkinson’s disease (PD), α-synuclein accumulates in neuronal perikarya (Lewy bodies) and neuronal processes (Lewy neurites). The disease process is multifocal and involves select central nervous system neurons and peripheral autonomic nervous system neurons. The particular set of neurons affected determines nonmotor clinical presentations. Multiple system atrophy (MSA) is the other major α-synucleinopathy. It is also associated with autonomic dysfunction and in some cases with cerebellar signs. The hallmark histopathologic feature of MSA is accumulation of α-synuclein within glial cytoplasmic inclusions (GCI). The most common of the Parkinsonian tauopathies is progressive supranuclear palsy (PSP), which is clinically associated with severe postural instability leading to early falls. The tau pathology of PSP also affects both neurons and glia. Given the population frequency of PD, α-synuclein pathology similar to that in PD, but not accompanied by neuronal loss, is relatively common (10% of people over 65 years of age) in neurologically normal individuals, leading to proposed staging schemes for PD progression. Although MSA-like and PSP-like pathology can be detected in neurologically normal individuals, such cases are too infrequent to permit assessment of patterns of disease progression.
All Parkinsonian disorders display nigrostriatal dopaminergic degeneration. But they are increasingly classified by underlying pathology (α-synucleinopathies, tauopathies, and TDP-43 proteinopathy).
Parkinson’s disease (PD) is a progressive neurological disorder defined by a characteristic clinical syndrome by bradykinesia, tremor, rigidity, and postural instability. There are a large number of different disorders that can have some or all of these clinical features, and the clinical syndrome is referred to as “parkinsonism.” Disorders in which parkinsonism is a prominent part are referred to as “parkinsonian disorders.” PD is but one of a host of parkinsonian disorders (Table 1). Some parkinsonian disorders are chronic and progressive and caused by an unknown degenerative disease process, whereas others may have clear genetic cause, such as cases driven by autosomal dominant mutations in the gene for α-synuclein. Others can be transient and caused by effects of toxins, metabolic disturbances, or drugs. The latter may have no telltale sign with standard pathological methods and can be considered “functional” rather than “structural” disorders. Some toxins that cause parkinsonism (e.g., MPTP induced parkinsonism) produce lasting brain damage that leaves structural changes.
Table 1.
Parkinsonian disorders
| Classification | Examples |
|---|---|
| Degenerative parkinsonism | |
| α-Synuclein | Parkinson’s disease, multiple system atrophy |
| Tau | Progressive supranuclear palsy, corticobasal degeneration, Guam parkinson dementia complex, chronic traumatic encephalopathy |
| TDP-43 | Frontotemporal lobar degeneration (FTLD-TDP) |
| Nondegenerative parkinsonism | |
| Vascular | Vascular parkinsonism |
| Toxic | MPTP, manganese poisoning |
| Drug-induced | Antipsychotic medications |
| Infectious | Influenza virus (postencephalitic parkinsonism) |
It is important to emphasize that it is not possible to diagnose parkinsonism with neuropathologic methods; it is only possible to describe pathologic findings—histologic, neurochemical, and molecular—that are frequently associated with parkinsonism.
Degenerative parkinsonian disorders can be inherited or sporadic, but are all characterized by neuronal loss in selective populations of vulnerable neurons. The common denominator of all degenerative parkinsonian disorders is loss of dopaminergic neurons of the substantia nigra that project to the putamen (i.e., dopaminergic nigrostriatal pathway) (Figs. 1 and 2).
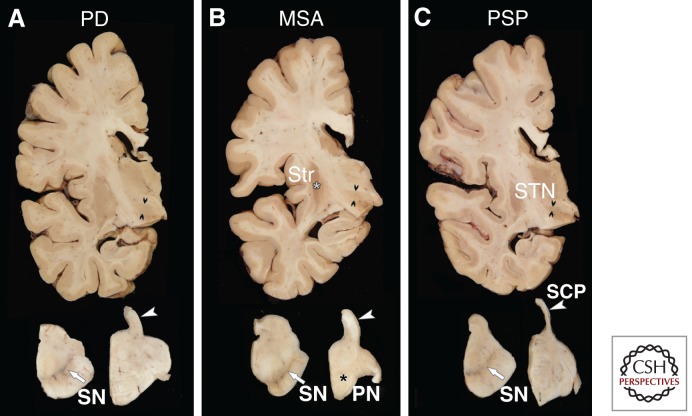
Figure 1.
Macroscopic appearance of the brain in PD (A), MSA (B), and PSP (C). There are distinguishing macroscopic features in these three major degenerative parkinsonian disorders, which is also the basis of neuroimaging biomarkers in the living patient. Transverse sections of the midbrain (lower left) and pons (lower right) shows pigment loss in the substantia nigra (white arrows) in all three disorders, which correlates with parkinsonism. In MSA there is atrophy of the pontine base (black asterisk in B), whereas the pontine base is unremarkable in PD and PSP. In PSP the superior cerebellar peduncle (compare structures marked with white arrowheads in A–C) has marked atrophy in C, whereas it has normal thickness in PD and MSA. Sections of the cerebrum at the level of the subthalamic nucleus show atrophy in PSP (C) (double black arrowheads mark the widest diameter of the nucleus), whereas the STN is normal in PD (A) and MSA (B). In MSA there is atrophy and dark discoloration of the posterior putamen (white asterisk in B), but no atrophy or discoloration is noted in PD or PSP. Abbreviations: SN, substantia nigra; Str, striatum; PN, pontine nuclei; SCP, superior cerebellar peduncle; STN, subthalamic nucleus.
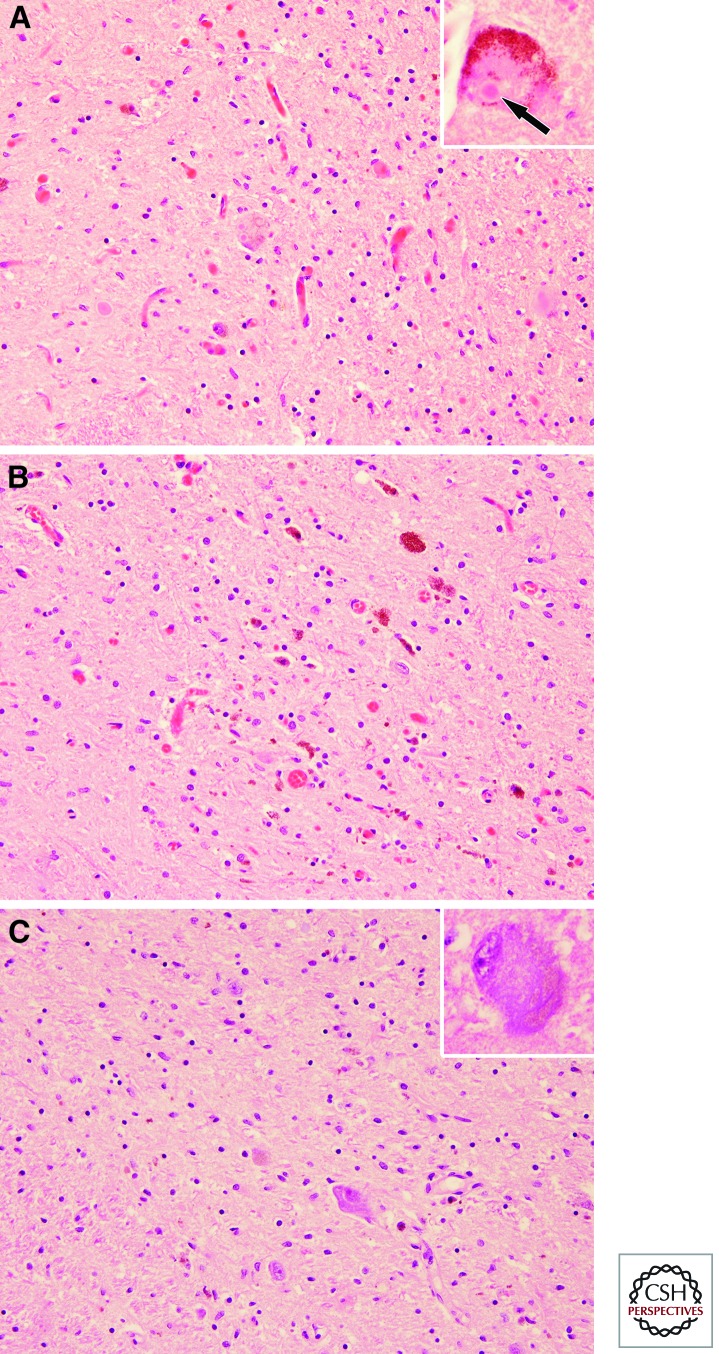
Figure 2.
Substantia nigra in PD (A), MSA (B), and PSP (C). All three major degenerative parkinsonian disorders have neuronal loss, extraneuronal neuromelanin pigment, and gliosis in the substantia nigra, especially the ventrolateral tier (shown here). There are no distinctive histologic features in MSA, but in PD there are typical hyaline cytoplasmic inclusions—Lewy bodies (inset in A), whereas PSP is characterized by basophilic globose shaped neurofibrillary tangles (inset in C).
Degenerative diseases can be classified a number of ways, but increasingly, pathologists classify them based on molecular mechanisms. Many of the most common degenerative diseases have onset in mid-to-late adulthood and have pathologic accumulations of normal cellular proteins within vulnerable neuronal populations. Most degenerative parkinsonian disorders fall into one of two molecular classes—tauopathies and α-synucleinopathies—based on pathologic accumulation of the microtubule associated protein tau or the presynaptic protein α-synuclein within vulnerable neurons and often within glial cells, as well.
PD is a degenerative parkinsonian disorder characterized by neuronal inclusions composed of α-synuclein. The inclusions are located in neuronal perikarya and referred to as Lewy bodies (Fig. 2A inset and Fig. 3A,B). Similar inclusions within neuronal cell processes are referred to as Lewy neurites (Fig. 3C). The combination of Lewy bodies and Lewy neurites is sometimes referred to as Lewy-related pathology (Dickson et al. 2009), because it is increasingly clear that abnormal α-synuclein accumulation in neuronal perikarya may be the tip of the iceberg, with evidence of accumulation not only in neuronal cell processes (Irizarry et al. 1998), but also with the synaptic compartment (Muntane et al. 2008; Schulz-Schaeffer 2010).
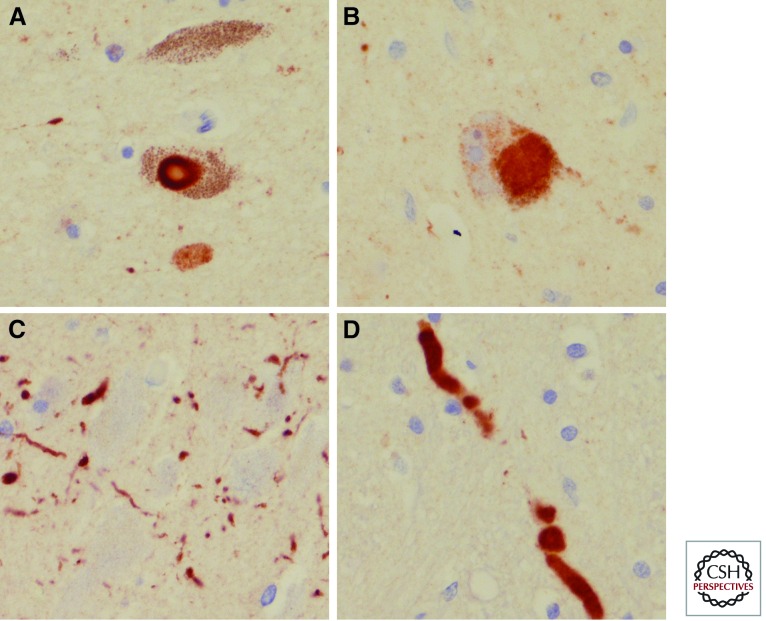
Figure 3.
Microscopic findings in PD with α-synuclein immunohistochemistry. A typical brainstem type Lewy body (A) and a pale staining “cortical type” Lewy body (B), Lewy neurites in CA2 sector of hippocampus (C), and intraneuritic Lewy bodies in medulla (D).
The other major degenerative parkinsonian disorder characterized by inclusions composed of α-synuclein is multiple system atrophy (MSA), a parkinsonian disorder that affects not only the nigrostriatal dopaminergic pathway, but also the cerebellar afferent pathways (pontocerebellar and olivocerebellar fibers). Neuronal inclusions in MSA (Fig. 4B,C), however, are a minor component of the pathology. In contrast, α-synuclein inclusions within the cytoplasm of oligodendroglial cells, so-called glial cytoplasmic inclusions (Lantos 1998), are the major finding (Fig. 4A). Interestingly, neurons in MSA may also have α-synuclein inclusions within their nuclei (Fig. 4D) (Lin et al. 2004), a feature not seen in affected neurons in PD.
Figure 4.
Microscopic findings in MSA with α-synuclein immunohistochemistry. Glial cytoplasmic inclusions in pencil fibers of the putamen (A), neuronal cytoplasmic inclusion in pontine nuclei (arrow in B), neuronal cytoplasmic inclusions and dystrophic neurites in the inferior olivary nucleus (C), and intranuclear inclusions (arrow) and dystrophic neuritics in the pontine nucleus (D).
The most common of the degenerative parkinsonian disorders associated with neuronal inclusions composed of tau protein is progressive supranuclear palsy (PSP), in which there are also tau inclusions within glial cells (both astrocytes and oligodendrocytes) (Fig. 5) (Dickson 2007). PSP is sometimes referred to as a “parkinsonism-plus” disorder in that the clinical features consistently include other neurologic features not clearly related to parkinsonism, such as eye movement disorder and dementia (Steele et al. 1964). This corresponds to involvement of brain regions beyond the dopaminergic neurons of the nigrostriatal pathway. MSA is also a “parkinsonism-plus” disorder because patients invariably have evidence of autonomic dysfunction and often signs of cerebellar dysfunction, such as nystagmus and ataxia.
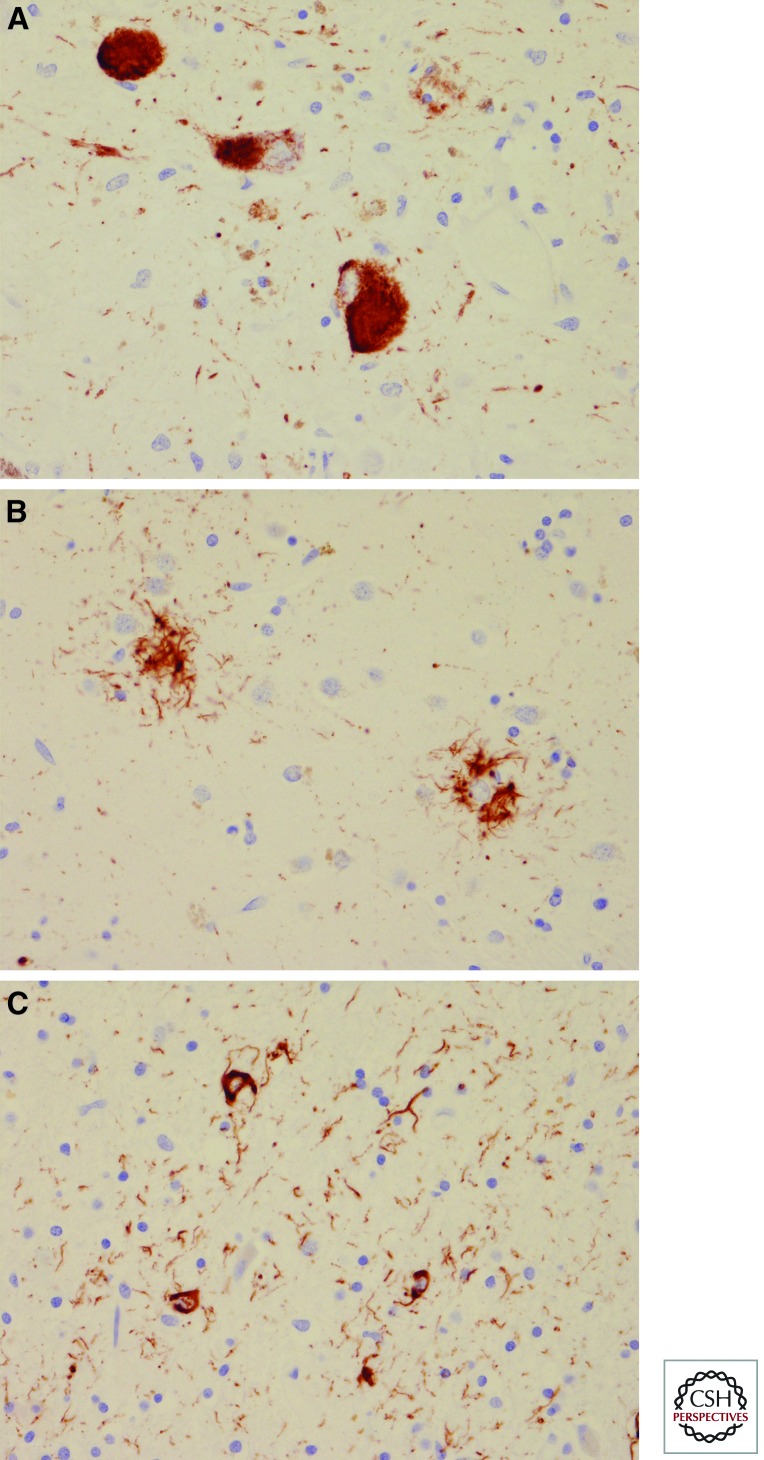
Figure 5.
Microscopic examination of PSP with tau immunohistochemistry. Globus neurofibrillary tangles (A), tufted astrocytes (B), and coiled bodies (C). Note also tau positive neurites in A and C.
The concept of as a “parkinsonism-plus” disorder has become increasingly muddled as it clear that most patients with PD also have nonparkinsonian clinical features, such as autonomic dysfunction, sleep disorders, and eventually dementia (Langston 2006). Indeed, Lewy bodies and Lewy neurites are not confined to the nigrostriatal system in PD, but can be widespread in peripheral and central autonomic neurons, even the cerebral cortex (Braak and Del Tredici 2009).
The focus of this article will be on the pathology of the most common of the degenerative parkinsonian disorders—PD, MSA, and PSP. The discussion will compare and contrast their clinical, macroscopic, and microscopic features. The rationale for limiting the discussion to these three disorders is that in autopsy series of parkinsonism, these are the most common diagnoses and clinically the most important differential diagnosis. The most common nondegenerative cause of parkinsonism found in autopsy series is cerebrovascular disease, which produces a disorder referred to as “vascular parkinsonism” (Zijlmans et al. 2004). Given heterogeneity of cerebrovascular pathology (e.g., infarcts, hemorrhages, and white matter pathology) and the neuroanatomical distribution of these lesions (e.g., basal ganglia, thalamus, and brainstem), the clinical and pathological criteria of vascular parkinsonism are less well established. The last section provides a brief overview of pathologic features other degenerative parkinsonian disorders.
CLINICAL COMPARISON OF PD, MSA, AND PSP
Characteristic Clinical Features of PD
PD can be diagnosed with considerable accuracy, particularly by neurologists specializing in diagnosis and management of movement disorders (Hughes et al. 2002), when robust clinical criteria are used such as those of the Queen Square Parkinson Disease Brain Bank, which have inclusion criteria (bradykinesia and at least one of rigidity, tremor, or postural instability) and exclusion criteria (absence of strokes, head injury, encephalitis, neuroleptic treatment, supranuclear gaze palsy, cerebellar signs, early severe autonomic dysfunction, early dementia pyramidal tract, exposure to toxins signs) as well as presence of supportive features (chronic progressive disease course, unilateral onset and asymmetry of signs during disease course, excellent and prolonged response to levodopa, late levodopa-induced dyskinesia). Asymmetry is an important supportive feature in that the other major degenerative parkinsonian disorders MSA and PSP are usually symmetrical. Response to dopamine replacement therapy (e.g., levodopa or dopamine agonists) is typical of PD, whereas MSA and PSP have limited response to such therapy. The exclusion criteria also include absence of family history of movement disorder, but this is criterion is often ignored today given increasing evidence of genetic determinants of PD (Farrer 2006).
Some of the exclusion criteria are meant to rule out PSP (e.g., supranuclear gaze palsy) and MSA (e.g., cerebellar signs and early severe autonomic dysfunction) (Table 2), but it is increasingly recognized that autonomic dysfunction is common in PD and that it may also be an early feature of the disease, given recent evidence that peripheral nerves and ganglia of the autonomic nervous system are affected early in PD and may actually be affected prior to significant brain involvement (Langston 2006; Lang 2007). It is of interest that epidemiologic studies indicate that autonomic symptoms may precede clinical PD by more than a decade (Abbott et al. 2001). Another clinical syndrome that may be a harbinger of PD is rapid eye movement behavior disorder (RBD), a condition that appears a number of years before PD (Schenck et al. 1996). The RBD syndrome appears to have its anatomic origins within lower brainstem nuclei (Kayama and Koyama 2003) that are consistently affected in PD. Olfactory dysfunction is common in PD (Hawkes et al. 1997), and it may also precede motor symptoms (Berendse et al. 2001). The later stages of PD have involvement of the cerebral cortex and at this state of the disease, PD is characterized by cognitive dysfunction or frank dementia, referred to as PD dementia. PD dementia is distinguished from dementia with Lewy bodies (McKeith et al. 2004), in which dementia is an early and prominent clinical feature.
Table 2.
Clinical differential diagnosis
| PD | MSA | PSP | |
|---|---|---|---|
| Asymmetrical motor signs | Almost always | Sometimes | Sometimes |
| Levodopa response | Almost always | Sometimes | Sometimes |
| Autonomic dysfunction | Sometimes | Always | Almost never |
| Dementia | Almost always, late in the disease | Almost never | Almost always, in some cases early |
Characteristic Clinical Features of MSA
MSA is a nonheritable neurodegenerative disorder characterized by parkinsonism, cerebellar ataxia and idiopathic orthostatic hypotension (also known as Shy-Drager syndrome), a syndrome complex first recognized by Oppenheimer, who noted overlap in the pathology of sporadic olivopontocerebellar atrophy and striatonigral degeneration (Oppenheimer 1976). Depending on the predominant signs and symptoms, MSA is subdivided into MSA-C, for those with predominant degeneration in cerebellar circuitry and ataxia, and MSA-P for those with predominant degeneration in the basal ganglia with parkinsonism (Gilman et al. 1999). Autonomic dysfunction is required for the clinical diagnosis of MSA, but as noted above can also be seen in PD. It is rare in PSP.
Characteristic Clinical Features of PSP
One of the earliest clinical features of PSP is unexplained falls. Eventually, most patients with PSP develop postural instability, vertical gaze paresis, nuchal and axial rigidity, and dysarthria. Despite many differences in clinical presentation, it is not uncommon for an individual to carry a diagnosis of PD for years before a correct diagnosis of PSP is made (Rajput et al. 1991; Josephs and Dickson 2003). Recently, it has been suggested that a subset of cases of pathologically confirmed PSP have parkinsonism with many similarities to PD, including asymmetry, tremor and partial response to levodopa, PSP-P (Williams et al. 2005). Many patients with PSP have cognitive problems or dementia, but this does not help to differentiate PSP from PD, because late in the disease process PD patients also frequently develop dementia (Hely et al. 2008) and even early in the disease, PD patients may have mild cognitive deficits compared to healthy individuals. On the other hand, most MSA patients have better preservation of cognition.
NEUROPATHOLOGY OF PARKINSONISM
Macroscopic Pathology—PD, MSA, PSP
PD is often unremarkable, with mild frontal atrophy in some cases. There is no significant atrophy of brainstem, and this can be useful in the differential diagnosis of PSP and MSA, in which there is midbrain atrophy in PSP and pontine atrophy in MSA. Sections of the brainstem usually reveal loss of the normally dark black pigment in the substantia nigra (Fig. 2) and locus ceruleus, but pigment loss in the substantia nigra is also characteristic of PSP and MSA. The loss of pigmentation correlates with neuronal loss of dopaminergic neurons in the substantia nigra and noradrenergic neurons in the locus ceruleus. Pigment loss in the locus ceruleus is consistent in PD, but less predictable in PSP and MSA.
MSA-P has atrophy and brownish discoloration of the posterolateral putamen (Fig. 1), the brown color correlating with increased iron pigment. In cases with significant cerebellar signs, there is also atrophy of the pontine base and atrophy and gray discoloration of the cerebellar white matter. More subtle atrophy is noted in the medulla (e.g., inferior olive) and the cerebellar cortex.
PSP has mild frontal cortical atrophy and often-marked atrophy of the midbrain. The latter is uncommon in PD and MSA. The cerebellar dentate nucleus usually has atrophy and discoloration of the white matter in the dentate hilus, with similar atrophy and discoloration in the cerebellar outflow pathway. This produces marked atrophy of the superior cerebellar peduncle (Fig. 1). The basal ganglia and thalamus are usually macroscopically unremarkable, but the subthalamic nucleus is almost always smaller than normal and often discolored (Fig. 1). The subthalamic nucleus and the superior cerebellar peduncle are not affected in PD or MSA.
MICROSCOPIC PATHOLOGY
Lewy Bodies and Lewy-Related Pathology in PD
Classical Lewy bodies have a hyaline appearance on H&E (Fig. 2A inset), whereas α-synuclein immunoreactive inclusions in less vulnerable neuronal populations, such as the amygdala and cortex, are pale staining and poorly circumscribed. These lesions are referred to as “cortical Lewy bodies” (Ikeda et al. 1978). A related pale staining neuronal cytoplasmic inclusion found in pigmented brainstem neurons of the substantia nigra and locus ceruleus is the “pale body” (Pappolla et al. 1988; Dale et al. 1992). Evidence suggests that cortical Lewy bodies and pale bodies may be early cytologic alterations that precede the classical Lewy body, so-called pre-Lewy bodies. In some cases with severe pathology, hyaline type inclusions consistent with classical Lewy bodies can be detected in the amygdala and cortex, particularly the limbic cortex. Although most of the α-synuclein immunoreactive cytopathology in PD is within neurons, α-synuclein immunoreactive glia, particularly oligodendroglia, can be detected in small numbers in the midbrain and basal ganglia (Wakabayashi and Takahashi 1996; Wakabayashi et al. 2000).
At the ultrastructural level, Lewy bodies are composed of dense granular material and straight filaments that are approximately 10–15nm in diameter (Forno 1969; Tiller-Borcich and Forno 1988; Galloway et al. 1992). Similar filaments can be created in the test tube with recombinant α-synuclein (Conway et al. 2000; Crowther et al. 2000). The presence of α-synuclein in cytoplasmic inclusions represents aberrant cytologic localization, because α-synuclein is normally a component of presynaptic terminals. The factors that give rise to the abnormal conformation remain to be determined, but several posttranslational modifications, including phosphorylation, truncation, and oxidative damage are implicated (reviewed by Dickson 2001). The composition of the dense granular material in Lewy bodies is unknown, but perhaps related to other components that have been shown to be present in Lewy bodies. Antibodies to neurofilament (Galvin et al. 1997), ubiquitin (Kuzuhara et al. 1988), and the ubiquitin binding protein p62 (Kuusisto et al. 2003) are among the most consistently detected proteins in Lewy bodies. A subset of Lewy bodies shows immunoreactivity with antibodies to tau protein (Ishizawa et al. 2003), but this is a small subset of Lewy bodies and almost always in neuronal populations that are inherently vulnerable to tau pathology. It is rare to find tau immunoreactivity in cortical type Lewy bodies in PD. Many other antibodies that inconsistently label Lewy bodies have been reported (Pollanen et al. 1993).
Glial Cytoplasmic Inclusions in MSA
Lantos and co-workers first described glial (oligodendroglial) cytoplasmic inclusions in MSA (6). Glial cytoplasmic inclusions can be detected with silver stains, in particular, the Gallyas silver stain, but are best seen with antibodies to α-synuclein (Fig. 4A) and ubiquitin, in which they appear as flame- or sickle-shaped inclusions in oligodendrocytes. At the ultrastructural level, glial cytoplasmic inclusions are nonmembrane-bound cytoplasmic inclusions composed of 10 to 20nm diameter coated filament similar to the filaments in Lewy bodies (Lin et al. 2004).
Although most α-synuclein inclusions in MSA are in oligodendroglial cells, certain neuronal populations are vulnerable to neuronal cytoplasmic and intranuclear inclusions, particularly those in the pontine base (Fig. 4A), inferior olive (Fig. 4C), and putamen. A few of the neuronal inclusions in MSA resemble Lewy bodies, but their anatomical distribution is distinct from neuronal populations vulnerable to Lewy bodies. Intranuclear α-synuclein-immunoreactive inclusions (Fig. 4D) (Lin et al. 2004) are not found in PD.
Neuronal and Glial Tau Pathology in PSP
PSP is a degenerative tauopathy characterized by accumulation of filamentous tau inclusions within neurons. Tau is a microtubule associated protein that is biochemically composed of six major isoforms related to alternative mRNA splicing, including three isoforms with four ∼32-amino acid conserved repeats (4R-tau) in the microtubule binding domain and three isoforms with three repeats (3R tau). In PSP, 4R tau preferentially accumulates, whereas in Alzheimer’s disease tau inclusions are composed on a nearly equal mixture of 3R and 4R tau. Monoclonal antibodies specific to 3R and 4R tau now permit assessment of the type of tau that accumulates within neuronal lesions with routine immunohistochemistry (de Silva et al. 2003).
In addition to neurofibrillary tangles (Fig. 5A), tau pathology of PSP is characterized by inclusions in astrocytes (so-called “tufted astrocytes”) (Fig. 5B) and in oligodendroglia (so-called “coiled bodies”) (Fig. 5C). The latter glial lesions are distinct from the glial cytoplasmic inclusions of MSA and the sparse glial lesions detected in PD, not only based on their immunoreactivity with tau, but also on their morphology. Tau also accumulates in cell processes (both neuronal and glial), referred to as tau-positive “threads.”
Distribution of Pathology
The hallmark of any neurodegenerative disease is selective neuronal loss (Table 3). Accompanying neuronal loss in all neurodegenerative disorders are reactive changes in astrocytes and microglia. Microglia express markers of activation, such as the class II major histocompatibility antigen HLA-DR (McGeer et al. 1988), and astrocytes become hypertrophic and accumulate the intermediate filament protein, glial fibrillary acidic protein. Dying neurons undergo phagocytosis by microglia, a term referred to as neuronophagia. In the substantia nigra and locus ceruleus, evidence of neuronophagia is neuromelanin pigment in the cytoplasm of microglia. In cases with very long disease duration, microglia migrate to blood vessels and exit the brain along with the neuromelanin pigment. Neuronal loss in the substantia nigra is most marked in the ventrolateral tier of neurons of the pars compacta (A9) in all parkinsonian disorders. In contrast, the dorsal and medial neuronal cell groups are less vulnerable. Loss of medial neuronal cell groups (e.g., ventral tegmental region or A10) may be increased in parkinsonian disorders with dementia (Rinne et al. 1989).
Table 3.
Pathologic comparison of PD, MSA, and PSP
| Region | PD | MSA | PSP |
|---|---|---|---|
| Amygdala | Consistent/severe | Spared | Spared |
| Hippocampus | Variable/moderate | Spared | Spared |
| Temporal cortex | Variable/moderate | Spared | Spared |
| Cingulate cortex | Variable/moderate | Uncommon/mild | Spared |
| Superior frontal gyrus | Uncommon/mild | Spared | Variable/moderate |
| Motor cortex | Spared | Variable/moderate | Consistent/severe |
| Caudate/putamen | Uncommon/mild | Consistent/severe | Consistent/severe |
| Globus pallidus | Spared | Variable/moderate | Consistent/severe |
| Basal nucleus of Meynert | Consistent/severe | Uncommon/mild | Consistent/severe |
| Hypothalamus | Consistent/severe | Uncommon/mild | Consistent/severe |
| Thalamus | Spared | Uncommon/mild | Consistent/severe |
| Subthalamic nucleus | Spared | Spared | Consistent/severe |
| Red nucleus | Spared | Spared | Variable/moderate |
| Substantia nigra | Consistent/severe | Consistent/severe | Consistent/severe |
| Oculomotor complex | Variable/moderate | Spared | Consistent/severe |
| Midbrain tectum | Spared | Spared | Consistent/severe |
| Locus ceruleus | Consistent/severe | Uncommon/mild | Consistent/severe |
| Pontine tegmentum (including raphe and pedunculopontine nuclei) | Variable/moderate | Uncommon/mild | Consistent/severe |
| Pontine nuclei (including pontocerebellar fibers) | Spared | Consistent/severe | Variable/moderate |
| Medullary tegmentum (including dorsal motor nucleus of vagus) | Consistent/severe | Consistent/severe | Consistent/severe |
| Inferior olive (including olivocerebellar fibers) | Spared | Consistent/severe | Variable/moderate |
| Dentate nucleus | Spared | Spared | Consistent/severe |
| Cerebellar white matter | Spared | Consistent/severe | Uncommon/mild |
Braak PD Staging Scheme
It has been known for many years that Lewy bodies in PD extend well beyond the substantia nigra (Jellinger 1991). Based on the distribution of α-synuclein pathology, Braak and co-workers have proposed a staging scheme for PD (Braak et al. 2004). In this scheme, neuronal pathology occurs early in the dorsal motor nucleus of the vagus in the medulla and the anterior olfactory nucleus in the olfactory bulb. As the disease progresses, locus ceruleus neurons in the pons and then dopaminergic neurons in the substantia nigra are affected. In later stages, pathology extends to the basal forebrain, amygdala and the medial temporal lobe structures, with convexity cortical areas affected in the last stages. Although the staging scheme is attractive, it should be remembered that this scheme is not based on distribution of neuronal loss, but on distribution of abnormal α-synuclein deposits and how it relates to progression of neuronal loss has not been rigorously studied. Thus, the proposed staging should be interpreted cautiously. The scheme was originally based on evaluation of brains of individuals that were not necessarily well characterized in life, and cases were chosen for further study if they had pathology in the medulla (Del Tredici et al. 2002), thus, biasing the results in favor of “early” pathology in the medulla. In more recent studies of prospectively studied individuals who have come to autopsy, the scheme proposed by Braak and co-workers does not always hold true. Some elderly individuals have Lewy bodies confined to the olfactory bulb (Fujishiro et al. 2008a; Beach et al. 2009) or the amygdala, the latter particularly true if associated with concurrent Alzheimer type pathology (Uchikado et al. 2006). Moreover, some neurologically normal individuals have sparse, but widespread Lewy body pathology, even involving the cortex (Parkkinen et al. 2005; Frigerio et al. 2011), which would seem to violate the theory of progression from brainstem and perhaps fit better with a multicentric disease process from the onset. Clearly, the observed distribution of Lewy bodies is dependent on case selection (Parkkinen et al. 2001).
Although the staging scheme of Braak and co-workers should be considered tentative, it nevertheless, has prompted considerable debate in the field and reawakened recognition of early nonmotor clinical features of PD (Jain 2011). Subsequent iterations of the Braak scheme proposed that autonomic neurons in peripheral nervous system may be affected before involvement of the central nervous system (Braak and Del Tredici 2009) and this has prompted recognition that PD is a multiorgan disease process, not merely a disorder of central nervous system (Beach et al. 2010). Moreover, it has fed the debate on cell-to-cell transmission of unknown putative disease factors (prion-like) (Hawkes et al. 2009), especially given the fact that fetal mesencephalic intrastriatal transplants to treat PD have been shown to develop Lewy body pathology (Kordower et al. 2008), possibly by cell-to-cell transmission (Kordower et al. 2011).
Jellinger Staging Scheme for MSA
It has been more challenging to stage pathology in MSA and PSP because of the rarity of these disorders and because of their inherent variability. Nevertheless, Jellinger has proposed a staging scheme for MSA that scores severity of striatonigral degeneration (SND) and olivopontocerebellar atrophy (OPCA), each on a three-point scale. The final classification is indicated by an OPCA + SND score (e.g., OPCA 1 + SND 3 for a typical MSA-P case and OPCA 3 + SND 1 for a typical MSA-C case). Halliday and co-workers (2011) proposed a similar scheme and graphically illustrated the two major MSA types, as well as the overlap in OPCA and SND system degenerations. Ozawa and colleagues used a semiquantitative scoring scheme for lesion density and found differences in the proportion of MSA types in Japanese compared to European autopsy cohorts, with far more OPCA in Japanese (Ozawa et al. 2004, 2010). Detection of MSA in neurologically normal individuals (“incidental MSA”) is extremely uncommon (Fujishiro et al. 2008b), and large numbers of such cases would be needed to determine the earliest sites of involvement to develop a staging scheme for MSA analogous to the Braak staging scheme for PD.
Distribution of Pathology in PSP
The distribution neuronal loss and neurofibrillary degeneration in PSP was beautifully documented in the original report by Steele, Richardson, and Olszewski based on classic silver staining methods (Steele et al. 1964). Modern neuropathologic methods with tau immunohistochemistry have extended these observations, by recognizing glial involvement, as well as greater cortical pathology than noted in the original report, particularly affecting motor and premotor cortices of the frontal lobe (Hauw et al. 1990). Nevertheless, the cardinal nuclei affected in PSP remain those originally described and include the globus pallidus, subthalamic nucleus, substantia nigra, midbrain tectum, periaqueductal gray, locus ceruleus, and the cerebellar dentate nucleus. Other regions that are consistently affected include corpus striatum, ventrolateral thalamus, red nucleus, pontine and medullary tegmentum, pontine base, and inferior olivary nucleus. Spinal cord involvement is also common, where neuronal inclusions can be found in intermediolateral cell columns. Heterogeneity in the distribution of tau pathology in PSP is increasingly recognized (Williams et al. 2005; Dickson et al. 2010), but a staging scheme remains to be defined. The presence of PSP-like pathology in neurologically normal individuals (“incidental PSP”) is uncommon (Evidente et al. 2011). As in MSA, the paucity of such cases precludes development of staging scheme for PSP.
OTHER DEGENERATIVE PARKINSONIAN DISORDERS
Other Parkinsonian Tauopathies
Corticobasal Degeneration
Corticobasal degeneration (CBD), which is also known as cortical basal ganglionic degeneration, is a parkinsonism-plus disorder with characteristic focal cortical signs in addition to atypical levodopa-nonresponsive parkinsonism (Litvan et al. 1997; Boeve et al. 1999). Patients with CBD may present with progressive asymmetrical rigidity and apraxia (i.e., the corticobasal syndrome), but other clinical syndromes are also reported such as progressive aphasia and progressive frontal lobe dementia (Litvan 1999). Parkinsonism is characterized by bradykinesia, rigidity, and dystonia, but most patients do not have tremor.
The pathologic correlate of focal cortical findings on clinical evaluations is focal cortical atrophy, which is uncommon in PD, MSA, and PD. Cortical atrophy in CBD is often most marked in the superior frontal gyrus, and the motor cortex may be severely affected. The midbrain does not have atrophy as in PSP, but pigment loss is common in the substantia nigra. In contrast to PSP, the superior cerebellar peduncle and the subthalamic nucleus are grossly normal (Dickson 1999).
Microscopically, the affected cortical areas have neuronal loss, spongiosis, and gliosis with swollen achromatic or ballooned neurons. Cortical neurons in affected areas have pleomorphic tau-immunoreactive inclusions, and there are invariably numerous tau positive threads in both gray and white matter of affected cortices, as well as in the basal ganglia (Dickson et al. 2002). The most characteristic lesion in CBD is an annular cluster of short, stubby processes with fuzzy outlines that represent tau accumulation in distal processes of astrocytes, a lesion referred to as an “astrocytic plaque” (Feany and Dickson 1995). Astrocytic plaques differ from the tufted astrocytes seen in PSP, and the two lesions do not coexist in the same brain (Komori 1999).
The globus pallidus and putamen show mild neuronal loss with gliosis. Thalamic nuclei may also be affected. The substantia nigra usually shows moderate to severe neuronal loss with extraneuronal neuromelanin, gliosis and tau immunoreactive neuronal lesions. The lower brainstem is less affected than in PSP (Dickson 1999, 2004).
Chronic Traumatic Encephalopathy
Individuals that suffer repeated closed head trauma may develop parkinsonism as well as dementia, a disorder currently referred to as chronic traumatic encephalopathy (CTE) (McKee et al. 2009). In the past, this syndrome was referred to as dementia pugilistica or “punch drunk” syndrome because it was often associated with dementia and parkinsonism in professional boxers. There may be evidence of increasing frequency of the syndrome in other contact sports. In addition to other signs of chronic head trauma, such as small contusions or chronic subdural membrane, patients with CTE also had tau pathology that patchy and predominant in gray matter at the depths of cortical sulci and in superficial cerebral white matter. In these areas, tau accumulates in both neurons and astrocytes. The tau protein that accumulates is biochemically similar to that found in Alzheimer’s disease.
Guam Parkinson-Dementia Complex
A characteristic Parkinsonism with dementia (Parkinson dementia complex [PDC]) with a number of features that overlap with PSP (Steele et al. 2002; Steele 2005) is common in the native Chamorro population of Guam and in the Kii peninsula of Japan (Kuzuhara and Kokubo 2005). The gross findings in PDC are notable for cortical atrophy affecting especially the medial temporal lobe, as well as atrophy of the hippocampus and the tegmentum of the rostral brainstem, which overlaps with atrophy seen in Alzheimer’s disease. These areas typically have neuronal loss and gliosis with many neurofibrillary tangles in residual neurons and extracellular neurofibrillary tangle are numerous (Hirano et al. 1961). The substantia nigra and locus ceruleus have neuronal loss and neurofibrillary tangles. The basal nucleus and large neurons in the striatum are also vulnerable to neurofibrillary tangle. Biochemically and morphologically, neurofibrillary tangles in Guam PDC are indistinguishable from those in Alzheimer’s disease (Buee-Scherrer et al. 1995; Morris et al. 1999).
TDP-43-Related Parkinsonism
In addition to α-synuclein and tau, a third major protein has been found to accumulate within neurons and glial is a number of neurodegenerative disorders, including amyotrophic lateral sclerosis, frontotemporal lobar degeneration (FTLD), Alzheimer’s disease and even some parkinsonian disorders. This protein is termed TDP-43 after TAR DNA binding protein of 43-kDa molecular weight, a protein originally found to bind to an HIV transactive response DNA binding protein. It is now known to be an RNA/DNA binding protein that has a number of functions, not all of which are currently known (Buratti and Baralle 2010). It is normally a nuclear protein and its accumulation in cytoplasmic inclusions is decidedly abnormal.
Frontotemporal lobar degenerations are clinically and pathologically heterogenous, and importantly fall into two major classes—TDP-43 proteinopathies and tauopathies (Mackenzie et al. 2010). In some classification schemes, CBD and PSP are included among FTLD-tau, although as noted above frontal lobe clinical features in both CBD and PSP may be overshadowed by atypical parkinsonism in individual cases. On the other hand, parkinsonism is often a minor component of FTLD-TDP. Nevertheless, in autopsy series of atypical parkinsonism some cases will inevitably have pathology of FTLD-TDP. These patients often present with mixed clinical syndromes: dementia with parkinsonism, parkinsonism-plus syndrome, or corticobasal syndrome (Josephs et al. 2007). Pathologic findings will be those of FTLD-TDP—focal cortical atrophy with neuronal loss, gliosis, spongiosis, and neuronal inclusions of TDP-43—with the additional findings of significant neuronal loss in the substantia nigra associated with TDP-43 neuronal inclusions. In many cases, there is also TDP-43 pathology in the basal ganglia, which may contribute to the movement disorder.
CONCLUSIONS
Parkinsonian disorders are increasingly classified according to underlying molecular pathology (Dickson et al. 2009), with α-synucleinopathies (PD, MSA) and tauopathies (PSP, CBD, Guam PDC, CTE) being the most common. Recently, another category as been recognized—Parkinsonism associated with TDP-43 proteinopathy. As genetic and molecular studies are increasingly used to further refine underlying disease processes, it is likely that other molecular forms of Parkinsonism will be identified. Currently, the one feature that unifies Parkinsonian disorders is nigrostriatal dopaminergic degeneration, but intriguing evidence from genetic studies (Hoglinger et al. 2011; Nalls et al. 2011) suggest that there may also be shared genetic risk factors (e.g., MAPT), but these remain largely unknown at present.
ACKNOWLEDGMENTS
Supported by NIH grants P50-NS72187, P50-AG25711, P50-AG16574, P01-AG17216, and P01-AG03949. The histological support of Virginia Phillips, Linda Rousseau, and Monica Casey-Castanedes is greatly appreciated. Dr. John Steele is acknowledged for his efforts to provide samples from his patients on Guam for neuropathologic study. The clinicians and geneticists involved in familial PD studies, including Drs. Zbigniew Wszolek, Katerina Gwinn Hardy, and Matt Farrer are acknowledged. These studies would not be possible without the generous donation of patients and their families toward research on Parkinsonism.
Footnotes
Editor: Serge Przedborski
Additional Perspectives on Parkinson’s Disease available at www.perspectivesinmedicine.org
REFERENCES
- Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW 2001. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57: 456–462 [DOI] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, et al. 2009. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117: 613–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, et al. 2010. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119: 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Booij J, Francot CM, Bergmans PL, Hijman R, Stoof JC, Wolters EC 2001. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol 50: 34–41 [DOI] [PubMed] [Google Scholar]
- Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, Dickson DW, Kokmen E, Petersen RC 1999. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 53: 795–800 [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K 2009. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol 201: 1–119 [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K 2004. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318: 121–134 [DOI] [PubMed] [Google Scholar]
- Buee-Scherrer V, Buee L, Hof PR, Leveugle B, Gilles C, Loerzel AJ, Perl DP, Delacourte A 1995. Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam. Immunochemical characterization of tau proteins. Am J Pathol 146: 924–932 [PMC free article] [PubMed] [Google Scholar]
- Buratti E, Baralle FE 2010. The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol 7: 420–429 [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT Jr 2000. Fibrils formed in vitro from α-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry 39: 2552–2563 [DOI] [PubMed] [Google Scholar]
- Crowther RA, Daniel SE, Goedert M 2000. Characterisation of isolated α-synuclein filaments from substantia nigra of Parkinson’s disease brain. Neurosci Lett 292: 128–130 [DOI] [PubMed] [Google Scholar]
- Dale GE, Probst A, Luthert P, Martin J, Anderton BH, Leigh PN 1992. Relationships between Lewy bodies and pale bodies in Parkinson’s disease. Acta Neuropathol (Berl) 83: 525–529 [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H 2002. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61: 413–426 [DOI] [PubMed] [Google Scholar]
- de Silva R, Lashley T, Gibb G, Hanger D, Hope A, Reid A, Bandopadhyay R, Utton M, Strand C, Jowett T, et al. 2003. Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol Appl Neurobiol 29: 288–302 [DOI] [PubMed] [Google Scholar]
- Dickson DW 1999. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246: 116–115 [DOI] [PubMed] [Google Scholar]
- Dickson DW 2001. α-Synuclein and the Lewy body disorders. Curr Opin Neurol 14: 423–432 [DOI] [PubMed] [Google Scholar]
- Dickson DW 2004. Sporadic tauopathies: Pick’s disease, corticobasal degeneration, progressive supranuclear palsy and argyrophilic grain disesase. In The neuropathology of dementia, 2nd ed. (ed. MM Esiri, VMY Lee, JQ Trojanowski), pp. 227–256 Cambridge University Press, New York [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, et al. 2002. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61: 935–946 [DOI] [PubMed] [Google Scholar]
- Dickson DW, Rademakers R, Hutton ML 2007. Progressive supranuclear palsy: Pathology and genetics. Brain Pathol 17: 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, et al. 2009. Neuropathological assessment of Parkinson’s disease: Refining the diagnostic criteria. Lancet Neurol 8: 1150–1157 [DOI] [PubMed] [Google Scholar]
- Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA 2010. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23: 394–400 [DOI] [PubMed] [Google Scholar]
- Evidente VG, Adler CH, Sabbagh MN, Connor DJ, Hentz JG, Caviness JN, Sue LI, Beach TG 2011. Neuropathological findings of PSP in the elderly without clinical PSP: Possible incidental PSP? Parkinsonism Relat Disord 17: 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer MJ 2006. Genetics of Parkinson disease: Paradigm shifts and future prospects. Nat Rev Genet 7: 306–318 [DOI] [PubMed] [Google Scholar]
- Feany MB, Dickson DW 1995. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146: 1388–1396 [PMC free article] [PubMed] [Google Scholar]
- Forno LS 1969. Concentric hyalin intraneuronal inclusions of Lewy type in the brains of elderly persons (50 incidental cases): Relationship to parkinsonism. J Am Geriatr Soc 17: 557–575 [DOI] [PubMed] [Google Scholar]
- Frigerio R, Fujishiro H, Ahn TB, Josephs KA, Maraganore DM, DelleDonne A, Parisi JE, Klos KJ, Boeve BF, Dickson DW, et al. 2011. Incidental Lewy body disease: Do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol Aging 32: 857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW 2008a. Co-localization of tau and α-synuclein in the olfactory bulb in Alzheimer’s disease with amygdala Lewy bodies. Acta Neuropathol 116: 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro H, Ahn TB, Frigerio R, DelleDonne A, Josephs KA, Parisi JE, Eric Ahlskog J, Dickson DW 2008b. Glial cytoplasmic inclusions in neurologically normal elderly: Prodromal multiple system atrophy? Acta Neuropathol 116: 269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway PG, Mulvihill P, Perry G 1992. Filaments of Lewy bodies contain insoluble cytoskeletal elements. Am J Pathol 140: 809–822 [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Lee VM, Baba M, Mann DM, Dickson DW, Yamaguchi H, Schmidt ML, Iwatsubo T, Trojanowski JQ 1997. Monoclonal antibodies to purified cortical Lewy bodies recognize the mid-size neurofilament subunit. Ann Neurol 42: 595–603 [DOI] [PubMed] [Google Scholar]
- Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, et al. 1999. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 163: 94–98 [DOI] [PubMed] [Google Scholar]
- Halliday GM, Holton JL, Revesz T, Dickson DW 2011. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol 122: 187–204 [DOI] [PubMed] [Google Scholar]
- Hauw JJ, Verny M, Delaere P, Cervera P, He Y, Duyckaerts C 1990. Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy. Basic differences with Alzheimer’s disease and aging. Neurosci Lett 119: 182–186 [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Shephard BC, Daniel SE 1997. Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 62: 436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H 2009. Parkinson’s disease: The dual hit theory revisited. Ann NY Acad Sci 1170: 615–622 [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG 2008. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov Disord 23: 837–844 [DOI] [PubMed] [Google Scholar]
- Hirano A, Kurland LT, Krooth RS, Lessell S 1961. Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain 84: 642–661 [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L, Rademakers R, de Silva R, Litvan I, Riley DE, et al. 2011. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet 43: 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ 2002. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125: 861–870 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ikeda S, Yoshimura T, Kato H, Namba M 1978. Idiopathic Parkinsonism with Lewy-type inclusions in cerebral cortex. A case report. Acta Neuropathol (Berl) 41: 165–168 [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Growdon W, Gomez-Isla T, Newell K, George JM, Clayton DF, Hyman BT 1998. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson’s disease and cortical Lewy body disease contain α-synuclein immunoreactivity. J Neuropathol Exp Neurol 57: 334–337 [DOI] [PubMed] [Google Scholar]
- Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW 2003. Colocalization of tau and α-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62: 389–397 [DOI] [PubMed] [Google Scholar]
- Jain S 2011. Multi-organ autonomic dysfunction in Parkinson disease. Parkinsonism Relat Disord 17: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA 1991. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14: 153–197 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW 2003. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord 18: 1018–1026 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve BF, Knopman DS, Petersen RC, Davies P, Duara R, Graff-Radford NR, et al. 2007. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 66: 142–151 [DOI] [PubMed] [Google Scholar]
- Kayama Y, Koyama Y 2003. Control of sleep and wakefulness by brainstem monoaminergic and cholinergic neurons. Acta Neurochir 87: 3–6 [DOI] [PubMed] [Google Scholar]
- Komori T 1999. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol 9: 663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW 2008. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14: 504–506 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, Sortwell C, Steece-Collier K, Collier TJ 2011. Transfer of host-derived α synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis 43: 552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ 2007. Presynaptic α-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci 27: 1405–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusisto E, Parkkinen L, Alafuzoff I 2003. Morphogenesis of Lewy bodies: Dissimilar incorporation of α-synuclein, ubiquitin, and 62. J Neuropathol Exp Neurol 62: 1241–1253 [DOI] [PubMed] [Google Scholar]
- Kuzuhara S, Kokubo Y 2005. Atypical parkinsonism of Japan: Amyotrophic lateral sclerosis-parkinsonism-dementia complex of the Kii peninsula of Japan (Muro disease): An update. Mov Disord 20: S108–S113 [DOI] [PubMed] [Google Scholar]
- Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y 1988. Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol (Berl) 75: 345–353 [DOI] [PubMed] [Google Scholar]
- Lang AE 2007. The progression of Parkinson disease: A hypothesis. Neurology 68: 948–952 [DOI] [PubMed] [Google Scholar]
- Langston JW 2006. The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann Neurol 59: 591–596 [DOI] [PubMed] [Google Scholar]
- Lantos PL 1998. The definition of multiple system atrophy: A review of recent developments. J Neuropathol Exp Neurol 57: 1099–1111 [DOI] [PubMed] [Google Scholar]
- Lin WL, DeLucia MW, Dickson DW 2004. α-Synuclein immunoreactivity in neuronal nuclear inclusions and neurites in multiple system atrophy. Neurosci Lett 354: 99–102 [DOI] [PubMed] [Google Scholar]
- Litvan I 1999. Recent advances in atypical parkinsonian disorders. Curr Opin Neurol 12: 441–446 [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, Lai EC, Verny M, Ray-Chaudhuri K, McKee A, et al. 1997. Accuracy of the clinical diagnosis of corticobasal degeneration: A clinicopathologic study. Neurology 48: 119–125 [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, et al. 2010. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol 119: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG 1988. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38: 1285–1291 [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA 2009. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68: 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, et al. 2004. Dementia with Lewy bodies. Lancet Neurol 3: 19–28 [DOI] [PubMed] [Google Scholar]
- Morris HR, Lees AJ, Wood NW 1999. Neurofibrillary tangle parkinsonian disorders—tau pathology and tau genetics. Mov Disord 14: 731–736 [DOI] [PubMed] [Google Scholar]
- Muntane G, Dalfo E, Martinez A, Ferrer I 2008. Phosphorylation of tau and α-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer’s disease, and in Parkinson’s disease and related α-synucleinopathies. Neuroscience 152: 913–923 [DOI] [PubMed] [Google Scholar]
- Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, et al. 2011. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet 377: 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer DR 1976. Diseases of the basal ganglia, cerebellum and motor neurons. In Greenfield’s neuropathology, 3rd ed (ed. Blackwood W, Corsellis JAN), pp. 608–651, Edward Arnold, London [Google Scholar]
- Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, Healy DG, Wood NW, Lees AJ, Holton JL, et al. 2004. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: Clinicopathological correlations. Brain 127: 2657–2671 [DOI] [PubMed] [Google Scholar]
- Ozawa T, Tada M, Kakita A, Onodera O, Ishihara T, Morita T, Shimohata T, Wakabayashi K, Takahashi H, Nishizawa M 2010. The phenotype spectrum of Japanese multiple system atrophy. J Neurol Neurosurg Psychiatry 81: 1253–1255 [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Shank DL, Alzofon J, Dudley AW 1988. Colloid (hyaline) inclusion bodies in the central nervous system: Their presence in the substantia nigra is diagnostic of Parkinson’s disease. Hum Pathol 19: 27–31 [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Soininen H, Laakso M, Alafuzoff I 2001. α-synuclein pathology is highly dependent on the case selection. Neuropathol Appl Neurobiol 27: 314–325 [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Pirttila T, Tervahauta M, Alafuzoff I 2005. Widespread and abundant α-synuclein pathology in a neurologically unimpaired subject. Neuropathology 25: 304–314 [DOI] [PubMed] [Google Scholar]
- Pollanen MS, Dickson DW, Bergeron C 1993. Pathology and biology of the Lewy body. J Neuropathol Exp Neurol 52: 183–191 [DOI] [PubMed] [Google Scholar]
- Rajput AH, Rozdilsky B, Rajput A 1991. Accuracy of clinical diagnosis in parkinsonism—a prospective study. Can J Neurol Sci 18: 275–278 [DOI] [PubMed] [Google Scholar]
- Rinne JO, Rummukainen J, Paljarvi L, Rinne UK 1989. Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Ann Neurol 26: 47–50 [DOI] [PubMed] [Google Scholar]
- Schenck CH, Bundlie SR, Mahowald MW 1996. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 46: 388–393 [DOI] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ 2010. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol 120: 131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JC 2005. Parkinsonism-dementia complex of Guam. Mov Disord 20: S99–S107 [DOI] [PubMed] [Google Scholar]
- Steele JC, Richardson JC, Olszewski J 1964. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10: 333–359 [DOI] [PubMed] [Google Scholar]
- Steele JC, Caparros-Lefebvre D, Lees AJ, Sacks OW 2002. Progressive supranuclear palsy and its relation to pacific foci of the parkinsonism-dementia complex and Guadeloupean parkinsonism. Parkinsonism Relat Disord 9: 39–54 [DOI] [PubMed] [Google Scholar]
- Tiller-Borcich JK, Forno LS 1988. Parkinson’s disease and dementia with neuronal inclusions in the cerebral cortex: Lewy bodies or Pick bodies. J Neuropathol Exp Neurol 47: 526–535 [DOI] [PubMed] [Google Scholar]
- Uchikado H, Lin WL, DeLucia MW, Dickson DW 2006. Alzheimer disease with amygdala Lewy bodies: A distinct form of α-synucleinopathy. J Neuropathol Exp Neurol 65: 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H 1996. Gallyas-positive, tau-negative glial inclusions in Parkinson’s disease midbrain. Neurosci Lett 217: 133–136 [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H 2000. NACP/α-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol (Berl) 99: 14–20 [DOI] [PubMed] [Google Scholar]
- Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ 2005. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 128: 1247–1258 [DOI] [PubMed] [Google Scholar]
- Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ 2004. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord 19: 630–640 [DOI] [PubMed] [Google Scholar]