Abstract
The fibroblast growth factor (FGF) signaling axis plays important roles in heart development. Yet, the molecular mechanism by which the FGF regulates cardiogenesis is not fully understood. Using genetically engineered mouse and in vitro cultured embryoid body (EB) models, we demonstrate that FGF signaling suppresses premature differentiation of heart progenitor cells, as well as autophagy in outflow tract (OFT) myocardiac cells. The FGF also promotes mesoderm differentiation in embryonic stem cells (ESCs) but inhibits cardiomyocyte differentiation of the mesoderm cells at later stages. Furthermore, inhibition of FGF signaling increases myocardial differentiation and autophagy in both ex vivo cultured embryos and EBs, whereas activation of autophagy promotes myocardial differentiation. Thus, a link between FGF signals preventing premature differentiation of heart progenitor cells and suppression of autophagy has been established. These findings provide the first evidence that autophagy plays a role in heart progenitor differentiation, and suggest a new venue to regulate stem/progenitor cell differentiation.
Keywords: FGF, autophagy, heart defect, heart development, premature differentiation, second heart field
Understanding the molecular mechanisms by which cardiac progenitor cell differentiation is regulated is essential for inducing stem cells to differentiate into functional cardiac cells, one of the key steps for developing stem cell therapies for cardiovascular disease, the most prevalent disease in the world. Mammalian heart morphogenesis begins with the generation of a single heart tube from cardiomyocytes originated from the first heart field (FHF), which then undergoes rightward looping, expansion, and formation of recognizable cardiac chambers. At the start of looping and throughout the process, cells from the the second heart field (SHF) are recruited to the heart and contribute to the establishment of the outflow tract, atria, and left ventricle.
The fibroblast growth factor signaling axis has been implicated in the development, recruitment, and differentiation of heart progenitors; disruption of FGF signaling leads to severe defects in heart development. Yet, how FGF signals regulate the contribution of SHF cells to the heart remains elusive. The FGF elicits its regulatory signals via activating the FGF receptor (FGFR) tyrosine kinases, which then activate downstream signaling cascades, including the MAP and the PtdIns 3-kinase pathways in a FGF receptor substrate 2α (FRS2α)-dependent manner. Ablation of Frs2α in heart progenitor cells leads to premature differentiation of the SHF cells and increases the autophagic activity in OFT myocardiac cells. Autophagy is a cellular process for degradation of bulky subcellular organelles and macromolecules to make way for new organelles or to recycle nutrients and needed building blocks for formation of new cellular apparatus, which plays important roles in development and metabolism. Defective autophagic activity leads to tumorigenesis and cardiovascular diseases. We previously reported that FRS2α is required for the FGFR to suppress autophagy in mouse embryonic fibroblasts via the mTOR-dependent pathway.
The ESC-derived EBs, which have been widely used for investigating cardiomyocyte differentiation, were employed to test the hypothesis that autophagy mediates FGF signals in regulating SHF cell differentiatiation. We showed that abrogating FGF signaling promotes myocardial differentiation. Interestingly, suppression of FGF signaling increases the abundance of LC3-II both in the presence or absence of NH4Cl, a lysosome inhibitor blocking LC3-II degradation. The results indicate that LC3-II conversion, and therefore autophagy initiation, is increased by inhibiting FGF signaling. In addition, the abundance of two key autophagy regulators, BECN1 and p27, are increased in the presence of the FGFR inhibitor. On the other hand, treating with autophagy inhibitors, including NH4Cl or bafilomycin A1, significantly inhibits formation of beating foci, while treating with rapamycin, an autophagy promoter, increases formation of beating foci. Furthermore, treating with NH4Cl diminishes the enhancement of beating focus formation by FGFR inhibitors, and treating with rapamycin abolishes the suppressive activity of FGF2 on beating focus formation. We further demonstrated that although suppression of either the ERK or PtdIns3K pathway increases autophagy activities and promotes beating focus formation, treating the EBs with the PtdIns3K inhibitor, but not the ERK inhibitor, upregulates expression of BECN1 and p27, suggesting that the two pathways regulate autophagy via non-overlapping mechanisms. Consistently, treating ex vivo cultured embryos with rapamycin induces premature expression of SMA and myosin in the SHF; ablation of Frs2α significantly increases the numbers of green fluorescent protein GFP-LC3 positive punctate foci in the OFT myocardium, especially in the presence of chloroquine, which is lysosome inhibitors. The results demonstrate that the formation of autophagosomes is increased in differentiating cardiomyocytes of mutant embryos. In addition, we also demonstrate that FRS2α-mediated signals inhibit maturation of ventricular cardiomyocytes and differentiation of cardiac mesenchymal cells as well. Together, the results suggest that FGF signaling suppresses cardiac cell differentiation by inhibiting autophagy.
Although the detailed molecular mechanisms still need to be investigated, we have provided the first evidence that autophagy positively regulates cardiac progenitor cell differentiation and cardiomyocyte maturation. This is the first report showing that autophagy plays a crucial role in mediating growth factor signaling pathways in regulating progenitor cell differentiation (Fig. 1).
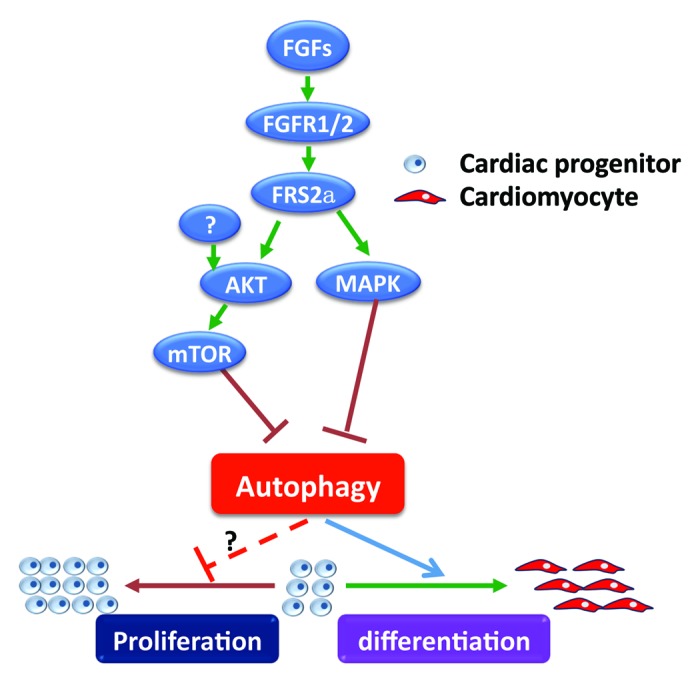
Figure 1. The FGF signaling axis inhibits premature differentiation of cardiac progenitor cells by suppressing autophagy. Binding of the FGF to the FGFR activates the FGFR tyrosine kinase, which then activates the AKT and MAPK kinase pathways in a FRS2α-dependent manner, suppresses autophagy, and inhibits differentiation of cardiac progenitor cells. Disruption of FGF signaling by deletion of Frs2α, or suppression of FGFR, PtdIns3K, or MAPK kinase activity promotes autophagy and differentiation of cardiac progenitor cells. Consistently, stimulation of autophagy promotes cardiac progenitor differentiation; suppression of autophagy prevents the differentiation. Although disruption of FGF signaling suppresses cardiac progenitor proliferation, whether autophagy directly plays a role in regulating cardiac progenitor proliferation remains to be investigated.
Acknowledgments
The work is supported by NIH-CA96824 and CA140388 from the NCI to F.W.; and AHA0655077Y to F.W. and 09PRE2010130 predoctoral fellowship to J.Z. from The American Heart Association.
Glossary
Abbreviations:
- EB
embryoid body
- ESC
embryonic stem cell
- FGF
fibroblast growth factor
- FGFR
FGF receptor
- FRS2α
FGFR substrate 2α
- FHF
first heart field
- NCCs
neural crest cells
- OFT
outflow tract
- SHF
second heart field
- SM
splanchnic mesoderm
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/19290


