Abstract
Background
There are few published studies on microtia-anotia frequency.
Methods
Using data from birth defects surveillance programs around the world, we conducted a systematic review on the frequency of microtia-anotia to further explore the differences in prevalence across countries. Ninety-two birth defects surveillance programs were evaluated with a total of 8,917 cases of microtia-anotia. We computed the prevalence per 10,000 births for each surveillance program for total cases of microtia-anotia (microtia types I to IV), microtia (types I to III), and anotia (type IV). Prevalence ratios were calculated by large geographical areas, race/ethnicity, and by surveillance methodologies.
Results
The overall prevalence were for microtia-anotia 2.06 (CI: 2.02–2.10), for microtia 1.55 (CI: 1.50–1.60), and for anotia 0.36 (CI: 0.34–0.38). Higher prevalence was observed in the Americas, Northern Europe and Asia, among Hispanics and Asians, and among active ascertainment and hospital-based surveillance programs.
Conclusions
We observed marked variation in the prevalence of microtia-anotia across surveillance programs and within countries. These results must be interpreted cautiously as this variability may be explained mainly by differences in surveillance methods. However, given the magnitude of some of the differences, other factors may also be involved. This study contributes to the knowledge on the prevalence of microtia-anotia by providing a critical analysis of the existing data. In addition, it supports the need for a coding system that allows complete phenotype characterization of microtia-anotia, including severity and laterality; as well as further studies on the variation of its frequency related to race and ethnicity.
Keywords: microtia-anotia, microtia, anotia, ear, prevalence, birth defects, epidemiology
Introduction
The external ear consists of the auricle, the external acoustic meatus and the tympanic membrane. Microtia-anotia is a spectrum of congenital anomalies of the auricle ranging from mild structural abnormalities to complete absence of the ear (anotia) (Carey et al., 2006). A well-accepted classification of microtia-anotia was proposed by Marx (1926). In the Marx classification, all of the features of a normal auricle are present in grade I, but the pinna is smaller than normal. In grade II, some anatomical structures are still recognizable. In the most common form, grade III (the peanut-shell type), only a rudiment of soft tissue is present. The extreme case where there is no external ear and auditory canal is called anotia or microtia grade IV.
There is no consensus in the literature in relation to the terminology used for these external ear malformations. Some authors prefer to use the term “microtia” (Alasti and Van Camp, 2009; Castilla and Orioli, 1986; Hunter et al., 2009; Suutarla et al., 2007) while others use “microtia-anotia” or “microtia/anotia” (Canfield et al., 2009; Carey et al., 2006; Forrester and Merz, 2005; Harris et al., 1996; Mastroiacovo et al., 1995; Shaw et al., 2004). Anotia, from the dysmorphology-descriptive standpoint, is the most severe end of the microtia spectrum. For the purpose of this paper we chose the term “microtia-anotia” because: (a) it was a common term used in the literature to identify these two anomalies, (b) some of the surveillance programs included in this study use this term to indicate that they do not differentiated between microtia and anotia, while others only report anotia (but not microtia types I to III). This term includes microtia types I to IV.
Microtia-anotia may occur as an isolated condition, or as part of a spectrum of anomalies or a syndrome. It can occur unilaterally (79–93% of cases) or bilaterally; in unilateral cases the right ear is more often affected (Suutarla et al., 2007). Individuals with unilateral microtia-anotia usually have normal hearing in the unaffected ear (Eavey, 1995; Kelley and Scholes, 2007). Therefore, speech and language development are typically normal, although children with microtia-anotia are at a greater risk of delayed language development and attention deficit disorders (Eavey, 1995; Kelley and Scholes, 2007). Children with microtia-anotia will have an associated anomaly or an identifiable syndrome pattern in 20–60% of cases (Carey et al., 2006; Castilla and Orioli, 1986; Kaye et al., 1989; Mastroiacovo et al., 1995; Shaw et al., 2004). The most common anomalies are facial cleft, facial asymmetry, renal abnormalities, cardiac defects, microphthalmia, polydactyly, and vertebral anomalies (Harris et al., 1996; Mastroiacovo et al., 1995).
The etiology of microtia-anotia is poorly understood. Much has been published regarding surgical treatments and their outcomes (Chang et al., 2006; Habiballah and Bamousa, 2000; Osorno, 2007; Tollefson, 2006), but few studies have focused on the environmental or genetic causes of microtia-anotia. There is strong evidence supporting the importance of environmental causes for microtia-anotia, such as altitude (Castilla et al., 1999; González-Andrade et al., 2010) and gestational exposure to retinoids, alcohol, thalidomide and, mycophenolate mofetil (Anderka et al., 2009; Carey et al., 2006; Klieger-Grossmann et al., 2010). More recently, periconceptional intake of folic-acid-containing supplements was associated with reduced risk of microtia-anotia among non-obese women (Ma et al., 2010).
Evidence for a genetic contribution to isolated microtia-anotia is based on: 1) higher concordance in monozygotic twins than in dizygotic twins; 38.5% and 4.5%, respectively (Artunduaga et al., 2009); 2) reported familial cases with autosomal recessive or dominant modes of inheritance with variable expression and incomplete penetrance (Balci, 1974; Balci et al., 2001; Gupta and Patton, 1995; Klockars et al., 2007; Strisciuglio et al., 1986); 3) population-based estimates of familial cases ranging from 3 to 34% (Castilla and Orioli, 1986; Llano-Rivas et al., 1999; Mastroiacovo et al., 1995; Okajima et al., 1996); 4) more than 18 different microtia-associated syndromes for which single-gene defects or chromosomal aberrations have been reported; and 5) mouse models demonstrating that specific genes are necessary for mouse ear development and that, when mutated, result in anotia or microtia. However, only few studies have focused on the possible genetic causes of isolated microtia-anotia in humans (Lin et al., 2009; Monks et al., 2010; Zhang et al., 2009) and no gene has been associated so far to microtia-anotia.
The first step to better understand the epidemiology and the etiology of any birth defect is to analyze its distribution among the various populations. There are few published studies on microtia-anotia frequency. The birth prevalence estimates vary greatly among countries ranging from 0.8 to 17.4 per 10,000 (Suutarla et al., 2007). Higher prevalence has been reported, in Ecuadorians (Castilla and Orioli, 1986; González-Andrade et al., 2010); Chileans (Nazer et al., 2006), and Finns (Suutarla et al., 2007); with a prevalence of respectively, 17.4, 8.8, and 4.3 per 10,000 births. In the US population higher prevalence has been observed for Hispanics, Asians, Pacific Islanders and Native Americans when compared to non-Hispanic Whites. Prevalence has been reported from 4 to 12.0 in Native Americans (Aase and Tegtmeier, 1977; Jaffe, 1969; Nelson and Berry, 1984), from 2.2 to 3.2 in Asians (Forrester and Merz, 2005; Harris et al., 1996; Shaw et al., 2004), 4.61 in Pacific Islanders (Forrester and Merz, 2005) and from 1.9 to 3.4 in US individuals of Hispanic descent (Canfield et al., 2009; Harris et al., 1996; Shaw et al., 2004; Yang et al., 2004). We conducted a systematic review on the frequency of microtia-anotia using data of the three existing networks of birth defects surveillance to further explore the differences in prevalence of microtia-anotia across countries.
Material and Methods
We used the term microtia-anotia when including microtia types I to IV, as already mentioned; microtia was used to indicate microtia types I to III, and anotia was used for type IV.
We used data from three sources: (a) Annual Reports of the International Clearinghouse for Birth Defects Surveillance and Research [ICBDSR] (International Clearinghouse for Birth Defects Surveillance and Research. Annual Report 2008, 2010) and the updated database stored at the ICBDSR Headquarter in Rome, Italy (b) Reports of the National Birth Defects Prevention Network [NBDPN] (Population-based Birth Defects Surveillance data from selected states, 2002–2006–2009), and (c) the website of the European Surveillance of Congenital Anomalies [EUROCAT] (European Surveillance of Congenital Anomalies, 2010). For US or European surveillance programs that contribute with data to NBDPN or EUROCAT and to ICBDSR concomitantly, we used the data from ICBDSR to avoid duplication of cases. A total of ninety-two birth defects surveillance programs were evaluated.
The specific methods used by the surveillance programs to identify, code, and report birth defects are available from the annual reports of ICBDSR (International Clearinghouse for Birth Defects Surveillance and Research. Annual Report 2008, 2010) and NBDPN (Population-based Birth Defects Surveillance data from selected states, 2002–2006–2009), and from the EUROCAT website (European Surveillance of Congenital Anomalies, 2010). Table 1 summarizes the main characteristics of these three surveillance networks.
Table 1.
Number of birth defects surveillance programs evaluated in this review and their main characteristics
| Birth Defects surveillance networks | Number of surveillance programs | Microtia-Anotia reporting | Ascertainment | Years considered | Pregnancy outcome ascertained |
|---|---|---|---|---|---|
| EUROCAT | 26 | Anotia only registered | Population-based | Longest available period up to 2007 | LB, SB, ETOPFA (b) |
| ICBDSR | 39 | Microtia and Anotia are differentiated (a) | Mostly population-based | Longest available period up to 2007 | LB, SB, ETOPFA (c) |
| NBDPN | 27 | Microtia and Anotia are not differentiated | Population-based | 2002–2006 | LB, SB, ETOPFA (d) |
LB = Livebirth, SB = Stillbirth, ETOPFA = Elective termination of pregnancy for fetal anomalies
Anotia and microtia are not differentiated in China CBDMN, Finland, Japan JAOG, and Mexico RYVEMCE
ETOPFA are not reported in Poland and Poland Wielkopolska
ETOPFA are not reported in Canada British Columbia, Canada National, China Beijing, China CBDMN, Italy Sicily, Japan JAOG, Spain ECEMC, and Ukraine
ETOPFA are not reported in Alaska, Arizona, Colorado, Florida, Illinois, Indiana, Kentucky, Massachusetts, Michigan, Mississippi, Nebraska, New Jersey, New York, Rhode Island, Tennessee, Virginia, and Wisconsin
In general the diagnosis of microtia-anotia was confirmed by clinical examination at local level, however validation of the diagnosis, particularly important for the distinction between a minor ear defect and microtia grade I or between microtia grade III and microtia grade IV (anotia) was not possible because there was no detailed description available.
Almost all the surveillance programs are population-based except for China CBDMN, Israel IBDSP, Japan JAOG, Mexico RYVEMCE, South America ECLAMC, Spain ECEMC and United Arab Emirates which are hospital-based. We included these hospital-based programs assuming that because microtia-anotia is rarely diagnosed prenatally and most of participant hospitals are not referral centers for birth defects, the cases are fairly representative of those in the general population, and ascertainment would be relatively unbiased. In addition, previous reporting of higher prevalence in Ecuador (Castilla and Orioli, 1986) and the current hypothesis of higher prevalence in US individuals of Hispanic descent made the analysis of the data from some of these programs essential.
Livebirths (LB), stillbirths (SB), and elective terminations of pregnancy for fetal anomalies (ETOPFA) were included in the surveillance programs where are permitted (see table 1 notes). We included the surveillance programs not reporting ETOPFA since the influence of ETOPFA on the total proportion of cases is very limited (4%), as evaluated from surveillance programs reporting them (data not shown, available from the original source).
We computed the prevalence per 10,000 births and 95% confidence intervals (CI) using the Poisson distribution and calculated medians and interquartile range (IQR) to describe the distribution of the prevalence across the surveillance programs. . Prevalence was calculated for total cases of microtia-anotia, microtia only, and anotia only for each surveillance program and for large geographical areas: North America, Central and South-America, Europe (North, West, East, and South), Asia and Australia. These geographical areas were named and defined according to the United Nations Statistic Division Classification (http://unstats.un.org/unsd/methods/m49/m49regin.htm). We also evaluated the prevalence of microtia-anotia by race/ethnicity using the NBDPN data, for ICBDSR and EUROCAT these data was not available. The numerator was the total number of cases of microtia-anotia, microtia, and anotia occurring in LB, SB, and ETOPFA, and the denominator was the total number of births (LB + SB).
Surveillance programs vary widely in the methodology used for conducting surveillance. The main differences are (a) population- or hospital-based, (b) active or passive ascertainment, (c) single or a multiple sources for ascertainment, (d) register or not ETOPFA, and (e) different periods of life covered for ascertainment (i.e.: during the perinatal period or up to 1–7 years of age). The validity of the data cannot be solely inferred by these characteristics because a number of other factors are related to it. However, the methodology employed by a program may be related to ascertainment. In order to evaluate the variation of microtia-anotia, microtia and anotia that could be due to different surveillance methodologies among the programs, we conducted an exploratory on surveillance methods comparing: (a): hospital-based versus population-based programs, (b) active versus passive surveillance programs, and (c) programs reporting ETOPFA or not. Data for the analysis comparing active versus passive surveillance programs was only available for the NBDPN network.
We calculated prevalence ratios (PR) and 95% CI to estimate the differences in prevalence by large areas, race/ethnicity and, surveillance methods. Results of these exploratory analyses are presented only for microtia-anotia since we obtained similar results for microtia only and anotia only.
Results
Microtia-anotia
The prevalence of microtia-anotia is shown in table 2 by surveillance programs ordered by large geographical areas and by alphabetic order in each large geographical area. For the 66 surveillance programs reporting microtia-anotia, among 43,279,396 births, 8,917 cases of microtia-anotia were diagnosed, with an overall prevalence of 2.06 per 10,000 births (CI: 2.02–2.10). The median value was 1.43 with the interquartile range between 0.79 and 2.21 and an overall range between zero (Malta, North Dakota and Rhode Island) and 7.19 (Mexico RYVEMCE).
Table 2.
Prevalence (per 10,000 births) of microtia-anotia (types I to IV) by large geographical areas and surveillance program
| Area | Registry | Network | Period | Total births | Total cases | % of Microtia on specified cases | Total Prevalence(a) | CI 95% | |
|---|---|---|---|---|---|---|---|---|---|
| Am-N | Canada Alberta | ICBDSR | 1997–2007 | 441,654 | 91 | 75.8 | 2.06 | 1.66 | 2.53 |
| Am-N | Canada British Columbia | ICBDSR | 2001–2007 | 289,180 | 70 | 71.0 | 2.42 | 1.89 | 3.06 |
| Am-N | Canada National | ICBDSR | 2006–2007 | 732,342 | 129 | 86.4 | 1.76 | 1.47 | 2.09 |
| Am-N | Alaska | NBDPN | 2002–2006 | 51,409 | 15 | n.c. | 2.92 | 1.63 | 4.81 |
| Am-N | Arizona | NBDPN | 2003–2007 | 378,789 | 70 | n.c. | 1.85 | 1.44 | 2.33 |
| Am-N | Arkansas | NBDPN | 2002–2006 | 194,000 | 36 | n.c. | 1.86 | 1.30 | 2.57 |
| Am-N | California | NBDPN | 2002–2006 | 325,906 | 100 | n.c. | 3.07 | 2.50 | 3.73 |
| Am-N | Colorado | NBDPN | 2002–2006 | 345,858 | 87 | n.c. | 2.52 | 2.01 | 3.10 |
| Am-N | Department of Defense | NBDPN | 2002–2006 | 495,815 | 85 | n.c. | 1.71 | 1.37 | 2.12 |
| Am-N | Florida | NBDPN | 2002–2006 | 1,095,992 | 66 | n.c. | 0.60 | 0.47 | 0.77 |
| Am-N | Georgia - Atlanta | ICBDSR | 1988–2007 | 905,118 | 140 | 86.4 | 1.54 | 1.30 | 1.82 |
| Am-N | Hawaii | NBDPN | 2002–2005 | 71,878 | 39 | n.c. | 5.43 | 3.86 | 7.42 |
| Am-N | Illinois | NBDPN | 2002–2006 | 902,988 | 89 | n.c. | 0.99 | 0.79 | 1.21 |
| Am-N | Indiana | NBDPN | 2002–2006 | 348,478 | 17 | n.c. | 0.49 | 0.28 | 0.78 |
| Am-N | Iowa | NBDPN | 2002–2006 | 193,929 | 35 | n.c. | 1.80 | 1.26 | 2.51 |
| Am-N | Kentucky | NBDPN | 2004–2006 | 169,727 | 15 | n.c. | 0.88 | 0.49 | 1.46 |
| Am-N | Massachusetts | NBDPN | 2002–2006 | 393,745 | 59 | n.c. | 1.50 | 1.14 | 1.93 |
| Am-N | Michigan | NBDPN | 2002–2006 | 645,941 | 83 | n.c. | 1.28 | 1.02 | 1.59 |
| Am-N | Mississippi | NBDPN | 2002–2006 | 172,687 | 11 | n.c. | 0.64 | 0.32 | 1.14 |
| Am-N | Nebraska | NBDPN | 2002–2006 | 130,470 | 5 | n.c. | 0.38 | 0.12 | 0.89 |
| Am-N | New Hampshire | NBDPN | 2003–2006 | 51,160 | 6 | n.c. | 1.17 | 0.43 | 2.55 |
| Am-N | New Jersey | NBDPN | 2002–2006 | 574,128 | 115 | n.c. | 2.00 | 1.65 | 2.40 |
| Am-N | New York | NBDPN | 2002–2006 | 1,234,971 | 83 | n.c. | 0.67 | 0.54 | 0.83 |
| Am-N | North Carolina | NBDPN | 2003–2006 | 488,751 | 90 | n.c. | 1.84 | 1.48 | 2.26 |
| Am-N | North Dakota | NBDPN | 2002–2006 | 40,887 | 0 | n.c. | 0.00 | 0.00 | 0.90 |
| Am-N | Oklahoma | NBDPN | 2002–2006 | 258,126 | 28 | n.c. | 1.08 | 0.72 | 1.57 |
| Am-N | Rhode Island | NBDPN | 2006 | 11,959 | 0 | n.c. | 0.00 | 0.00 | 3.08 |
| Am-N | Tennesse | NBDPN | 2002–2006 | 401,873 | 21 | n.c. | 0.52 | 0.32 | 0.80 |
| Am-N | Texas | ICBDSR | 1996–2007 | 4,017,567 | 1,179 | 90.7 | 2.93 | 2.77 | 3.11 |
| Am-N | Utah | ICBDSR | 1999–2007 | 453,386 | 126 | 94.4 | 2.78 | 2.32 | 3.31 |
| Am-N | Virginia | NBDPN | 2002–2006 | 514,588 | 32 | n.c. | 0.62 | 0.43 | 0.88 |
| Am-N | West Virginia | NBDPN | 2002–2006 | 92,823 | 3 | n.c. | 0.32 | 0.07 | 0.94 |
| Am-N | Wisconsin | NBDPN | 2002–2006 | 332,596 | 19 | n.c. | 0.57 | 0.34 | 0.89 |
| Am C-S | Chile | ICBDSR | 2002–2007 | 78,743 | 22 | 90.9 | 2.79 | 1.75 | 4.23 |
| Am C-S | Costa Rica | ICBDSR | 2001–2007 | 508,303 | 141 | 80.1 | 2.77 | 2.34 | 3.27 |
| Am C-S | Cuba (§) | ICBDSR | 2002–2007 | 732,614 | 48 | 79.5 | 0.66 | 0.48 | 0.87 |
| Am C-S | Mexico RYVEMCE (§) | ICBDSR | 1988–2007 | 777,415 | 559 | n.c. | 7.19 | 6.61 | 7.81 |
| Am C-S | South America ECLAMC (§) | ICBDSR | 1997–2007 | 1,953,313 | 1,066 | 93.3 | 5.45 | 5.13 | 5.79 |
| Eu-N | Denmark Odense | EUROCAT | 1988–2007 | 111,365 | 3 | ---- | 0.27 | 0.06 | 0.79 |
| Eu-N | Finland | ICBDSR | 1993–2007 | 889,387 | 405 | n.c. | 4.55 | 4.12 | 5.02 |
| Eu-N | Ireland Cork and Kerry | EUROCAT | 1996–2005 | 80,288 | 0 | ---- | 0.00 | 0.00 | 0.46 |
| Eu-N | Ireland Dublin | ICBDSR | 2002–2007 | 143,328 | 4 | 50.0 | 0.28 | 0.08 | 0.71 |
| Eu-N | Ireland Galway | EUROCAT | 1988–1999 | 31,409 | 0 | ---- | 0.00 | 0.00 | 1.17 |
| Eu-N | Ireland SE | EUROCAT | 1997–2007 | 69,289 | 0 | ---- | 0.00 | 0.00 | 0.53 |
| Eu-N | Norway | ICBDSR | 1994–2007 | 826,518 | 60 | 70.0 | 0.73 | 0.55 | 0.93 |
| Eu-N | Sweden | ICBDSR | 1999–2007 | 877,634 | 103 | 15.7 | 1.17 | 0.96 | 1.42 |
| Eu-N | UK East Midlands & South Yorkshire | EUROCAT | 1998–2007 | 622,064 | 4 | ---- | 0.06 | 0.02 | 0.16 |
| Eu-N | UK Glasgow | EUROCAT | 1988–2000 | 148,213 | 2 | ---- | 0.13 | 0.02 | 0.49 |
| Eu-N | UK Merseyside & Cheshire | EUROCAT | 1995–1999 | 139,867 | 1 | ---- | 0.07 | 0.00 | 0.40 |
| Eu-N | UK N W Thames | EUROCAT | 1991–2004 | 661,527 | 3 | ---- | 0.05 | 0.01 | 0.13 |
| Eu-N | UK Northern England | EUROCAT | 2000–2007 | 247,091 | 17 | ---- | 0.69 | 0.40 | 1.10 |
| Eu-N | UK Thames Valley | EUROCAT | 1991–2007 | 169,919 | 3 | ---- | 0.18 | 0.04 | 0.52 |
| Eu-N | UK Wessex | EUROCAT | 1994–2007 | 370,122 | 0 | ---- | 0.00 | 0.00 | 0.10 |
| Eu-N | Wales | ICBDSR | 1998–2007 | 323,422 | 36 | 63.9 | 1.11 | 0.78 | 1.54 |
| Eu-W | Austria Styria | EUROCAT | 1988–2007 | 235,491 | 4 | ---- | 0.17 | 0.05 | 0.43 |
| Eu-W | Belgium Antwerp | EUROCAT | 1990–2007 | 256,747 | 10 | ---- | 0.39 | 0.19 | 0.72 |
| Eu-W | Belgium Hainaut | EUROCAT | 1988–2007 | 247,765 | 8 | ---- | 0.32 | 0.14 | 0.64 |
| Eu-W | France Auvergne | EUROCAT | 2002 | 13,397 | 0 | ---- | 0.00 | 0.00 | 2.75 |
| Eu-W | France Isle de la Reunion | EUROCAT | 2002–2007 | 88,025 | 8 | ---- | 0.91 | 0.39 | 1.79 |
| Eu-W | France Paris | ICBDSR | 1994–2007 | 511,225 | 76 | 44.7 | 1.49 | 1.17 | 1.86 |
| Eu-W | France Rhone-Alps | ICBDSR | 1994–2007 | 1,358,786 | 204 | 56.0 | 1.50 | 1.30 | 1.72 |
| Eu-W | France Strasbourg | ICBDSR | 1994–2007 | 198,059 | 48 | 77.1 | 2.42 | 1.79 | 3.21 |
| Eu-W | Germany Mainz | EUROCAT | 1990–2006 | 59,403 | 2 | ---- | 0.34 | 0.04 | 1.22 |
| Eu-W | Germany Saxony Anhalt | ICBDSR | 1988–2007 | 267,212 | 21 | 81.0 | 0.79 | 0.49 | 1.20 |
| Eu-W | Northern Netherlands | ICBDSR | 1988–2007 | 380,881 | 73 | 41.1 | 1.92 | 1.50 | 2.41 |
| Eu-W | Switzerland Vaud | EUROCAT | 1989–2007 | 142,366 | 5 | ---- | 0.35 | 0.11 | 0.82 |
| Eu-E | Bulgaria Sofia | EUROCAT | 1996–1999 | 38,257 | 1 | ---- | 0.26 | 0.01 | 1.46 |
| Eu-E | Hungary | ICBDSR | 1995–2007 | 1,292,228 | 80 | 12.5 | 0.62 | 0.49 | 0.77 |
| Eu-E | Poland | EUROCAT | 1999–2007 | 2,043,322 | 74 | ---- | 0.36 | 0.28 | 0.45 |
| Eu-E | Poland Wielkopolska | EUROCAT | 1999–2007 | 316,838 | 12 | ---- | 0.38 | 0.20 | 0.66 |
| Eu-E | Russia Moscow | ICBDSR | 2001–2007 | 379,216 | 40 | 61.5 | 1.05 | 0.75 | 1.44 |
| Eu-E | Slovak Republic | ICBDSR | 1995–2007 | 722,742 | 59 | 72.4 | 0.82 | 0.62 | 1.05 |
| Eu-E | Ukraine | ICBDSR | 2000–2007 | 212,825 | 47 | 83.0 | 2.21 | 1.62 | 2.94 |
| Eu-S | Croatia Zagreb | EUROCAT | 1988–2007 | 126,293 | 4 | ---- | 0.32 | 0.09 | 0.81 |
| Eu-S | Italy Campania | ICBDSR | 1995–2007 | 701,448 | 80 | 55.0 | 1.14 | 0.90 | 1.42 |
| Eu-S | Italy Emilia Romagna | ICBDSR | 1995–2007 | 373,068 | 54 | 48.1 | 1.45 | 1.09 | 1.89 |
| Eu-S | Italy North East | ICBDSR | 1988–2007 | 1,068,721 | 199 | 88.2 | 1.89 | 1.63 | 2.17 |
| Eu-S | Italy Sicily | ICBDSR | 1998–2005 | 142,806 | 12 | 41.7 | 0.84 | 0.43 | 1.47 |
| Eu-S | Italy Tuscany | ICBDSR | 1992–2007 | 427,214 | 35 | 62.9 | 0.82 | 0.57 | 1.14 |
| Eu-S | Malta | ICBDSR | 1993–2007 | 64,817 | 0 | n.c. | 0.00 | 0.00 | 0.57 |
| Eu-S | S Portugal | EUROCAT | 1990–2007 | 263,540 | 1 | ---- | 0.04 | 0.00 | 0.21 |
| Eu-S | Spain Asturias | EUROCAT | 1990–2004 | 103,732 | 0 | ---- | 0.00 | 0.00 | 0.36 |
| Eu-S | Spain Barcelona | EUROCAT | 1992–2007 | 211,022 | 1 | ---- | 0.05 | 0.00 | 0.26 |
| Eu-S | Spain Basque Country | EUROCAT | 1990–2006 | 293,473 | 5 | ---- | 0.17 | 0.06 | 0.40 |
| Eu-S | Spain ECEMC (§) | ICBDSR | 1988–2007 | 1,865,293 | 297 | 92.9 | 1.59 | 1.42 | 1.78 |
| As | China Beijing (§) | ICBDSR | 1997–2005 | 1,228,542 | 333 | 92.5 | 2.71 | 2.43 | 3.02 |
| As | China CBDMN (§) | ICBDSR | 1996–2006 | 3,932,069 | 1,221 | n.c. | 3.11 | 2.93 | 3.28 |
| As | Israel IBDSP (§) | ICBDSR | 1988–2007 | 450,890 | 59 | 98.3 | 1.31 | 1.00 | 1.69 |
| As | Japan JAOG (§) | ICBDSR | 1988–2007 | 1,958,245 | 245 | n.c. | 1.25 | 1.10 | 1.42 |
| As | United Arab Emirates | ICBDSR | 1996–2003 | 63,606 | 9 | 100.0 | 1.41 | 0.65 | 2.69 |
| Au | Australia Victoria | ICBDSR | 1988–2007 | 1,293,664 | 172 | 37.8 | 1.34 | 1.14 | 1.54 |
| Au | Australia Western | ICBDSR | 1988–2007 | 516,441 | 165 | 38.2 | 3.18 | 2.73 | 3.72 |
Hospital based n.c. = not computable
Prevalence in bold = prevalence of anotia-microtia (microtia I-IV grade)
---- = microtia not registered, the prevalence given is the prevalence of anotia (microtia I) only
Am-N = Northern America, Am C-S = Central & Southern America, Eu-N = Northern Europe, Eu-W = Western Europe, Eu-E = Eastern Europe, Eu-S = Southern Europe, As = Asia, Au = Australia
Elective terminations of pregnancy are not permitted for the following surveillance programs: Ireland Cork and Kerry, Ireland Galway, Ireland SE (EUROCAT); Chile Maule, Costa Rica, Mexico RYVEMCE, Ireland Dublin, Malta, United Arab Emirates, South America ECLAMC (ICBDSR). The values for elective termination of pregnancy are not available and have been substituted by 0 for the calculation of total cases and prevalence rates, for the following surveillance programs: Poland Wielkopolska, Poland (EUROCAT); Canada British Columbia, Canada National, China Beijing, China CBDMN, Italy Sicily, Japan JAOG, Spain ECEMC, Ukraine (ICBDSR); Alaska, Arizona, Colorado, Florida, Illinois, Indiana, Kentucky, Massachusetts, Michigan, Mississippi, Nebraska, New Jersey, New York, Rhode Island, Tennesse, Virginia, Wisconsin (NBDPN). The values for Stillbirths are not available and have been substituted by 0 for the calculation of total cases and prevalence rates, for the following surveillance programs: Alaska, Department of Defense, Florida, Indiana, New Jersey, New York, Rhode Island, Virginia.
Microtia only
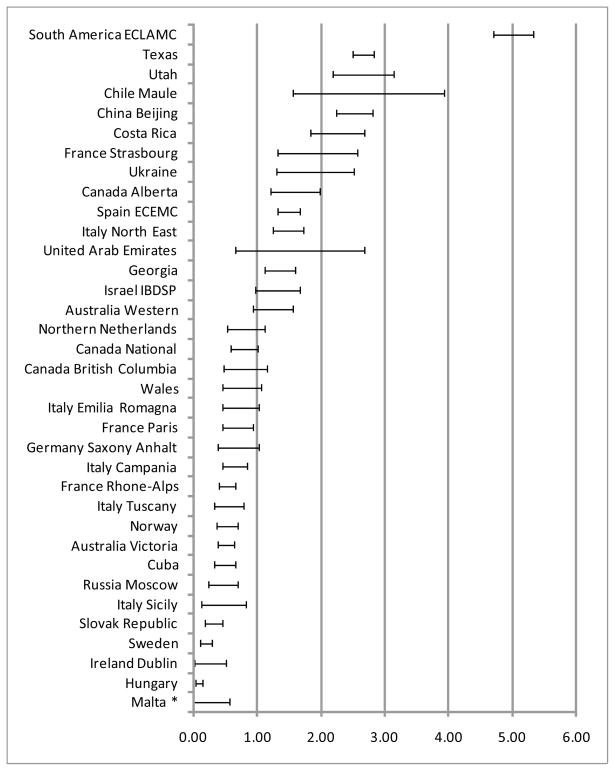
For the 35 surveillance programs that reported microtia-anotia and discriminated cases in microtia and anotia, among 25,802,806 births; 3,993 cases of microtia were diagnosed with an overall prevalence of 1.55 per 10,000 births (CI: 1.50–1.60). The median value was 0.76 with an interquartile range between 0.50 and 1.56 and an overall range between zero (Malta) and 5.01 (South America ECLAMC). The highest rates (last decile) were observed, in order of increasing prevalence, in Chile Maule (2.54), Utah -USA (2.62), Texas-USA (2.66), and South America ECLAMC (5.01) programs (Figure 1).
Figure 1.
Prevalence (per 10,000 births) of microtia across surveillance programs
* Zero case
Anotia only
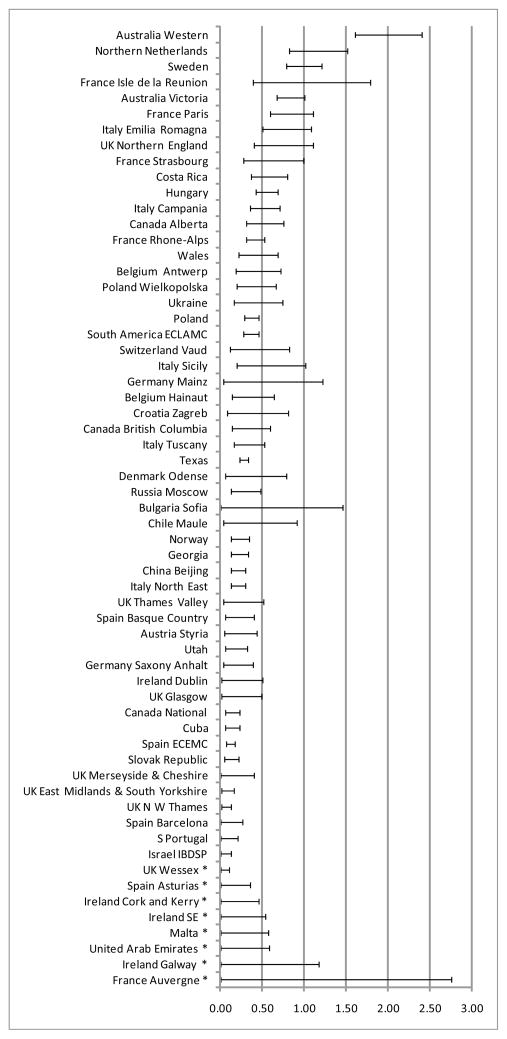
For the 61 surveillance programs that reported only anotia or anotia discriminated from microtia, among 32,893,632 births; 1,184 cases of anotia were diagnosed, with an overall prevalence of 0.36 per 10,000 births (CI: 0.34–0.38). The median value was 0.26 with an interquartile range between 0.11 and 0.39 and overall range between zero, in eight surveillance programs (UK Wessex, Spain Asturias, Ireland Cork and Kerry, Ireland SE, Malta, United Arab Emirates, Ireland Galway, France Auvergne), and 1.98 (Western Australia). The highest rates (last decile) were found, in order of increasing prevalence, in Paris, France (0.82); Victoria, Australia (0.83); Isle de la Reunion, France (0.91); Sweden (0.98); Northern Netherlands (1.13); and Western Australia (1.98) (Figure 2).
Figure 2.
Prevalence (per 10,000 births) of anotia across surveillance programs
* Zero case
Proportion of cases of microtia only
Among the surveillance programs with data for anotia and microtia (n=35), the most frequently observed proportion of microtia was 90% or higher (n=8). This high proportion was observed in USA-Texas, Chile Maule, China Beijing, Spain ECEMC, South America ECLAMC, USA-Utah, Israel IBDSP, and United Arab Emirates. The four surveillance programs with a proportion of microtia less than 40% were Hungary, Sweden, Australia Victoria, and Western Australia (Table 2).
Variation of prevalence of microtia-anotia by large geographical areas and race/ethnicity
The evaluation of prevalence of microtia-anotia by large geographical areas (Table 3) showed a higher prevalence in Central and South America (PR=2.58, CI 2.43–2.74), Asia (PR=1.39, CI 1.31–1.48), Northern Europe (PR=1.13, 1.04–1.23) and a lower rate in Eastern Europe (PR=0.49, CI 0.43–0.56), Southern Europe (PR=0.83, CI 0.76–0.90) and Western Europe (PR=0.88, CI 0.80–0.98) as compared to North America.
Table 3.
Prevalence (per 10,000 births) and prevalence ratio of microtia-anotia by large geographical areas and by race/ethnicity
| No. of programs | No. of cases | Prevalence (per 10,000 births) | Prevalence ratio | 95% CI | ||
|---|---|---|---|---|---|---|
| Geographical areas | ||||||
| Northern America | 33 | 2,944 | 1.76 | 1.00 | Referent | |
| Central and Southern America | 5 | 1,836 | 4.53 | 2.58 | 2.43 | 2.74 |
| Northern Europe | 5 | 608 | 1.99 | 1.13 | 1.04 | 1.23 |
| Western Europe | 5 | 422 | 1.55 | 0.88 | 0.80 | 0.98 |
| Eastern Europe | 4 | 226 | 0.87 | 0.49 | 0.43 | 0.56 |
| Southern Europe | 7 | 677 | 1.46 | 0.83 | 0.76 | 0.90 |
| Asia | 5 | 1,867 | 2.45 | 1.39 | 1.31 | 1.48 |
| Australia | 2 | 337 | 1.86 | 1.06 | 0.95 | 1.19 |
| Ethnicity a | 30 | |||||
| Non-Hispanic White | 775 | 1.13 | 1.00 | Referent | ||
| American Indian or Alaskan Native | 38 | 3.18 | 2.81 | 2.03 | 3.88 | |
| Hispanic | 832 | 2.89 | 2.55 | 2.31 | 2.81 | |
| Asian or Pacific Islander | 117 | 2.08 | 1.83 | 1.51 | 2.23 | |
| Non-Hispanic Black or African | 109 | 0.60 | 0.53 | 0.44 | 0.65 | |
Data available only in 30 surveillance programs of the NBDPN
The analysis by ethnicity, performed only with the NBDPN data, showed a higher prevalence in American Indians or Alaskan Natives (PR=2.81, CI 2.03–3.88), Hispanics (PR=2.55, CI 2.31–2.81) and Asian or Pacific Islanders (PR=1.83, CI 1.51–2.23) and a lower prevalence among non-Hispanic Black or African (PR=0.53, CI 0.44–0.65) as compared to non-Hispanic whites (PR= 1.13) (Table 3).
Variation of prevalence of microtia-anotia by methodological characteristics
Using all programs with data for microtia-anotia the group of eight hospital-based programs showed a higher prevalence as compared to the 58 population-based programs (PR=1.77, CI 1.70–1.85). Among the 8 hospital-based programs there were 3 programs from Central and South America. The comparison of programs which reported ETOPFA with those that did not, showed a lower prevalence in the programs not reporting ETOPFA (PR=0.95, CI 0.90–0.99). In the NBDPN, there was a lower prevalence of microtia-anotia in the surveillance programs with passive ascertainment (PR=0.38, CI 0.35–0.41) (Table 4).
Table 4.
Prevalence (per 10,000 births) and prevalence ratio of microtia-anotia by main surveillance program methodological characteristics
| No. of programs | No. of cases | Prevalence (per 10,000 births) | Prevalence ratio | 95% CI | ||
|---|---|---|---|---|---|---|
| Coverage | ||||||
| Population-based | 58 | 5,089 | 1.68 | 1.00 | Referent | |
| Hospital-based | 8 | 3,828 | 2.97 | 1.77 | 1.70 | 1.85 |
| ETOPFA a | ||||||
| Reported | 34 | 3,975 | 1.84 | 1.00 | Referent | |
| Not reported | 25 | 3,141 | 1.74 | 0.95 | 0.90 | 0.99 |
| Type of surveillance system b | ||||||
| Active | 11 | 1,902 | 2.48 | 1.00 | Referent | |
| Passive | 13 | 630 | 0.94 | 0.38 | 0.35 | 0.41 |
ETOPFA = elective termination of pregnancy for a fetal anomaly. ETOPFA not permitted in seven surveillance programs
Classification used only by the US programs
Discussion
In this study, we conducted a systematic review of the data on the prevalence of microtia-anotia. The data used, although published in the annual reports of the surveillance networks, had not been previously critically analyzed or discussed in the context of the scientific literature on microtia-anotia. Equally important, this study highlights the variation in reporting and classification and the challenges this poses for surveillance and etiologic studies.
This study provides a global assessment of prevalence of microtia-anotia, as observed in 92 surveillance programs in Europe, the Americas, in addition to United Arab Emirates, China, Japan, and Australia. The findings add considerably to the available literature on microtia-anotia. For example, compared to recent studies of microtia-anotia (Canfield et al., 2009; Forrester and Merz, 2005; Harris et al., 1996; Mastroiacovo et al., 1995; Shaw et al., 2004; Suutarla et al., 2007) in which data from different birth defects programs were analyzed, this report examines more than 8,000 cases and adds further years to the assessment of rates from surveillance programs with published data.
For most surveillance programs here analyzed the prevalence was similar to reports from earlier population-based studies conducted in Italy, France, Sweden, Finland and United States (Canfield et al., 2009; Forrester and Merz, 2005; Harris et al., 1996; Shaw et al., 2004; Suutarla et al., 2007), where prevalence rates ranged between 0.83 and 4.34 per 10,000 births (Table 5). There was marked variation in the prevalence of microtia-anotia across surveillance programs, from zero to 7.19 and with the interquartile range between 0.79 and 2.21, with a similar magnitude in variation observed for microtia and anotia when they were analyzed separately. Heterogeneous rates were observed even within countries where there was more than one surveillance program, such as in Italy, US, France and Australia. This variation may have been due to registry methodological artifacts, i.e., differences among the surveillance programs relating to their inclusion criteria and/or their case definition and ascertainment. Our analysis showed a higher prevalence in hospital-based and in active ascertainment surveillance programs, this most probably account for a proportion of the differences in prevalence. Although it is important to note that three, out of the eight, hospital-based programs are in South and Central America, and the Hispanic population has apparently a higher prevalence of microtia. In addition, microtia-anotia is an external anomaly, easily recognized on physical examination after delivery; however, the less severe form of microtia (i.e. type I according to Marx classification) is difficult to define and the term may be used with considerable variability in clinical settings and in medical records. This could lead to under or over reporting of microtia-anotia resulting in a false exaggeration of the geographical variation in prevalence. Although we would expect that anotia is unambiguous, as it is clearly defined as “absence of the ear”, it may also be used incorrectly and explain the wide range in prevalence also observed in anotia.
Table 5.
Prevalence of microtia-anotia reported in the literature from 1960–2010.
| Registry | Age of Ascertainment | Prevalence Microtia-Anotia | Prevalence Anotia | Prevalence Microtia | Types of microtia included (c) | Authors | Year |
|---|---|---|---|---|---|---|---|
| Navajo (USA) | > 21 years old | 9.7 | nr | nr | nr | Jaffe et al | 1967 |
| New Mexico (USA) | Any age | 1.3 | nr | nr | I–IV | Aase et al | 1977 |
| Navajo (USA) | 4–14 years old | 12.0 | nr | nr | nr | Nelson et al | 1984 |
| South America(b) | LB | 3.2 | nr | nr | I–IV | Castilla et al | 1986 |
| Italy | LB+SB | 1.5 | 0.3 | 1.2 | I–IV | Mastroiacovo et al | 1995 |
| Central East France | LB+SB | 0.8 | 0.4 | 0.4 | I–IV | Harris et al (a) | 1996 |
| California (USA) | LB+SB | 2.0 | 0.2 | 1.8 | I–IV | Harris et al (a) | 1996 |
| Sweden | LB+SB | 2.4 | 0.2 | 2.1 | I–IV | Harris et al (a) | 1996 |
| China | LB+SB | 1.4 | nr | nr | nr | Zhu et al | 2000 |
| California (USA) | LB+SB | 2.2 | nr | nr | nr | Shaw et al (a) | 2004 |
| Hawaii (USA) | LB+SB+ETOP | 3.8 | 0.3 | 3.5 | II–IV | Forrester et al | 2005 |
| Chile | LB+SB | 8.8 | 0.5 | 8.3 | I–IV | Nazer et al | 2006 |
| Finland | LB+SB | 4.3 | 0.2 | 4.1 | I–IV | Suurtala et al | 2007 |
| Texas (USA) | LB+SB+ETOP | 2.8 | nr | nr | nr | Husain et al | 2008 |
| Texas (USA) | LB+SB+ETOP | 2.9 | 0.2 | 2.7 | II–IV | Canfield et al | 2009 |
All prevalence rates are per 10,000 births
LB=Livebirth, SB=Stillbirth, ETOPFA=Elective termination of pregnancy for fetal anomalies
nr= not reported
Excluded cases with known chromosomal anomalies
Argentina, Brazil, Chile, Ecuador, Peru, Uruguay, and Venezuela
According to Marx Classification
Our findings also suggest that, with few exceptions, the rates of microtia seem to be higher in the Americas and Asia when compared to Europe. And, within the Americas, we observed an increasing trend towards regions where the proportion of Native American ancestry is higher like Texas and Mexico, compared to Atlanta and Cuba, where the proportion of Native American ancestry is reported to be lower. In the NBDPN data, there was a higher prevalence among Native Americans, Hispanics, and Asians or Pacific Islanders. These results expand on the previous studies in California, Texas, and Hawaii that had previously demonstrated a significantly greater risk for microtia-anotia among the Hispanic and Asian population compared with non-Hispanic African American and Caucasian populations. (Forrester and Merz, 2005; Harris et al., 1996; Husain et al., 2008; Shaw et al., 2004). Based on these observations, we cannot exclude the possibility that some attribute of Hispanics and Asians, either environmental or genetic factors, could explain some of the variability found besides the methodological differences among the surveillance programs.
“Hispanic” is a term used in the United States for people with origins in Spanish–speaking countries from Central and South America living in the US. This term suffers from some flaws such as conveying that there is a high uniformity into this group, i.e., combining Cubans, Mexicans, Peruvians, and Argentineans, among others, in the ethnic Hispanic group. It is well established that the various Hispanic American communities have varying degrees of admixture of three different ancestral roots: Native American, African and European as a consequence of, among other things, the size and number of migratory waves to specific regions and the native population density in the areas being occupied. Some regions had high initial native population density (e.g., Mexico, Central America and the central Andes) while others received large waves of immigration from Europe and Asia very recently (e.g., parts of southern South America). Studies that analyzed genetic ancestry informative markers in individuals who self identified themselves as Hispanics have confirmed that this group represents a continuous along the Europeans and Indigenous Americans axes, and that the term “Hispanic” shows little correspondence with genetic ancestry or even ancestral admixture since not all Hispanics exhibit substantial Native American ancestry (Halder et al., 2008).
Reports of higher prevalence of microtia-anotia in Navajos (Aase and Tegtmeier, 1977; Jaffe, 1969), the second largest Native American tribe of Northern America and the fact that Mexicans, largest percentage of persons of Hispanic descent in the US, have a high proportion of Native ancestry in their population could indicate that Native ancestry may be confounding the association of Hispanic ethnicity and prevalence of microtia-anotia reported in US studies. Furthermore, anthropological, archaeological, linguistic, and molecular data have demonstrated that the peopling of the American continent took place by migrations originating in northeast Asia and entering America via Beringia, giving rise to Alaskan Natives and Amerindians among other populations in the Americas(Jobling et al., 2004). In summary, we are compelled to speculate that the Native American shared gene pool may play a role in the occurrence of microtia-anotia making plausible to consider a role of ethnicity in microtia-anotia; this will be further pursued using ancestry informative markers.
The analysis of microtia and anotia separately, into two clinical subgroups, showed that in most of the surveillance programs, from the total cases of microtia-anotia, above 80% were cases of microtia. This finding is consistent with other studies’ findings where anotia occurs less commonly than microtia, although most studies have observed lower proportions of anotia cases than we did ranging between 2–14% (Canfield et al., 2009; Forrester and Merz, 2005; Harris et al., 1996; Shaw et al., 2004; Suutarla et al., 2007). In Italy and Central-east France, previous studies showed a higher proportion of anotia cases, 22% and 44%, respectively (Harris et al., 1996; Mastroiacovo et al., 1995). In our data set, the prevalence of anotia was unexpectedly much higher than the prevalence of microtia in some surveillance programs; this could be due to underreporting of microtia, a less severe type of birth defect than anotia, or that the term “anotia” is more frequently used by some surveillance programs. Another possibility is a truly high prevalence of anotia in those areas.
The findings of this study should be interpreted cautiously. There were some limitations in case classification, as we were not able to distinguish between isolated, multiple malformed, and syndromic cases. Nevertheless, in a preliminary analysis of the database from ECLAMC, using data from 1982 to 2008, and analyzing only isolated cases, the trend in prevalence remained the same across the countries (Luquetti et al., 2010). A second limitation is that further splitting may have been desirable (e.g., into types I through IV, presence or absence of canal atresia) because of likely differences in etiology and developmental mechanisms. Aggregating such groups (for example, types I to III under “microtia”) in the analysis may have limited the ability to identify difference in prevalence rates that may occur in one of such subgroups. However, limitation in phenotypic details from most surveillance programs made such further splitting not feasible.
A second potential limitation pertains to variable ascertainment among surveillance programs and the associated variations in prevalence rates of congenital anomalies. Programs with active ascertainment presented higher prevalence; which was not surprising as this methodological characteristic is usually related to better validity of data. Lastly, some surveillance program do not report ETOPFA; however we believe that this had minimal impact on the results because the frequency of this birth defect in ETOPFA in the other surveillance programs was very low, accounting for only 4% of the cases. In addition, the comparison between the programs that report ETOPFA and those which do not did not show any significant difference in the prevalence.
On the other hand, there were several strengths to this study. It drew cases from an unprecedented number of live and still births (> 50 million). An additional strength relates to the fact that this is the first descriptive study about microtia-anotia conducted in a global scale. Previous studies reporting higher risk in “Hispanics”, used data on immigrants in the US or US individuals of Hispanic descent from Texas and California, looking at the prevalence of microtia among smaller racial/ethnic groups, i.e. there was a small size and representativeness of the sample. In this study we extended the analysis to surveillance programs based on Spanish speaking countries, like Spain, Costa Rica, Cuba and most countries of South America. We observed a marked difference within these countries that may challenge the current concept of higher risk in the US census broad category defined as “Hispanic”.
As we already mentioned, the prevalence could not be analyzed by type of microtia, because currently few surveillance programs have coding systems that specifies different types of microtia-anotia. Most birth defects surveillance programs, including those from ICBDSR network, handle their congenital anomaly type data coded by either ICD-9 or ICD-10, with or without British Pediatric Association extension. Some surveillance programs classify anotia and microtia in a single code. Both the four-digit ICD-9 classification and the alphanumeric ICD-10 have no information on severity or side(s) affected. Both revisions of ICD have one code for microtia (744.2 and Q17.2) and one code for anotia (744.0 and Q16.0). The British Pediatric Association modified this system to a five-digit code (744.01 for anotia and 744.21 for microtia), however, for microtia-anotia the fifth digit does not provide additional phenotypic refinement. In summary, the ICD coding system is not sufficiently specific in its codes for microtia-anotia. Therefore, the study of sub-phenotypes of microtia and anotia is currently only possible in the material reported from the few surveillance programs with verbatim description and photographic documentation of the reported cases.
The difficulties in further characterization of the heterogeneity of the different sub-phenotypes of microtia-anotia emphasizes the continuing need to strengthen coding training efforts, and, more important, to improve the classification of microtia-anotia to include severity and laterality information. The ICD classification has proven insufficient for the classification of microtia-anotia, as for most birth defects. Therefore, the creation of new codes, or the expansion of codes by adding new digits that would differentiate microtia types I to IV, seems urgent. In order to generate etiological hypotheses research from data from surveillance programs, good phenotypic information must be available. The potential for research, which could lead to primary prevention strategies, based on birth defects surveillance programs require and justify modifications in the ICD. For instance, if the assumption that the different degrees of severity of microtia-anotia represent the same risk factors with different levels of exposure, then studies based on surveillance data or case-control data can test that hypothesis.
Using data from the three birth defects surveillance networks, this study found that there is heterogeneity of the prevalence of microtia-anotia in different areas of the world that is not easily explained by surveillance methodologies differences among surveillance programs. Great care must be taken when comparing prevalence across surveillance programs because differences in ascertainment may be the major source of the variation in rates. However, the magnitude of the difference in the prevalence may indicate that there may be genuine differences in rates in these countries and that the reasons for such variations should, therefore, be further explored. There is a clear need of prospective studies with better phenotype ascertainment, with a case by case analysis to understand better the source of this variability. Additionally we strongly suggest modifications in ICD classification to achieve better phenotypic classification of microtia-anotia.
Acknowledgments
We thank Dr. Adolfo Correa and Dr. Mark A. Canfield for their helpful comments. This work was supported by the Center for Disease Control and Prevention (grant number 1U50DD000524-02) and Seattle Children’s Craniofacial Center Research Fellow grant.
Literature cited
- Aase JM, Tegtmeier RE. Microtia in New Mexico: evidence for multifactorial causation. Birth Defects Orig Artic Ser. 1977;13(3A):113–116. [PubMed] [Google Scholar]
- Alasti F, Van Camp G. Genetics of microtia and associated syndromes. J Med Genet. 2009;46(6):361–369. doi: 10.1136/jmg.2008.062158. [DOI] [PubMed] [Google Scholar]
- Anderka MT, Lin AE, Abuelo DN, et al. Reviewing the evidence for mycophenolate mofetil as a new teratogen: case report and review of the literature. Am J Med Genet A. 2009;149A(6):1241–1248. doi: 10.1002/ajmg.a.32685. [DOI] [PubMed] [Google Scholar]
- Artunduaga MA, Quintanilla-Dieck MD, Greenway S, et al. A Classic Twin Study of External Ear Malformations, Including Microtia. N Engl J Med. 2009;361(12):1216–1218. doi: 10.1056/NEJMc0902556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci S. Familial microtia with meatal atresia in father and son. Turk J Pediatr. 1974;16(3):140–143. [PubMed] [Google Scholar]
- Balci S, Boduroglu K, Kaya S. Familial microtia in four generations with variable expressivity and incomplete penetrance in association with type I syndactyly. Turk J Pediatr. 2001;43(4):362–365. [PubMed] [Google Scholar]
- Canfield MA, Langlois PH, Nguyen LM, et al. Epidemiologic features and clinical subgroups of anotia/microtia in Texas. Birth Defects Res A Clin Mol Teratol. 2009;85:905–913. doi: 10.1002/bdra.20626. [DOI] [PubMed] [Google Scholar]
- Carey JC, Park AH, Muntz HR. External Ear. In: Stevenson RE, editor. Human malformations and related anomalies. Oxford; New York: Oxford University Press; 2006. pp. 329–338. [Google Scholar]
- Castilla EE, Lopez-Camelo JS, Campana H. Altitude as a risk factor for congenital anomalies. Am J Med Genet. 1999;86(1):9–14. doi: 10.1002/(sici)1096-8628(19990903)86:1<9::aid-ajmg3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Castilla EE, Orioli IM. Prevalence rates of microtia in South America. Int J Epidemiol. 1986;15(3):364–368. doi: 10.1093/ije/15.3.364. [DOI] [PubMed] [Google Scholar]
- Chang SO, Choi BY, Hur DG. Analysis of the long-term hearing results after the surgical repair of aural atresia. Laryngoscope. 2006;116(10):1835–1841. doi: 10.1097/01.mlg.0000233703.52308.73. [DOI] [PubMed] [Google Scholar]
- Eavey RD. Microtia and significant auricular malformation. Ninety-two pediatric patients. Arch Otolaryngol Head Neck Surg. 1995;121(1):57–62. doi: 10.1001/archotol.1995.01890010045008. [DOI] [PubMed] [Google Scholar]
- European Surveillance of Congenital Anomalies. 2010 Retrieved 01 February 2010; Available from: http://www.eurocat.ulster.ac.uk.
- Forrester MB, Merz RD. Descriptive epidemiology of anotia and microtia, Hawaii, 1986–2002. Congenit Anom (Kyoto) 2005;45(4):119–124. doi: 10.1111/j.1741-4520.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- González-Andrade F, López-Pulles R, Espín VH, et al. High altitude and microtia in Ecuadorian patients. Journal of Neonatal-Perinatal Medicine. 2010;3(2):109–116. [Google Scholar]
- Gupta A, Patton MA. Familial microtia with meatal atresia and conductive deafness in five generations. Am J Med Genet. 1995;59(2):238–241. doi: 10.1002/ajmg.1320590223. [DOI] [PubMed] [Google Scholar]
- Habiballah JA, Bamousa A. Allograftic and alloplastic auricular reconstruction. Saudi Med J. 2000;21(12):1173–1177. [PubMed] [Google Scholar]
- Halder I, Shriver M, Thomas M, et al. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: Utility and applications. Human Mutation. 2008;29(5):648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- Harris J, Kallen B, Robert E. The epidemiology of anotia and microtia. J Med Genet. 1996;33(10):809–813. doi: 10.1136/jmg.33.10.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A, Frias JL, Gillessen-Kaesbach G, et al. Elements of morphology: standard terminology for the ear. Am J Med Genet A. 2009;149A(1):40–60. doi: 10.1002/ajmg.a.32599. [DOI] [PubMed] [Google Scholar]
- Husain T, Langlois PH, Sever LE, et al. Descriptive epidemiologic features shared by birth defects thought to be related to vascular disruption in Texas, 1996–2002. Birth Defects Res A Clin Mol Teratol. 2008;82(6):435–440. doi: 10.1002/bdra.20449. [DOI] [PubMed] [Google Scholar]
- International Clearinghouse for Birth Defects Surveillance and Research. Annual Report 2008. 2010 Retrieved [2010 01 February 2010; Available from: http://www.icbdsr.org.
- Jaffe BF. Incidence of ear diseases in Navajo Indians. Laryngoscope. 1969;79(12):2126. doi: 10.1288/00005537-196912000-00007. [DOI] [PubMed] [Google Scholar]
- Jobling MA, Hurles M, Tyler-Smith C. In: Human Evolutionary Genetics: Origins, Peoples and Disease. Science G, editor. New York: 2004. p. 523. [Google Scholar]
- Kaye CI, Rollnick BR, Hauck WW, et al. Microtia and associated anomalies: statistical analysis. Am J Med Genet. 1989;34(4):574–578. doi: 10.1002/ajmg.1320340424. [DOI] [PubMed] [Google Scholar]
- Kelley PE, Scholes MA. Microtia and congenital aural atresia. Otolaryngol Clin North Am. 2007;40(1):61–80. vi. doi: 10.1016/j.otc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Klieger-Grossmann C, Chitayat D, Lavign S, et al. Prenatal exposure to mycophenolate mofetil: an updated estimate. J Obstet Gynaecol Can. 2010;32(8):794–797. doi: 10.1016/s1701-2163(16)34622-9. [DOI] [PubMed] [Google Scholar]
- Klockars T, Suutarla S, Kentala E, et al. Inheritance of microtia in the Finnish population. Int J Pediatr Otorhinolaryngol. 2007;71(11):1783–1788. doi: 10.1016/j.ijporl.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Lin L, Pan B, Jiang HY, et al. Study of methylation of promoter of EYA1 gene in microtia. Zhonghua Zheng Xing Wai Ke Za Zhi. 2009;25(6):436–439. [PubMed] [Google Scholar]
- Llano-Rivas I, Gonzalez-del Angel A, del Castillo V, et al. Microtia: a clinical and genetic study at the National Institute of Pediatrics in Mexico City. Arch Med Res. 1999;30(2):120–124. doi: 10.1016/s0188-0128(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Luquetti DV, Cunningham ML, Castilla EE. Prevalence Analysis of 1,379 Cases of Isolated Microtia from South America. 31st Annual David W Smith Workshop on Malformations and Morphogenesis; Alderbrook Resort, Union, WA. 2010. [Google Scholar]
- Ma C, Carmichael SL, Scheuerle AE, et al. Association of Microtia With Maternal Obesity and Periconceptional Folic Acid Use. Am J Med Genet A. 2010;152A(11):2756–2761. doi: 10.1002/ajmg.a.33694. [DOI] [PubMed] [Google Scholar]
- Marx H. Die Missbildungen des ohres. In: Denker AKO, editor. Handbuch der Spez Path Anatomie Histologie. Berlin, Germany: Springer; 1926. p. 131. [Google Scholar]
- Mastroiacovo P, Corchia C, Botto LD, et al. Epidemiology and genetics of microtia-anotia: a registry based study on over one million births. J Med Genet. 1995;32(6):453–457. doi: 10.1136/jmg.32.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks DC, Jahangir A, Shanske AL, et al. Mutational analysis of HOXA2 and SIX2 in a Bronx population with isolated microtia. Int J Pediatr Otorhinolaryngol. 2010;74(8):878–882. doi: 10.1016/j.ijporl.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Nazer J, Lay-Son G, Cifuentes L. Prevalence of microtia and anotia at the maternity of the University of Chile Clinical Hospital. Rev Med Chil. 2006;134(10):1295–1301. doi: 10.4067/s0034-98872006001000012. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Berry RI. Ear disease and hearing loss among Navajo children--a mass survey. Laryngoscope. 1984;94(3):316–323. doi: 10.1288/00005537-198403000-00005. [DOI] [PubMed] [Google Scholar]
- Okajima H, Takeichi Y, Umeda K, et al. Clinical analysis of 592 patients with microtia. Acta Otolaryngol Suppl. 1996;525:18–24. [PubMed] [Google Scholar]
- Osorno G. A 20-year experience with the Brent technique of auricular reconstruction: pearls and pitfalls. Plast Reconstr Surg. 2007;119(5):1447–1463. doi: 10.1097/01.prs.0000258572.57161.d8. [DOI] [PubMed] [Google Scholar]
- Population-based Birth Defects Surveillance data from selected states, 2002–2006. Birth Defects Res A Clin Mol Teratol. 2009;85(12):939–1055. doi: 10.1002/bdra.20638. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Kaidarova Z, et al. Epidemiologic characteristics of anotia and microtia in California, 1989–1997. Birth Defects Res A Clin Mol Teratol. 2004;70(7):472–475. doi: 10.1002/bdra.20042. [DOI] [PubMed] [Google Scholar]
- Strisciuglio P, Ballabio A, Parenti G. Microtia with meatal atresia and conductive deafness: mild and severe manifestations within the same sibship. J Med Genet. 1986;23(5):459–460. doi: 10.1136/jmg.23.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suutarla S, Rautio J, Ritvanen A, et al. Microtia in Finland: comparison of characteristics in different populations. Int J Pediatr Otorhinolaryngol. 2007;71(8):1211–1217. doi: 10.1016/j.ijporl.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Tollefson TT. Advances in the treatment of microtia. Curr Opin Otolaryngol Head Neck Surg. 2006;14(6):412–422. doi: 10.1097/MOO.0b013e328010633a. [DOI] [PubMed] [Google Scholar]
- Yang J, Carmichael SL, Kaidarova Z, et al. Risks of selected congenital malformations among offspring of mixed race-ethnicity. Birth Defects Res A Clin Mol Teratol. 2004;70(10):820–824. doi: 10.1002/bdra.20054. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Zhang J, Yu P, et al. Environmental and Genetic Factors Associated with Congenital Microtia: A Case-Control Study in Jiangsu, China, 2004 to 2007. Plastic and Reconstructive Surgery. 2009;124(4):1157–1164. doi: 10.1097/PRS.0b013e3181b454d8. [DOI] [PubMed] [Google Scholar]