Abstract
Wnt signaling is activated by wounding and participates in every subsequent stage of the healing process from the control of inflammation and programmed cell death, to the mobilization of stem cell reservoirs within the wound site. In this review we summarize recent data elucidating the roles that the Wnt pathway plays in the injury repair process. These data provide a foundation for potential Wnt-based therapeutic strategies aimed at stimulating tissue regeneration.
Endogenous Wnt signaling regulates stem cell recruitment and differentiation during wound repair. Modulation of Wnt signaling (e.g., via small molecules) may be used to therapeutically stimulate tissue regeneration.
The Oxford English Dictionary’s definition of repair is to “restore to good or proper condition by replacing or fixing parts.” In contrast, regeneration is the process of complete renewal, characterized by a full restoration of form and function. Why do some organisms regenerate tissues after injury, while others do not? And is it possible to induce regeneration in cases where repair is the norm? In this article we discuss some of the recent discoveries that have been made by investigators seeking to understand these differences, and how they have used this information to develop therapeutic strategies aimed at stimulating a regenerative response to acute injury.
The “reproduction of lost parts” has long been a subject of intense curiosity. In the 18th century the Swiss naturalist Abraham Trembley demonstrated that when the Cnidarian polyp Hydra was divided into small fragments, each piece “grew again into perfect hydrae” (Trembley 1744). The Scottish biologist and mathematician D’Arcy Thompson was fascinated by these early observations, and wrote that “the ability of an animal to regenerate lost parts [had] excited the interest of crowned heads…ambassadors, and state-couriers, who carried it through Europe” (Thompson 1884). Some years later the Vice President of the British Medical Association voiced a similar interest and wrote, “In these days of strife and stress, when fire and water play havoc with men’s lives and limbs, limb regeneration…would be of inestimable value.” Despite the fact that 238 years have elapsed since those initial observations, our curiosity about regeneration—and the possibility that we could harness this regenerative potential to aid in the healing of our own tissues—has not waned.
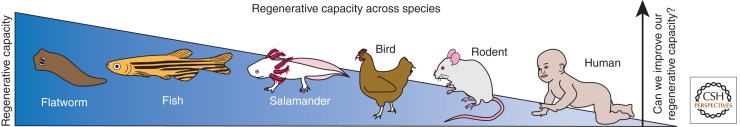
Our own regenerative capacities are remarkably limited. Early observers (Dinsmore 1992) who were interested in distinguishing “repair” from “regeneration” recognized that animals did not respond uniformly to an act of injury. In some cases, adult animals mount an injury response that results in the complete regeneration of the damaged tissue or organ; the appendage of the aquatic axolotl or the fins of a zebrafish are excellent examples (see Fig. 1) (Poss et al. 2000; Straube et al. 2004; Kawakami et al. 2006; Stewart et al. 2009; Satoh et al. 2010). In other cases, the same tissue or organ in a different species had a very limited regenerative response; for example, reptiles seem to lack this regenerative response, at least in their limbs (Galis et al. 2003), but most species retain the ability to regenerate their tails (McLean and Vickaryous 2011). In contrast, most mammalian tissues respond to injury by generating scar tissue (Harty et al. 2003), which is composed of granulation and fibrous tissues that are poorly organized and lack functionality.
Figure 1.
Regenerative capacity across species. The ability to regenerate tissues varies across species. Planarians can regenerate their entire body from a single fragment, fish and salamanders can regenerate their fins and limbs, while mammals have a relatively poor repair mechanism resulting in inadequate tissue replacement and scarring. A common goal of doctors and scientists is to develop biologic therapies to increase mammalian regenerative potential.
One might interpret these observations to mean that an animal’s regenerative capacity is species specific, but this is an oversimplification; some animals begin life with a robust regenerative response that wanes over time. Tadpoles, for instance, exhibit a regenerative capacity only up to metamorphosis; after this developmental event frogs cannot regenerate an amputated limb (Kawakami et al. 2006; Yokoyama et al. 2011). Avian embryos have some ability to regenerate a damaged retina but this potential is lost by the time they are hatchlings (Fischer and Reh 2000; Fischer and Reh 2001). Mammalian embryos also appear to possess an early regenerative ability that erodes by birth (Rinkevich et al. 2011). Why is there such variation in regenerative ability of animals, and between different stages of postnatal life? Such a question has obvious clinical importance, because if we understood the key features that distinguish human healing from that of our amphibian ancestors, then we could potentially “jump-start” a similar regenerative program (Kragl et al. 2009).
The observation that “simple animals” such as sponges and flatworms regenerate, whereas more complex organisms do not (Gurtner et al. 2008), is the basis for some theories that propose there is an association between regenerative capacity and tissue complexity (Sanchez Alvarado 2000). Many examples challenge this theory: the teleost, avian, and mammalian retinas exhibit very similar cellular architecture (Reh and Levine 1998). Yet fish can completely regenerate the neural retina (Cameron et al. 1999; Cameron 2000; Raymond et al. 2006), as can birds up to the hatchling stage (Coulombre and Coulombre 1965, 1970), but rodents and primates retain no such inherent regenerative capacity (Tropepe et al. 2000). Another theory proposes that regenerative abilities wane as the immune system matures (Mescher and Neff 2005, 2006). This theory is largely based on the observation that fetal wounds heal without scar formation, and this developmental period is associated with an immature immune system. A third theory posits that the loss of regenerative ability has evolved to curtail inappropriate cell division and malignant transformation (Gardiner 2005; Levesque et al. 2010). Perhaps most relevant to humans is the observation that across multiple species, regenerative capacity diminishes with age (reviewed in Silva and Conboy 2008; but see Eguchi et al. 2011 for conflicting data). The inference from these observations is that aging depletes a stem cell population from which new body parts arise. But this interpretation has been repeatedly challenged, which leaves open an obvious question: if they are not from a reservoir, where do the cells come from that reform the missing or damaged tissues?
New data demonstrate that adult cells at the edges of the wound dedifferentiate to generate the new tissues (Straube et al. 2004; Kragl et al. 2009; Azevedo et al. 2011). This feature is not unique to mammals: The wound blastema of an axolotl is composed of tissue-specific progenitor cells that arise from the partial dedifferentiation of neurons, cartilage, and muscle (Kragl et al. 2009). What factors are responsible for stimulating this dedifferentiation of adult cells to a stemlike state or the recruitment of stem/progenitor cells into the regenerating tissue? Growing evidence implicates the Wnt pathway in this critical event.
In the following sections we present a summation of recent data supporting a model whereby the act of injury triggers the endogenous Wnt pathway, and that this pathway activity is essential for subsequent healing. Most data suggest that within a damaged tissue, the endogenous Wnt signal activates tissue-resident stem cells, and these cells contribute to the repair and/or regeneration of the damaged tissue. Augmenting this endogenous Wnt signal appears to enhance the healing response; consequently, a number of approaches are being tested that aim to activate Wnt signaling in a local, transient manner to stimulate tissue regeneration in humans.
WNT SIGNALING IS NECESSARY FOR TISSUE REGENERATION
Generally speaking, when Wnt signaling is reduced in an animal with robust regenerative capacities, their inherent regenerative abilities are impaired. For example, blocking Wnt signaling in planarians disrupts the polarity of the regenerate, resulting in inappropriate anatomical generation (i.e., the posterior half of the flatworm regenerates another posterior [tail] segment, and the anterior half generates another anterior [head] segment) (Gurley et al. 2008; Petersen and Reddien 2008, 2009; Yazawa et al. 2009). Likewise, blocking Wnt signaling after amputation of the dorsal fin in zebrafish impairs their normal fin regeneration (Kawakami et al. 2006). Inhibiting Wnt signaling in the eyes of animals with continually regenerating retinas results in an abrupt cessation in this regenerative ability (Kubo et al. 2003; Cho and Cepko 2006; Stephens et al. 2010; Ramachandran et al. 2011).
Which aspects of these regenerative responses are Wnt dependent? The early consensus seems to be that Wnt signaling blockade disrupts the recruitment of stem/progenitor cells to the injury site (Liu et al. 2007b; Das et al. 2008; Denayer et al. 2008; Stephens et al. 2010; Ramachandran et al. 2011) and adversely affects the proliferative phase of the healing process (Qyang et al. 2007; Stoick-Cooper et al. 2007). The formation of a blastema, the mass of multipotent cells at the tip of the wounded limb (Bryant et al. 2002), depends upon recruitment and proliferation (Agata et al. 2007). Consequently, perturbations in Wnt signaling in these regenerating animals manifest as disruptions in the aggregation of morphologically undifferentiated cells that comprise the blastema.
In mammalian organs and tissues with limited regenerative capacity, Wnt signaling is still necessary for the repair process. For example, when Wnt signaling is blocked after skeletal fracture, the result is a nonunion of the injured bone (Chen et al. 2007; Kim et al. 2007; Leucht et al. 2008a). Inhibition of Wnt signaling during skin wounding prevents formation of epithelial appendages including hair and sweat glands, which results in prominent scarring of the epidermis (Ito et al. 2007). Myocardial infarctions in mammals produce a region of scar tissue surrounding the occluded vessels and if Wnt signaling is repressed, the scarring worsens and the result is myocardial rupture (Chen et al. 2004).
If Wnt signaling is required for tissue regeneration, can elevating Wnt signaling in animals with limited regenerative capacity improve the healing response? Current data support this hypothesis. For example, when the limb of a postmetamorphic frog is amputated, the result is a “spike,” an outgrowth that lacks any discernible digits. This spike can be converted into a fully functional limb, complete with all appendages, provided that Wnt signaling is elevated at the time of amputation (Kawakami et al. 2006) (although, see Yokoyama et al. 2011 for a differing opinion). Mammalian skin wounds typically heal by scarring; however, elevating Wnt signaling within the wound site leads to the growth of new hair follicles, a hallmark of a fully functional epidermis (Ito et al. 2007).
Even in diseases that are characterized by tissue destruction, elevating Wnt signaling can induce a repair of sorts. For example, multiple myeloma is a cancer of the plasma cells in the bone marrow that is distinguished by severe osteolytic lesions (Sezer 2005). This osteolysis has been linked to elevated levels of Dkk1 in the bone marrow cavity (Tian et al. 2003; Kaiser et al. 2008). Phase I/II clinical trials are now under way to test the efficacy of enhancing Wnt signaling via antibody-mediated repression of Dkk1 as a means to stimulate new bone formation in multiple myeloma patients (Yaccoby et al. 2007; Fulciniti et al. 2009). Likewise, some inflammatory bowel diseases are characterized by reduced Wnt pathway activity (Wehkamp et al. 2007) and reduced turnover of stem cells in the intestinal crypt (Sato et al. 2009). In these types of debilitating chronic injury states, transiently elevating Wnt signaling may be beneficial (reviewed in Anderson and Wong 2010). Indeed, such a Wnt-based therapeutic approach has been attempted. Oral mucositis is a frequent occurrence following chemotherapy, and is characterized by inflammation and ulceration of the mucosal lining of the digestive tract. Investigators demonstrated that they could stimulate regeneration of the mucous membranes in mice using systemic administration of the Wnt agonist, R-spondin (Zhao et al. 2009a).
Collectively, these data demonstrate that endogenous Wnt signaling is a prerequisite for tissue repair, but there are obvious caveats. Most experimental methods used to study Wnt signaling in tissue healing rely on techniques that, in general, produce unrestrained Wnt pathway activation. A well-documented effect of constitutively active Wnt signaling, however, is cancer (Chan et al. 1999; de Lau et al. 2007; Vermeulen et al. 2010), so experimental methods to test the role of Wnt signaling in healing must take this into account. Likewise, increasing Wnt signaling, via genetic deletion of negative regulators such as Ror2 (Brunetti-Pierri et al. 2008) and Axin2 (Liu et al. 2007a; Minear et al. 2010), or mutations in Wnt coreceptors such as Lrp6 (Boyden et al. 2002) or Wnt ligands themselves (Liang et al. 2003), have their own adverse sequelae. Clearly, what is required is a means to activate the pathway in a local, transient manner with the goal of restoring the endogenous Wnt response that is necessary for normal healing. But when should the Wnt pathway be activated? And what, precisely, does Wnt signaling contribute to the injury repair response? The answers to these questions will undoubtedly inform strategies that aim to take advantage of the Wnt pathway for regenerative medicine therapies, and thus we review recent data addressing these questions in the following paragraphs.
WNT SIGNALING AT THE INITIAL STAGE OF THE INJURY RESPONSE
When the body is injured, a cascade of events is set into motion that have as their most fundamental objective the cessation of bleeding, followed by an attempt to wall off the injured tissues from the rest of the body to maintain homeostasis. This vascular response occurs within minutes of tissue damage, and is largely due to the hypoxic condition that develops in tissues deprived of a vascular supply.
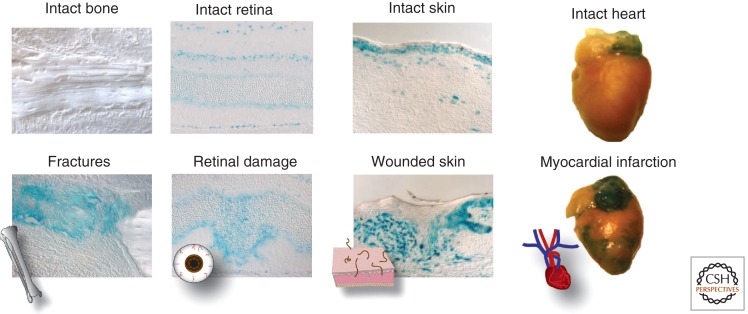
The activation of β-catenin-dependent Wnt signaling appears to be one of the initial molecular responses to injury. This activation is typically rapid and spatially restricted, in most cases to the site of damage. For example, severing a planarian in half leads to the rapid up-regulation in Wnt gene expression specifically at the cut site (Petersen and Reddien 2009). Wnt signaling is also initiated by bone fracture (Chen et al. 2007; Kim et al. 2007; Leucht et al. 2008a), by exposure to ionizing radiation (Gurung et al. 2009), by inhalation injury to the lung (Villar et al. 2011; also reviewed in Beers and Morrisey 2011), skin wounding, laser damage to the retina, myocardial infarction, and stroke (see Fig. 2, showing unpublished results from multiple laboratories).
Figure 2.
Wnt signaling is activated in response to injury. Damage in most tissues up-regulates Wnt signaling transiently at the injury site. While too much Wnt signaling has been shown to be deleterious, a number of studies have shown that the exogenous addition of Wnt signaling stimulates healing in a number of different injuries, including bone fractures, retinal damage, skin wounding, and myocardial infarction. (Wnt response seen here in blue, by X-gal staining of murine AxinLacZ/+ tissue.) (Heart images are thanks to Professor Joseph Wu.)
What features of injury lead to activation of the endogenous Wnt pathway? One intriguing possibility is oxygen tension: Embryonic stem cells—but not differentiated cells—respond to hypoxic conditions by up-regulating the expression of hypoxia-inducible factor (HIF)-1α and in turn, HIF1α regulates expression of the Wnt target genes Lef1 and Tcf1 (Mazumdar et al. 2010). If this in vitro observation is validated in vivo, then changes in oxygen availability and, by extension, HIF-1α activity, may be the direct trigger between injury and activation of the Wnt pathway.
INFLAMMATION AND WNT SIGNALING
Within minutes of tissue injury, platelets aggregate at a wound site to form a clot that both protects surrounding tissues from the invasion of microorganisms, and acts as a reservoir for growth factors that will eventually serve to recruit cells to repair the damage (reviewed in Midwood et al. 2004; Nurden et al. 2008). Although the role of Wnt signaling in platelet regulation during wound healing in vivo is unclear, one study suggests that β-catenin-dependent Wnt signaling inhibits platelet aggregation, at least in vitro (Steele et al. 2009).
Within hours of injury, the inflammatory response ensues: Neutrophils, monocytes, and lymphocytes invade the wound bed in an attempt to phagocytose cell debris and release growth factors and cytokines (Newton et al. 2004; Martin and Leibovich 2005). The inflammatory response is crucial for fighting infection but a state of chronic inflammation is largely considered to be detrimental, both to healing and to the program of regeneration. Some speculate that the mammalian immune system has evolved in such a way as to optimize tissue repair through fibrosis but that these modifications have been detrimental to the program of tissue regeneration (Mescher and Neff 2005). However, direct demonstration of a causal relationship between the inflammatory response and tissue regeneration is lacking. By selectively depleting cells of the neutrophil lineage from a wound site, investigators demonstrated that inflammation can retard wound closure (Dovi et al. 2003; Martin and Leibovich 2005), but how this affects tissue regeneration remains unknown.
The role(s) of Wnt signaling in the inflammatory response is poorly understood. β-catenin-independent Wnt signaling may be a proinflammatory stimulus, based on the observation that Wnt5a expression is increased in the sera of patients suffering from severe sepsis (Pereira et al. 2009). β-Catenin-independent Wnt signaling has also been implicated in a number of chronic inflammatory diseases such as rheumatoid arthritis (Sen et al. 2000) and atherosclerosis (Polzer et al. 2008), but in these cases a causal link between Wnt function and etiology of the inflammatory disease has not been established.
β-Catenin-dependent Wnt signaling, on the other hand, may inhibit or restrict the inflammatory response, based on the observation that inhibitors of the pathway increase the expression of some genes associated with inflammation (Kim et al. 2010). A more direct link has come with the demonstration that by elevating Wnt signaling through inhibition of Dkk1, the bone-resorbing phenotype observed in a mouse model of rheumatoid arthritis could be replaced with the bone-forming pattern of osteoarthritis (Diarra et al. 2007; reviewed in Baker-LePain et al. 2011). The theory that a reduction in Wnt signaling causes inflammatory bone loss is further supported by the observation that osteolytic lesions in multiple myeloma are associated with unrestricted Dkk1 activity (Tian et al. 2003). But it must be emphasized that these effects of Wnt signaling are largely attributed to a pro-osteogenic influence and not because Wnt signaling directly impacts inflammatory mediators. For example, in the condition of rheumatoid arthritis, tumor necrosis factor (TNF) contributes to the disease pathology because the cytokine increases the activity of bone-resorbing osteoclasts; Wnt signals counter this effect by stimulating osteoblast activity.
WNT SIGNALING AND THE PROLIFERATIVE PHASE OF WOUND REPAIR
Within days or months of an injury, the proliferative phase develops, and it is here that the difference between repair and regeneration becomes most obvious. In the repair program, granulation tissue accumulates at the wound site, which is composed of new blood vessels and highly proliferative fibroblasts that produce copious amounts of fibronectin, collagen type III, glycosaminoglycans, elastin, glycoprotein, proteoglycans and hyaluronan (Buchanan et al. 2009). This matrix appears to support cell migration but it is transient; gradually, a collagen type III-rich matrix that provides tensile strength to the wound site replaces the granulation tissue. In the regeneration program, the hyaluronan content of the extracellular matrix is much higher, and investigators speculate that this may curtail or reduce that amount of collagen deposited at the wound site by overproliferative fibroblasts (Carre et al. 2010). In support of this theory, investigators showed that hyaluronan removal results in fibrotic scarring (West et al. 1997), and that Wnt3a induces hyaluronan synthesis (Larson et al. 2010).
In the intestine, Wnt signaling maintains crypt architecture (reviewed in Clevers 2006; Sansom et al. 2007; van der Flier and Clevers 2008) and does so by being a positive stimulus for proliferation. Investigators demonstrated that the Wnt inhibitor Dkk1 is induced by inflammatory cytokines during colitis. Dkk1 causes further tissue damage by stimulating epithelial cell apoptosis. Blocking Dkk1 function results in elevated Wnt signaling and the induction of cell proliferation, which promotes wound repair after colitis (Koch et al. 2011). The experimental approach in this particular study, however, resulted in abolition of Dkk1 function and therefore unrestricted Wnt signaling, which had its own set of side effects, namely, hyperproliferation of the epithelial cells lining the crypt (Koch et al. 2011). These and other studies in skin wound healing (Ito et al. 2007) and bone formation (French et al. 2004; Friedman et al. 2009) and repair (Kim et al. 2007) emphasize that the proliferative effects of β-catenin-dependent Wnt signaling must be transient and localized to be beneficial in the wound repair/regeneration process.
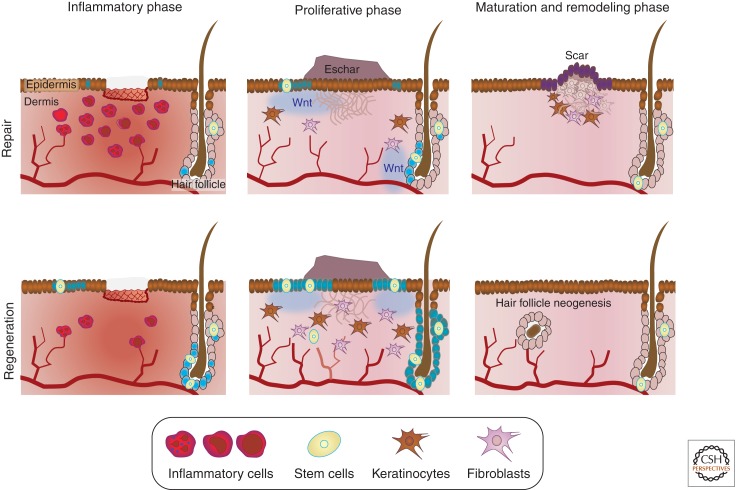
In the final stages of wound repair, extensive extracellular matrix remodeling occurs. In repair, the granulation tissue is converted to mature scar tissue through collagen synthesis and catabolism (Chang et al. 2004). Type III collagen is gradually degraded and is replaced by the stronger type I collagen. Proapoptotic signals at the injury site can regulate collagen degradation both by decreasing fibroblast numbers and by initiation of collagenase activity (Rai et al. 2005). These collagen fibers are rearranged, cross-linked, and aligned providing increased strength to the wound. Over time, this densely packed collagen develops into an inelastic white collagen scar. Regeneration, on the other hand, has no such fibrous tissue. A schematic of the stages of wound repair is shown in Figure 3.
Figure 3.
Wnt signaling drives tissue repair. In both repair and regeneration, wound healing is characterized by three main phases. During the inflammatory phase there is an influx of inflammatory cells and local Wnt signaling begins to increase. During the proliferative phase a scab (eschar) is formed and the wound is reepithelialized. This phase includes an increased local Wnt response, extracellular matrix deposition, angiogenesis, and the recruitment and proliferation of multiple cell types including stem cells, keratinocytes, and fibroblasts. The third phase, the maturation and remodeling phase, is characterized by extensive extracellular matrix remodeling. Whereas repair leads to scarring, or functionally inadequate tissue, increased Wnt signaling during regeneration leads to a reduced inflammatory response, increased angiogenesis, increased cellular proliferation and recruitment, as well as differences in matrix composition, leading to the complete reconstruction of the original tissue architecture. (Increase in blue color represents an increase in Wnt signaling.)
CHRONIC INJURY, CANCER, AND WNT SIGNALING
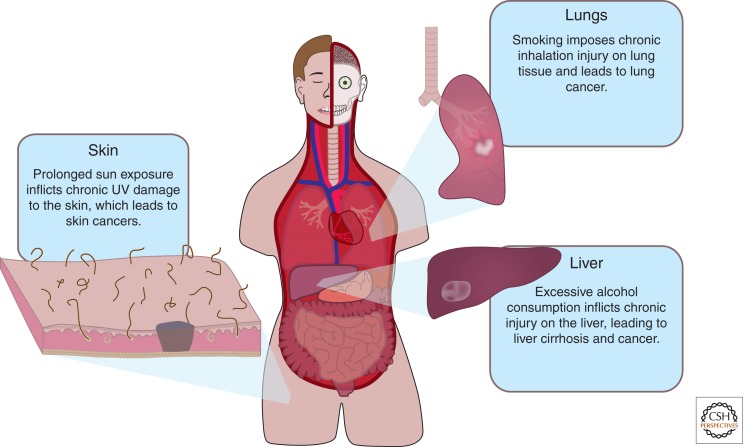
Multiple lines of evidence demonstrate that overactivation of the Wnt pathway causes hyperproliferation and cancer (reviewed in Barker and Clevers 2006). The majority of these cancers are due to loss of function mutations in Wnt pathway inhibitors. There are also clear examples of an intimate relationship between chronic injury and cancer initiation (see Fig. 4). For example, smoking inflicts chronic injury of the trachea and lung tissue, resulting in lung cancer (Kersting et al. 2000), extensive sun exposure leads to prolonged inflammation of the dermis and can result in melanomas and other skin cancers (El Ghissassi et al. 2009), and chronic alcohol consumption causes inflammation and death of hepatocytes, which can result in liver cancer (McKillop and Schrum 2005).
Figure 4.
Does chronic injury lead to prolonged Wnt signaling and cancer? Injury results in a local increase in Wnt signaling and chronic injury leads to cancer. Many cancers also have up-regulated Wnt signaling. The Wnt pathway is best known to promote proliferation; an intriguing possibility is that chronic injury may induce a prolonged Wnt response leading to uncontrolled cell proliferation and cancer. In the lungs, cigarette smokers inflict chronic injury to the trachea and lung tissue, which may result in lung cancer. Relating to the skin, prolonged UV exposure leads to chronic inflammation of the dermis, which may result in skin cancer. In the liver, chronic alcohol consumption subjects the tissue to constant damage, which may lead to liver cancer.
Abundant data demonstrate that injury locally activates Wnt signaling (see previous paragraphs), but does chronic injury cause inappropriately prolonged Wnt signaling, thus heightening the risk of cell transformation at the site of injury? Growing data support this hypothesis: Wnt1 is overexpressed in nonsmall cell lung carcinoma (He et al. 2004) and some melanoma cell lines exhibit abnormally high levels of β-catenin (Rubinfeld et al. 1997). More recently, investigators demonstrated that melanoma cells have evolved a mechanism to activate β-catenin-dependent signaling that does not require high levels of Wnt ligands (Sinnberg et al. 2010; reviewed in Vaid et al. 2011). Thus, the connection between protracted, repeated injury, persistent Wnt pathway activation, and tumorogenesis is compelling, and represents a novel hypothesis to explain the molecular etiologies of injury-related cancers.
HARNESSING THE WNT PATHWAY FOR THERAPEUTIC APPLICATIONS IN INJURY REPAIR
There is significant interest in finding ways in which the Wnt pathway can be modulated for therapeutic purposes (see Fig. 5). A number of small molecules have been identified that can activate or inhibit the pathway but thus far, they all depend upon Wnt ligands to exert their effects (reviewed in Leucht et al. 2008b). Recombinant proteins such as the Wnt agonist R-spondin (Kazanskaya et al. 2004; Kim et al. 2008) have been used to treat oral mucositis (Zhao et al. 2009a), and recombinant Wnt3a protein has been used to transiently elevate Wnt signaling in bone injury sites and in cases of implant osseointegration (Zhao et al. 2009b; Minear et al. 2010; Popelut et al. 2010). In these cases, the hydrophobic Wnt protein has been packaged into a lipid vesicle that maintains its activity in vivo (Morrell et al. 2008).
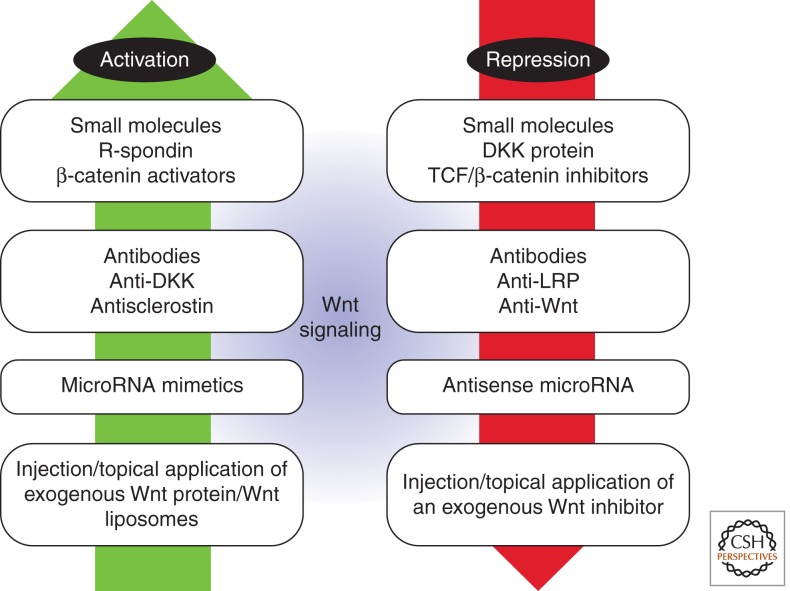
Figure 5.
Harnessing Wnt signaling for therapeutic applications in injury repair. There are many biological ways to activate or repress the Wnt signaling pathway; these may be therapeutic options for increasing a healing response or reducing uncontrolled proliferation. The use of small molecules, microRNAs, genetically modified cells, as well as ligands and inhibitors, are all potential future therapeutics for both activating and repressing the Wnt signaling pathway.
Anti-Dkk1 antibodies have been employed to treat the osteolytic lesions associated with multiple myeloma (Fulciniti et al. 2009), and antisclerostin antibodies are in phase I trials for the treatment of rheumatoid arthritis (Choi et al. 2009). Although microRNAs and small interfering RNAs have been identified that effectively block activity of Wnt pathway components (Korpal et al. 2008; Thatcher et al. 2008; Hashimi et al. 2009), it is unclear whether they have the ability to regulate Wnt signaling at sites of injury. Such an approach is likely to be challenging since a single miRNA can have multiple mRNA targets, each target can be regulated by several miRNAs, and the effects of a specific miRNA are variable depending on the cell context.
CONCLUDING REMARKS
Converting tissue repair to tissue regeneration remains a lofty goal, but growing evidence suggests it is a realistic objective. We now know that almost every adult tissue harbors stem cells with potential to regenerate damaged or diseased tissues. But there are problems: adult stem cells are usually found in low abundance, and their response to stress and aging typically diminishes their ability to self-renew and proliferate. Endogenous Wnt signaling regulates stem cell recruitment and differentiation during the wound repair process, which suggests that modulation of Wnt signaling can positively influence the injury repair process. Strict attention will have to be paid to controlling Wnt signaling in a spatial and temporal manner in order to avoid the well-documented untoward effects of unrestrained Wnt pathway activity.
ACKNOWLEDGMENTS
We thank Wilfred Manzano and Edward Wang for their help in compiling the manuscript. This work is supported by grants from the California Institute for Regenerative Medicine (CIRM) to J.A.H. and to A.A.S.
Footnotes
Editors: Roel Nusse, Xi He, and Renee van Amerongen
Additional Perspectives on Wnt Signaling available at www.cshperspectives.org
REFERENCES
- Agata K, Saito Y, Nakajima E 2007. Unifying principles of regeneration I: Epimorphosis versus morphallaxis. Dev Growth Differ 49: 73–78 [DOI] [PubMed] [Google Scholar]
- Anderson EC, Wong MH 2010. Caught in the Akt: Regulation of Wnt signaling in the intestine. Gastroenterology 139: 718–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo AS, Grotek B, Jacinto A, Weidinger G, Saude L 2011. The regenerative capacity of the zebrafish caudal fin is not affected by repeated amputations. PLoS ONE 6: e22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain JC, Nakamura MC, Lane NE 2011. Effects of inflammation on bone: An update. Curr Opin Rheumatol 23: 389–395 [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H 2006. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov 5: 997–1014 [DOI] [PubMed] [Google Scholar]
- Beers MF, Morrisey EE 2011. The three R’s of lung health and disease: Repair, remodeling, and regeneration. J Clin Invest 121: 2065–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346: 1513–1521 [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Del Gaudio D, Peters H, Justino H, Ott CE, Mundlos S, Bacino CA 2008. Robinow syndrome: Phenotypic variability in a family with a novel intragenic ROR2 mutation. Am J Med Genet A 146A: 2804–2809 [DOI] [PubMed] [Google Scholar]
- Buchanan EP, Longaker MT, Lorenz HP 2009. Fetal skin wound healing. Adv Clin Chem 48: 137–161 [DOI] [PubMed] [Google Scholar]
- Cameron DA 2000. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis Neurosci 17: 789–797 [DOI] [PubMed] [Google Scholar]
- Cameron DA, Vafai H, White JA 1999. Analysis of dendritic arbors of native and regenerated ganglion cells in the goldfish retina. Vis Neurosci 16: 253–261 [DOI] [PubMed] [Google Scholar]
- Carre AL, James AW, MacLeod L, Kong W, Kawai K, Longaker MT, Lorenz HP 2010. Interaction of wingless protein (Wnt), transforming growth factor-β1, and hyaluronan production in fetal and postnatal fibroblasts. Plast Reconstr Surg 125: 74–88 [DOI] [PubMed] [Google Scholar]
- Chan EF, Gat U, McNiff JM, Fuchs E 1999. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet 21: 410–413 [DOI] [PubMed] [Google Scholar]
- Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO 2004. Gene expression signature of fibroblast serum response predicts human cancer progression: Similarities between tumors and wounds. PLoS Biol 2: E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wu Q, Guo F, Xia B, Zuo J 2004. Expression of Dishevelled-1 in wound healing after acute myocardial infarction: Possible involvement in myofibroblast proliferation and migration. J Cell Mol Med 8: 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA 2007. β-Catenin signaling plays a disparate role in different phases of fracture repair: Implications for therapy to improve bone healing. PLoS Med 4: e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Cepko CL 2006. Wnt2b/β-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development 133: 3167–3177 [DOI] [PubMed] [Google Scholar]
- Choi Y, Arron JR, Townsend MJ 2009. Promising bone-related therapeutic targets for rheumatoid arthritis. Nat Rev Rheumatol 5: 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H 2006. Wnt/β-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ 1965. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol 12: 79–92 [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ 1970. Influence of mouse neural retina on regeneration of chick neural retina from chick embryonic pigmented epithelium. Nature 228: 559–560 [DOI] [PubMed] [Google Scholar]
- Das AV, Bhattacharya S, Zhao X, Hegde G, Mallya K, Eudy JD, Ahmad I 2008. The canonical Wnt pathway regulates retinal stem cells/progenitors in concert with Notch signaling. Dev Neurosci 30: 389–409 [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Clevers H 2007. WNT signaling in the normal intestine and colorectal cancer. Front Biosci 12: 471–491 [DOI] [PubMed] [Google Scholar]
- Denayer T, Locker M, Borday C, Deroo T, Janssens S, Hecht A, van Roy F, Perron M, Vleminckx K 2008. Canonical Wnt signaling controls proliferation of retinal stem/progenitor cells in postembryonic Xenopus eyes. Stem Cells 26: 2063–2074 [DOI] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, et al. 2007. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13: 156–163 [DOI] [PubMed] [Google Scholar]
- Dinsmore CE 1992. The foundations of contemporary regeneration research: Historical perspectives. Monogr Dev Biol 23: 1–27 [PubMed] [Google Scholar]
- Dovi JV, He LK, DiPietro LA 2003. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol 73: 448–455 [DOI] [PubMed] [Google Scholar]
- Eguchi G, Eguchi Y, Nakamura K, Yadav MC, Millan JL, Tsonis PA 2011. Regenerative capacity in newts is not altered by repeated regeneration and ageing. Nat Commun 2: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, et al. 2009. A review of human carcinogens—part D: Radiation. Lancet Oncol 10: 751–752 [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA 2000. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol 220: 197–210 [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA 2001. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci 4: 247–252 [DOI] [PubMed] [Google Scholar]
- French DM, Kaul RJ, D’Souza AL, Crowley CW, Bao M, Frantz GD, Filvaroff EH, Desnoyers L 2004. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol 165: 855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MS, Oyserman SM, Hankenson KD 2009. Wnt11 promotes osteoblast maturation and mineralization through R-spondin 2. J Biol Chem 284: 14117–14125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, et al. 2009. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114: 371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis F, Wagner GP, Jockusch EL 2003. Why is limb regeneration possible in amphibians but not in reptiles, birds, and mammals? Evol Dev 5: 208–220 [DOI] [PubMed] [Google Scholar]
- Gardiner DM 2005. Ontogenetic decline of regenerative ability and the stimulation of human regeneration. Rejuvenation Res 8: 141–153 [DOI] [PubMed] [Google Scholar]
- Gurley KA, Rink JC, Sanchez Alvarado A 2008. β-Catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319: 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT 2008. Wound repair and regeneration. Nature 453: 314–321 [DOI] [PubMed] [Google Scholar]
- Gurung A, Uddin F, Hill RP, Ferguson PC, Alman BA 2009. β-Catenin is a mediator of the response of fibroblasts to irradiation. Am J Pathol 174: 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, Mescher AL 2003. Regeneration or scarring: An immunologic perspective. Dev Dyn 226: 268–279 [DOI] [PubMed] [Google Scholar]
- Hashimi ST, Fulcher JA, Chang MH, Gov L, Wang S, Lee B 2009. MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differentiation. Blood 114: 404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM 2004. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia 6: 7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G 2007. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316–320 [DOI] [PubMed] [Google Scholar]
- Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, et al. 2008. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol 80: 490–494 [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Marti M, Dubova I, Izpisua Belmonte JC 2006. Wnt/β-catenin signaling regulates vertebrate limb regeneration. Genes Dev 20: 3232–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W 2004. R-Spondin2 is a secreted activator of Wnt/β-catenin signaling and is required for Xenopus myogenesis. Dev Cell 7: 525–534 [DOI] [PubMed] [Google Scholar]
- Kersting M, Friedl C, Kraus A, Behn M, Pankow W, Schuermann M 2000. Differential frequencies of p16(INK4a) promoter hypermethylation, p53 mutation, and K-ras mutation in exfoliative material mark the development of lung cancer in symptomatic chronic smokers. J Clin Oncol 18: 3221–3229 [DOI] [PubMed] [Google Scholar]
- Kim JB, Leucht P, Lam K, Luppen C, Ten Berge D, Nusse R, Helms JA 2007. Bone regeneration is regulated by Wnt signaling. J Bone Miner Res 22: 1913–1923 [DOI] [PubMed] [Google Scholar]
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, et al. 2008. R-Spondin family members regulate the Wnt pathway by a common mechanism. Molec Biol Cell 19: 2588–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim J, Kim DW, Ha Y, Ihm MH, Kim H, Song K, Lee I 2010. Wnt5a induces endothelial inflammation via β-catenin-independent signaling. J Immunol 185: 1274–1282 [DOI] [PubMed] [Google Scholar]
- Koch S, Nava P, Addis C, Kim W, Denning TL, Li L, Parkos CA, Nusrat A 2011. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology 141: 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y 2008. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 283: 14910–14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM 2009. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460: 60–65 [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S 2003. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development 130: 587–598 [DOI] [PubMed] [Google Scholar]
- Larson BJ, Longaker MT, Lorenz HP 2010. Scarless fetal wound healing: A basic science review. Plast Reconstr Surg 126: 1172–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Helms JA 2008a. β-Catenin-dependent Wnt signaling in mandibular bone regeneration. J Bone Joint Surg Am 90 Suppl 1: 3–8 [DOI] [PubMed] [Google Scholar]
- Leucht P, Minear S, Ten Berge D, Nusse R, Helms JA 2008b. Translating insights from development into regenerative medicine: The function of Wnts in bone biology. Semin Cell Dev Biol 19: 434–443 [DOI] [PubMed] [Google Scholar]
- Levesque M, Villiard E, Roy S 2010. Skin wound healing in axolotls: A scarless process. J Exp Zool B Mol Dev Evol 314: 684–697 [DOI] [PubMed] [Google Scholar]
- Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN 2003. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 4: 349–360 [DOI] [PubMed] [Google Scholar]
- Liu B, Yu HM, Hsu W 2007a. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of β-catenin in proliferation and differentiation. Dev Biol 301: 298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xu S, Wang Y, Mazerolle C, Thurig S, Coles BL, Ren JC, Taketo MM, van der Kooy D, Wallace VA 2007b. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol 308: 54–67 [DOI] [PubMed] [Google Scholar]
- Martin P, Leibovich SJ 2005. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol 15: 599–607 [DOI] [PubMed] [Google Scholar]
- Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC 2010. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol 12: 1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop IH, Schrum LW 2005. Alcohol and liver cancer. Alcohol 35: 195–203 [DOI] [PubMed] [Google Scholar]
- McLean KE, Vickaryous MK 2011. A novel amniote model of epimorphic regeneration: The leopard gecko, Eublepharis macularius. BMC Dev Biol 11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Neff AW 2005. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol 93: 39–66 [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW 2006. Limb regeneration in amphibians: Immunological considerations. Sci World J. 6 Suppl 1: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood KS, Williams LV, Schwarzbauer JE 2004. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 36: 1031–1037 [DOI] [PubMed] [Google Scholar]
- Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA 2010. Wnt proteins promote bone regeneration. Sci Transl Med 2: 29ra30. [DOI] [PubMed] [Google Scholar]
- Morrell NT, Leucht P, Zhao L, Kim JB, ten Berge D, Ponnusamy K, Carre AL, Dudek H, Zachlederova M, McElhaney M, et al. 2008. Liposomal packaging generates Wnt protein with in vivo biological activity. PLoS ONE 3: e2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Watson JA, Wolowacz RG, Wood EJ 2004. Macrophages restrain contraction of an in vitro wound healing model. Inflammation 28: 207–214 [DOI] [PubMed] [Google Scholar]
- Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E 2008. Platelets and wound healing. Front Biosci 13: 3532–3548 [DOI] [PubMed] [Google Scholar]
- Pereira CP, Bachli EB, Schoedon G 2009. The wnt pathway: A macrophage effector molecule that triggers inflammation. Curr Atheroscler Rep 11: 236–242 [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW 2008. Smed-βcatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319: 327–330 [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW 2009. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci 106: 17061–17066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzer K, Diarra D, Zwerina J, Schett G 2008. Inflammation and destruction of the joints—The Wnt pathway. Joint Bone Spine 75: 105–107 [DOI] [PubMed] [Google Scholar]
- Popelut A, Rooker SM, Leucht P, Medio M, Brunski JB, Helms JA 2010. The acceleration of implant osseointegration by liposomal Wnt3a. Biomaterials 31: 9173–9181 [DOI] [PubMed] [Google Scholar]
- Poss KD, Shen J, Keating MT 2000. Induction of lef1 during zebrafish fin regeneration. Dev Dyn 219: 282–286 [DOI] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, et al. 2007. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/β-catenin pathway. Cell Stem Cell 1: 165–179 [DOI] [PubMed] [Google Scholar]
- Rai NK, Tripathi K, Sharma D, Shukla VK 2005. Apoptosis: A basic physiologic process in wound healing. Int J Low Extrem Wounds 4: 138–144 [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D 2011. Ascl1a/Dkk/β-catenin signaling pathway is necessary and glycogen synthase kinase-3β inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci 108: 15858–15863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ 2006. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Levine EM 1998. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol 36: 206–220 [PubMed] [Google Scholar]
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL 2011. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476: 409–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P 1997. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 275: 1790–1792 [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A 2000. Regeneration in the metazoans: Why does it happen? Bioessays 22: 578–590 [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR 2007. Myc deletion rescues Apc deficiency in the small intestine. Nature 446: 676–679 [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Satoh A, Cummings GM, Bryant SV, Gardiner DM 2010. Regulation of proximal-distal intercalation during limb regeneration in the axolotl (Ambystoma mexicanum). Dev Growth Differ 52: 785–798 [DOI] [PubMed] [Google Scholar]
- Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA 2000. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci 97: 2791–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezer O 2005. Myeloma bone disease. Hematology 10 Suppl 1: 19–24 [DOI] [PubMed] [Google Scholar]
- Silva H, Conboy IM 2008. Aging and stem cell renewal. In StemBook, Cambridge, MA: [PubMed] [Google Scholar]
- Sinnberg T, Menzel M, Kaesler S, Biedermann T, Sauer B, Nahnsen S, Schwarz M, Garbe C, Schittek B 2010. Suppression of casein kinase 1α in melanoma cells induces a switch in β-catenin signaling to promote metastasis. Cancer Res 70: 6999–7009 [DOI] [PubMed] [Google Scholar]
- Steele BM, Harper MT, Macaulay IC, Morrell CN, Perez-Tamayo A, Foy M, Habas R, Poole AW, Fitzgerald DJ, Maguire PB 2009. Canonical Wnt signaling negatively regulates platelet function. Proc Natl Acad Sci 106: 19836–19841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens WZ, Senecal M, Nguyen M, Piotrowski T 2010. Loss of adenomatous polyposis coli (apc) results in an expanded ciliary marginal zone in the zebrafish eye. Dev Dyn 239: 2066–2077 [DOI] [PubMed] [Google Scholar]
- Stewart S, Tsun ZY, Izpisua Belmonte JC 2009. A histone demethylase is necessary for regeneration in zebrafish. Proc Natl Acad Sci 106: 19889–19894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT 2007. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134: 479–489 [DOI] [PubMed] [Google Scholar]
- Straube WL, Brockes JP, Drechsel DN, Tanaka EM 2004. Plasticity and reprogramming of differentiated cells in amphibian regeneration: Partial purification of a serum factor that triggers cell cycle re-entry in differentiated muscle cells. Cloning Stem Cells 6: 333–344 [DOI] [PubMed] [Google Scholar]
- Thatcher EJ, Paydar I, Anderson KK, Patton JG 2008. Regulation of zebrafish fin regeneration by microRNAs. Proc Natl Acad Sci 105: 18384–18389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA 1884. The regeneration of lost parts in animals. Mind 9: 415–420 [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr 2003. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349: 2483–2494 [DOI] [PubMed] [Google Scholar]
- Trembley A 1744. Memoires pour servir a l’histoire d’un genre de polypes d’eau douce, a bras en forme de cornes. Verbeek, Leide [Google Scholar]
- Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D 2000. Retinal stem cells in the adult mammalian eye. Science 287: 2032–2036 [DOI] [PubMed] [Google Scholar]
- Vaid M, Prasad R, Sun Q, Katiyar SK 2011. Silymarin targets β-catenin signaling in blocking migration/invasion of human melanoma cells. PLoS ONE 6: e23000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- van der Flier LG, Clevers H 2008. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260 [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. 2010. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 12: 468–476 [DOI] [PubMed] [Google Scholar]
- Villar J, Cabrera NE, Valladares F, Casula M, Flores C, Blanch L, Quilez ME, Santana-Rodriguez N, Kacmarek RM, Slutsky AS 2011. Activation of the Wnt/β-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS ONE 6: e23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Wang G, Kubler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M, et al. 2007. The Paneth cell α-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-4. J Immunol 179: 3109–3118 [DOI] [PubMed] [Google Scholar]
- West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT 1997. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int J Biochem Cell Biol 29: 201–210 [DOI] [PubMed] [Google Scholar]
- Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr 2007. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood 109: 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa S, Umesono Y, Hayashi T, Tarui H, Agata K 2009. Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proc Natl Acad Sci 106: 22329–22334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Maruoka T, Ochi H, Aruga A, Ohgo S, Ogino H, Tamura K 2011. Different requirement for Wnt/β-catenin signaling in limb regeneration of larval and adult Xenopus. PLoS ONE 6: e21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kim KA, De Vera J, Palencia S, Wagle M, Abo A 2009a. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/β-catenin pathway. Proc Natl Acad Sci 106: 2331–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Rooker SM, Morrell N, Leucht P, Simanovskii D, Helms JA 2009b. Controlling the in vivo activity of Wnt liposomes. Methods Enzymol 465: 331–347 [DOI] [PubMed] [Google Scholar]