Abstract
Among various types of ion species, carbon ions are considered to have the most balanced, optimal properties in terms of possessing physically and biologically effective dose localization in the body. This is due to the fact that when compared with photon beams, carbon ion beams offer improved dose distribution, leading to the concentration of the sufficient dose within a target volume while minimizing the dose in the surrounding normal tissues. In addition, carbon ions, being heavier than protons, provide a higher biological effectiveness, which increases with depth, reaching the maximum at the end of the beam's range. This is practically an ideal property from the standpoint of cancer radiotherapy. Clinical studies have been carried out in the world to confirm the efficacy of carbon ions against a variety of tumors as well as to develop effective techniques for delivering an efficient dose to the tumor. Through clinical experiences of carbon ion radiotherapy at the National Institute of Radiological Sciences and Gesellschaft für Schwerionenforschung, a significant reduction in the overall treatment time with acceptable toxicities has been obtained in almost all types of tumors. This means that carbon ion radiotherapy has meanwhile achieved for itself a solid place in general practice. This review describes clinical results of carbon ion radiotherapy together with physical, biological and technological aspects of carbon ions.
Keywords: radiotherapy, carbon ions, protons, dose distribution, relative biological effectiveness
INTRODUCTION
The primary principle of radiotherapy (RT) lies on precise localization of sufficient dose in the target lesion while minimizing the damage to the surrounding normal tissues. The success of the treatment therefore largely depends on the performance and capacity of the accelerator, treatment planning system and other related devices, as well as the quality of the radiation beams employed. This was proved by the fact that when the energy of photons (X-rays and gamma rays) reached the order of megavoltage in the 1950s, which marked the beginning of a modern RT, a significant improvement in local control (LC) had been obtained. The question is then raised whether improved LC could be associated with improved survival, despite the fact that many patients eventually succumb to distant metastases. There has been ample evidence that in a large number of malignancies, the local recurrence or relapse is correlated with distant metastasis and the impact of improved LC on survival is mediated via a reduction in deaths caused by local progression and a reduction in distant metastasis (1).
In this context, ion beams such as protons and carbon ions, when compared with photons, provide beneficial dose distribution and, in the case of carbon ions being heavier than protons, a larger relative biological effectiveness (RBE), leading to a higher probability of tumor control with the lesser volume of the surrounding normal tissues irradiated and reduction in the frequency and severity of radiation morbidity.
CHARACTERISTICS OF CARBON IONS
Physical Aspects
When compared with photons and fast neutrons, which are characterized by an exponential absorption of dose with depth, ion beams demonstrate an increase in energy deposition with penetration depth up to the sharp maximum at the end of their range, known as a Bragg peak. The peak is typically narrow, a few millimeters at the 80% level, and the dose at the peak is several times greater than the dose in the plateau. The particle range is determined by the energy of the incoming particles.
The quality of dose distribution is affected by the energy spread and range straggling, whose magnitude is smaller for carbon ions than protons, as well as by the degree of lateral sharpness (penumbra) that is dependent upon the Coulomb scattering and becomes smaller with increasing the mass of particles (2). Therefore, when comparing dose distributions between carbon ion beams and proton beams, the lateral fall-off around the target volume is more rapid in carbon ion beams than proton beams. In the region beyond the distal end of the peak, however, almost no dose is deposited in protons but a small dose in carbon ions because the primary carbon ions undergo nuclear interactions and fragment into particles with lower atomic number, producing a fragmentation tail beyond the peak. The biological effect of this fragmentation tail is small because the tail contains only fragments with low atomic number.
Since the original peak is too narrow and sharp to be used directly for the treatment of lesions with different shapes and sizes, broadening of the narrow peak is necessary to conform to the size and shape of the lesions. This has been achieved in two ways: a beam-scattering method with a passive beam delivery system (2,3) and a beam-scanning method with an active beam delivery system (4,5). In a beam-scattering method, the narrow peaks are swept over an extended region by a ridge filter to create the spread-out Bragg peak (SOBP) corresponding to the size of the target volume (Fig. 1). The shape of the ridge filter is designed to apply a gradient to the physical dose in order to achieve a uniform cell killing or isoeffect over the SOBP. For placing the SOBP precisely to the target, a combination of a range modulator, collimator and compensator is used in this method. In a beam-scanning method, on the other hand, the peak position is dynamically moved within the target by changing the beam energy in the accelerator or changing the beam penetration using absorbers, by which the sufficient dose can be precisely conformed to the target volume.
Figure 1.
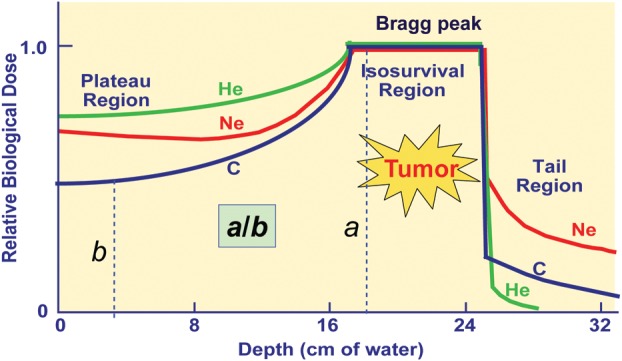
Dose distributions of ion beams. The ionization density increases with depth and the relative biological effectiveness (RBE) increases as they travel deeper in the body. The ratio of the RBE for the peak to plateau of carbon ions becomes larger than that of proton beams.
Radiobiological Aspects
The rate at which particle beams lose energy when penetrating into the tissue increases with the mass of the particles and is known as linear energy transfer (LET). Photons, electrons and protons are sparsely ionizing radiations and referred to as low-LET radiations, while fast neutrons and carbon ions are densely ionizing high-LET radiations. The LET has been used to evaluate the biological effects of radiations based on the fact that as the LET increases, the RBE also increases (6,7). Here, the RBE is defined as the ratio of two doses of different radiations given under identical conditions including dose fractionation and the tissue irradiated. In contrast to neutron beams whose LET remains uniformly high at any depth, the LET of carbon ion beams increases steadily with increasing the depth to reach the maximum in the peak region. This property is extremely advantageous from the therapeutic point of view, as the RBE of carbon ion beams also increases as they advance deeper to the tumor-lying region (Fig. 1).
Tepper et al. (8) and Goldstein et al. (9) investigated RBE values for single and fractionated doses for jejunal crypt cell survival after irradiation with various ions at different positions of the SOBP. They observed an increasing effectiveness of ions with increasing ion charge (and mass). As the ion mass increased, the increase in the RBE was first observed in the peak region and then extended to the plateau region. When different ion species were compared, carbon ions were characterized by the highest peak-to-plateau RBE ratio, which was also confirmed in early skin reactions of the mouse after carbon ion irradiation (10,11). Carbon ion beams are therefore considered to have the ‘best balance’ in terms of both the physical dose distribution and biological effect. This opens up a promising potential for the highly effective use of carbon ions in such tumors that are deeply located and resistant to photon beams.
The tumors with low radioresponsiveness against low-LET radiations are assumed to have a high proportion of hypoxic cells, poor reoxygenation pattern and high intrinsic repair capacity. It is also assumed that such tumors could benefit from high-LET radiations because the reduction in the oxygen enhancement ratio (OER) is achieved with increasing LET, together with the reduction in differences in radiosensitivity related to the position of the cells in the cell cycle, providing the rationale of introducing high-LET carbon ions in cancer therapy. Local values for the RBE can be as high as 2.0–3.5 for carbon ions and depend on many factors, which have to be addressed during the treatment planning.
CLINICAL ADVANTAGES OF CARBON IONS
Improved Therapeutic Gain
Although the RBE of high-LET carbon ions is greater than that of low-LET protons or photons, the clinical interest lies in the existence of differential effects between the tumor and normal tissue that favor the normal tissue. In this regard, radiobiological advantages expected from the use of carbon ions are: the repair of radiation damage is less, repopulation of the tissue is suppressed, OER is reduced and cell cycle dependency of radiosensitivity is reduced. These characteristics become the maximum at the peak region and, combined with improved physical dose localization, may play a major role in improving the therapeutic ratio of carbon ion beams when compared with proton and photon beams.
Batterman et al. (12) investigated the relationship between the RBE values and the volume-doubling time in lung metastases after fast neutron irradiation. They found that the RBE of fast neutron beams was larger with greater volume-doubling time and that neutron beams had a higher RBE value than photon beams for slow-growing tumors such as salivary gland tumor, prostate cancer and bone/soft tissue sarcomas. In the case of salivary gland tumors, the RBE for fractionated RT was found to be ∼8.0 compared with the values in the range of 3.0–3.5 expected for late damage in most normal tissues. Laramore et al. (13) summarized those tumor types and clinical situations in which fast neutron therapy offered an advantage. These findings could also be applied to carbon ion beams whose RBE is similarly high as that of fast neutron beams, and thereby, carbon ion RT could be effective against locally advanced, photon-resistant tumors as well as those located near critical structures.
Hypofractionated Radiotherapy
Because of its unique physical and biological properties, it is theoretically possible in carbon ion RT to perform hypofractionated RT with significantly smaller number of fractions than has been used in standard photon RT. Experiments with fast neutron beams have demonstrated that increasing the dose per fraction tended to lower the RBE for both tumor and normal tissues, but the RBE for the tumor did not decrease as rapidly as the RBE for normal tissues (14). These experiments led to the assumption that the therapeutic ratio would increase rather than decrease, even though the fraction dose was increased. Similar results have been obtained in experiments conducted with carbon ions (11,15), providing the biological rationale for the validity of the short-course, hypofractionated regimen in carbon ion RT.
Progress in dose escalation has been made at the National Institute of Radiological Sciences (NIRS) in Japan on a scale that permits the RT for Stage I lung cancer and liver cancer to be completed in one and two fractions, respectively. Even for other tumors such as head and neck cancer, prostate cancer and bone/soft tissue sarcomas that generally require a relatively prolonged irradiation time, it has been possible to accomplish the definitive treatment in16 fractions over 4 weeks in carbon ion RT. Currently, the average number of fractions and the treatment time per patient is 13 fractions in ∼3 weeks without enhancing toxicity (16–18).
Potential Suppression of Metastases
Ogata et al. (19) reported that carbon ion irradiation induced DNA damage, which possibly suppressed the metastatic capabilities of tumor cells, leading to suppression of pulmonary metastases in vivo. They postulated that the suppression of metastases might have been caused by carbon ion irradiation producing a higher proportion of double-strand DNA breaks than does X-ray irradiation. Their findings have been also confirmed by Tamaki et al. (20) and Akino et al. (21). This may be an advantage of carbon ion RT, although further studies are warranted to confirm these findings. Secondary cancer induction after neutron and carbon ion RT, however, is of particular concern since valid clinical data are not yet available.
TREATMENT PLANNING
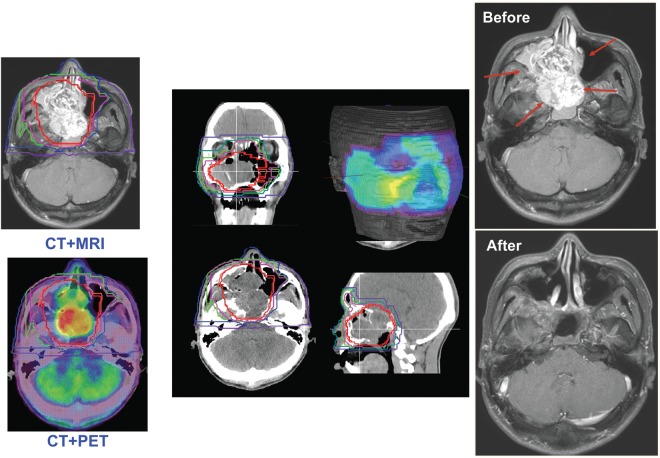
The first preparatory procedure to ensure the proper administration of carbon ion RT is the fabrication of immobilization devices for each particular patient. Computed tomography (CT) scans for treatment planning are then taken with the patient wearing these devices. For the determination of target volume, fusion images using CT, magnetic resonance imaging and positron emission tomography images have been frequently employed (Fig. 2). For the treatment of moving organs, the respiratory-gated irradiation devices have also been applied at the time of CT scans (22). At present, the respiration-synchronized irradiation is only feasible in a passive method but is under development in an active scanning method. The CT image data obtained in this manner are then transferred to the treatment planning system. At this stage, the irradiation parameters in terms of the number of irradiation portals and their directions are determined in conjunction with delineation of the target volume.
Figure 2.
In treatment planning, fusion images using the computed tomography, magnetic resonance imaging and positron emission tomography are commonly used (left). Dose distributions of the different planes and facial surface (middle). The patient with adenoid cystic cancer before and after carbon ion radiotherapy (right).
Treatment planning for carbon ion RT has been performed by the beam-scattering method (broad beam) developed at NIRS and the beam-scanning method developed at the Gesellschaft für Schwerionenforschung (GSI). The NIRS approach is based on clinical experience with high-LET neutron beams, in which the estimation of the clinically relevant RBE values is implemented as a two-step procedure; biological RBE is distinguished from the ‘clinical RBE’. For shaping the SOBP, the human salivary gland (HSG) tumor cell line was selected as an in vitro model system because the initial patients to receive carbon ion RT were those with adenocarcinomas (3). The GSI approach for selection of the RBE is based on the Local Effect Model, which allows the calculation of the biological effectiveness essentially based on two sets of input data, including physical characterization for radiation fields and biological characterization for the response of the cells or tissues, parameterized by a modified linear-quadratic approach (23). The treatment planning for the NIRS approach is performed by HIPLAN (24).
CARBON ION THERAPY FACILITIES
Historically, ion beam RT was begun using proton beams at the Lawrence Berkeley National Laboratory (LBNL) in 1954. Since then, the efficacy of heavier charged nuclei such as helium, carbon, nitrogen, neon, silicon and argon had been assessed for clinical use at LBNL. The major pioneering work for heavy ions was done at LBNL between 1977 and 1992, in which most patients were treated with helium and neon ions (25,26). In 1994, the clinical study on Carbon ion RT was started in NIRS using carbon ions generated by HIMAC, which was the world's first accelerator complex dedicated to cancer therapy. At present, there are more than 30 proton therapy facilities in operation, while carbon ion RT is performed in 5 facilities worldwide. Following the HIMAC/NIRS, the GSI in Darmstadt, Germany, started carbon ion RT in 1997, which terminated clinical application and was succeeded by Heidelberg Ion-Beam Therapy Center (HIT) in 2009. The HIT is the facility having both protons and carbon ions for clinical use (27). The Hyogo Ion Beam Medical Center (HIBMC) in Japan, established in 2001, is the first facility dedicated to proton/carbon treatment. At the Institute of Modern Physics (IMP) in Lanzhou, China, clinical trials have been performed since 2006, where carbon ion beams with energy up to 100 MeV/u have been supplied only for the treatment of superficial tumors. Based on technological research and development at NIRS, a downsized carbon-ion facility was realized as the Gunma University Heavy Ion Medical Center (GHMC), Gunma University in Japan, where a clinical study took place in 2010 (28).
In the Foundation CNAO, Italy, the accelerator complex was completed for proton/carbon treatment, in which the clinical study on proton therapy started in October 2011, and carbon ion RT is due to start in about 1 year. Under the license agreement between the GSI and the vendor company, proton/carbon facilities modeled on HIT are under construction in Marburg and Kiel, Germany, as well as in Shanghai, China. At present, however, there is uncertainty whether these institutions will be really built as has been planned. There are four more institutions with a carbon ion facility under construction: two in Japan, one in Austria and one in Lanzhow, China. Among them, the SAGA-HIMAT in Tosu, Japan, is unique in terms of its construction being based on a public–private partnership. The other facility is Kanagawa Cancer Center, Japan, in which both a passive and an active beam delivery system will be installed.
CLINICAL RESULTS OF CARBON ION RT
By the end of 1988, a total of 239 patients received a minimum neon physical dose of 10 Gy (median follow-up for survivors 32 months) at LBNL. Compared with historical results, the improved outcome was obtained for several types of tumors including advanced or recurrent macroscopic salivary gland carcinomas, paranasal sinus tumors, advanced soft tissue sarcomas, macroscopic sarcomas of the bone, locally advanced prostate carcinomas and biliary tract carcinomas (25,26). However, the treatment results of malignant gliomas, pancreatic, gastric, esophageal, lung and advanced or recurrent head and neck cancer appeared no better than those achieved with conventional X-ray therapy. Unfortunately, clinical research at LBNL was terminated in 1992 as the result of budget constraints in addition to the aging of the machine.
Stimulated by the promising results of ion beam therapy at LBNL, carbon ion RT was started at NIRS in 1994, where all patients have been treated within prospective Phase I/II or Phase II studies. In dose-escalation Phase I/II studies, the same rule of fixing both the total number of fractions and overall treatment time has been employed, and the total dose has been escalated in incremental steps of 5 or 10%. When the recommended dose was thus established in the Phase I/II study, it was then used in the following Phase II study. Since 1994, more than 6500 patients have been treated with carbon ions, demonstrating the benefit of carbon ion RT over other modalities in various types of tumors in terms of high LC and survival rates. A significant reduction in the overall treatment time with acceptable toxicities has been achieved in most cases (16–18). At GSI, the first patient was treated in 1997 using the beam line of GSI's heavy-ion synchrotron that had been primarily used for physics research. The clinical study at GSI was terminated in 2008; until then, over 450 patients were treated with carbon ions. The main indications were patients with chordomas and chondrosarcomas of the skull base, locally advanced adenoid cystic carcinomas (ACCs) as well as the chordoma and chondrosarcoma of the sacrum and prostate cancer (27).
Head and Neck Tumors
Results of proton therapy for head and neck tumors are difficult to evaluate because it has been frequently used as a boost therapy or combined with surgery with a variety of pathological types reported as a whole (29,30). Carbon ion RT has been found to offer radiobiological advantages in histologically non-squamous cell tumors such as ACC, adenocarcinoma, malignant melanoma and various sarcomas in prospective dose-escalation trials (31–33).
Adenoid cystic carcinoma
ACC is a rare form of adenocarcinoma, which is a broad term describing any cancer arising from glandular tissues. ACC is found mainly in the head and neck region and most commonly occurs in the salivary glands. Regardless of where it starts, ACC tends to spread along nerves (perineural invasion) or through the bloodstream. The most common site of metastasis is the lung. It spreads to the lymph nodes in only ∼5–10% of cases. The results of treatment for photon RT or surgery ranged from 27 to 72% for the 5-year LC rate and from 25 to 85% for the 5-year survival rate (34,35). In Table 1, an experience of proton therapy on 23 patients is reported, in which about half of the patients were treated with surgical resection followed by proton irradiation (36). There were three Grade III and one Grade IV late toxicities (17% ≥ Grade III late reactions). In contrast, there were no patients with ≥Grade III toxicities for a total of 151 patients with locally advanced ACC involving the oropharynx and the paranasal sinus treated with carbon ion RT alone at NIRS (32). The 5-year LC rate was 74% in spite of including 78 cases (52%) of T4 and 40 cases (26%) with recurrent tumors after surgery and/or chemotherapy. For 32 patients who seemed to be locally operable, the 5-year LC rate was improved to 96%. At GSI, patients with paranasal sinus cancers were treated with combined stereotactic photon RT to the clinical target volume, followed by a carbon ion boost to the gross target volume (33). Locoregional control rates for the combined photon and carbon ions were better than those observed in historical series of patients treated with photon intensity-modulated RT (IMRT) alone, although the difference was not statistically significant.
Table 1.
Adenoid cystic carcinomas of the head and neck
| Institutions | No. of patients | Treatment | 5-year local control (%) | 5-year overall survival (%) | Late ≥GIII injury |
|---|---|---|---|---|---|
| Iowa, 2009 (34) | 54 | Surgery alone | 72 | 85 | — |
| 10 | Photon alone | 27 | 25 | — | |
| Florida, 2004 (35) | 101 | Photon alone | 56 | 57 | 12.9% |
| MGH, 2006 (36) | 23 | Proton ± surgery | 93 | 77 | 17% |
| Heidelberg, 2001 (37) | 29 | Neutron ± surgery | 75 | 59 | 19% |
| GSI, 2005 (33) | 34 | Photon alone | 25 (4 years) | 78 (4 years) | <5% |
| 29 | Photon + carbon boost | 78 (4 years) | 76 (4 years) | ||
| NIRS, 2011 (32) | 151 | Carbon alone (all pats) | 74 | 72 | None |
| 32 | Carbon alone (T1–T3) | 96 | 92 | ||
| 119 | Carbon alone (T4 or recurrences) | 71 | 69 |
Mucosal malignant melanoma
Mucosal malignant melanoma of the head and neck (MMHN) is a rare type of tumor. Radical local excision used to be the treatment that provided a chance of cure, although the prognosis was generally grave. Adding RT to surgery in tumors that could be radically excised did not confer any statistically significant advantage in reducing local recurrence or improving survival (38–40). There has been no reported data for proton therapy, possibly because MMHN is considered to be resistant to proton beams. From 1997 to 2010, a total of 198 patients with MMHN were treated with carbon ions with or without chemotherapy at NIRS (32). In the initial study, 102 patients were treated with carbon ion RT alone with 57.6 GyE in 16 fractions over 4 weeks (41). The 5-year survival rate was 35%, which was similar to the most favorable results of surgery combined with or without RT or chemotherapy. This study strongly suggested the need of adding systemic therapy for the prevention of distant metastasis. Therefore, in the subsequent study for 96 patients, concomitant chemotherapy (DAV: Day 1: dacarbazine (DTIC) 120 mg/m2 + nimustine 70 mg/m2 + vincristine 0.7 mg/m2; Days 2–5: DTIC 120 mg/m2) with a 4-week interval, a total of five courses, was administered for the first course at the start of carbon ion RT, second course at the completion of RT and three courses thereafter (32). Although the LC rate remained almost the same, the 5-year survival rate improved by 23% and became 58% (Table 2). These results would be the best so far reported in the literature.
Table 2.
Mucosal malignant melanomas of the head and neck
Bone and soft tissue sarcomas of the head and neck
Bone and soft tissue sarcomas of the head and neck are rare mesenchymal malignant neoplasms accounting for <10% of all bone and soft tissue sarcomas and ∼1% of all head-and-neck neoplasms. Wide resection with enough safety margins is usually difficult, and delivery of high radiation dose is limited by the vicinity of critical normal tissue structures including the spinal cord, brain stem and eyes. Accordingly, the LC rates for these tumors are lower compared with the extremities (42). In resectable cases, the 5-year LC rate of combined surgery and RT, surgery alone and RT alone are 60–70, around 54 and 43–50%, respectively (43); however, in unresectable sarcomas, the prognosis is miserable (44). There are no reported data for proton therapy as a sole treatment, possibly because these types of tumors are considered difficult to treat with low-LET protons. In a dose-escalation study on carbon ion RT conducted at NIRS, the 5-year LC rate using 64.0 or 57.6 GyE (n = 16) was only 24%, whereas for the patients receiving 70.4 GyE in 16 fractions (n = 39), the 5-year LC rate was improved significantly to 73% and the 5-year survival rate was 48% with acceptable toxicities (32,45). These results would be the best so far reported in the literature for unresectable sarcomas.
Skull Base and Upper Cervical Spine Tumors
Major types of tumors of the skull base and the upper cervical spine include chordoma, chondrosarcoma, olfactory neurogenic tumor and meningioma, for which complete resection is rarely achieved because of the vicinity to the critical normal structures (46). They are generally resistant to photon beams. While improvements have been achieved with proton and helium ion therapy, the variance of LC rates among different centers is significant, possibly due to patient selection and differences in treatment techniques employed (47–52). It is of note that in the case of chordoma, there are still many cases who develop recurrences even after 5 years. Munzenrider and Liebsch (48) reported that the LC rate was 73% at 5 years after proton therapy, whereas it was only 54% at 10 years. In this regard, carbon ion RT holds a promising potential of improving long-term results, most likely due to increased biological effects of carbon ions as well as the sharp lateral fall-off permitting better sparing of critical organs (53–55).
A total of 76 patients with skull base and paracervical tumors including 44 chordoma, 14 chondrosarcoma, 9 olfactory neuroblastoma, 7 malignant meningioma, 1 giant cell tumor and 1 neuroendocrine carcinoma were treated with carbon ions at NIRS. There were no patients who developed serious acute (Grade ≥4) or late (Grade ≥3) reactions. The 5-year LC and survival rates for all 76 patients were 88 and 82%, respectively (55).
In the current study, at NIRS for 47 chordoma patients who received 60.8 GyE in 16 fractions, the 5-year survival rate was 87%. As shown in Table 3, the 5- and 10-year LC rates for these patients were 88 and 80%, respectively, without serious toxic reactions. When comparing the treatment results of different modalities reported in the literature for chordoma, the observation period of 5 years may not be long enough and an even longer period may be needed. In this context, when compared with proton therapy, the better LC rates at 5 and 10 years after carbon ion RT should have confirmed the radiobiological advantage of carbon ions. The GSI reported that cumulative LC and survival rates at 5 years were 70 and 86% for chordomas and 87 and 100% at 4 years for chondrosarcomas, respectively. Severe late toxicity was observed in <5% of all patients, while the overall treatment time was significantly reduced to 3 weeks (46,53).
Table 3.
Chordomas of the skull base and upper cervical spine
| Ions | Author | Year | No. of patients | Median dose | Median follow-up (years) | Local control |
Late ≥GIII injury | |
|---|---|---|---|---|---|---|---|---|
| 5 years | 10 years | |||||||
| Proton ± photon | Hug et al. (47) (Loma Linda) | 1999 | 33 | 72 | 59% | 7% | ||
| Munzenrider and Liebsch (48) (MGH) | 1999 | 169 | 66–83 | 3.4 | 73% | 54% | ||
| Noël et al. (49) (CPO) | 2003 | 100 | 67.0 | 2.6 | 54% (4 years) | 6% | ||
| Igaki et al. (50) (Tsukuba) | 2004 | 13 | 72.0 | 5.8 | 46% | |||
| Ares et al. (51) (PSI) | 2009 | 42 | 73.5 | 3.2 (mean) | 62% | — | 6% | |
| Helium | Castro et al. (52) (LB) | 1994 | 53 | 65.0 | 4.3 | 63% | ||
| Carbon | Schulz-Ertner et al. (53) (GSI) | 2007 | 96 | 60.0 | 2.6 (Ave.) | 70% | — | 5% |
| Mizoe et al. (54) (NIRS) | 2009 | 39 | 48–60.8 | 4.7 | 82% | 82% | None | |
| Current study (NIRS) | 2012 | 47 | 60.8 | 3.7 | 88% | 80% | None | |
Non-small Cell Lung Cancer
For localized non-small cell lung cancer (NSCLC), the standard treatment used to be surgical resection. In recent years, however, an increasing number of patients have been treated with hypofractionated stereotactic body RT (SBRT) and ion beam therapy (56–59). Timmerman et al. (59) reported 3-year results from RTOG 0236, where 55 patients with biopsy-proven peripheral T1-T2N0M0 NSCLC measuring <5 cm in diameter (T1; 44, T2; 11) were treated using the prescribed dose of 18 Gy × 3 fractions (54 Gy total) in 1.5–2 weeks. The estimated 3-year primary tumor control rate and 3-year overall survival rate were 97.6 and 55.8%, respectively (Table 4). These are highly favorable results; however, protocol-specified treatment-related adverse events were quite high; Grades 3 and 4 were reported in seven (12.7%) and two (3.6%) patients, respectively. For SBRT as reported, toxicity appears to be a more significant problem, although 2-year LC rates are similar to proton therapy.
Table 4.
Stage I NSCLC
| Author | Dose fractionation | No. of patients (IA:IB) | Overall survival | Local control | Late ≥GIII |
|---|---|---|---|---|---|
| Stereotactic radiotherapy | |||||
| Baumann et al. (56) (Sweden, 2009) | 45–66 Gy/3 fr | 57 (40:17) | 60% (3 years) | 92% (3 years) | 28% |
| Fakiris et al. (57) (Indiana, 2009) | T1: 60 Gy/3 fr, T2: 66 Gy/3 fr | 70 (34:36) | 42.7% (3 years) | 88.1% (3 years), T1: 100%, T2: 77% | 10% |
| Ricardi et al. (58) (Torino, 2009) | 45 Gy/3 fr | 62 (43:19) | 57.1% (3 years) | 87.8% (3 years) | <10% |
| Timmerman et al. (59) (RTOG, 2010) | 54 Gy/3 fr/1.5–2 weeks | 55 (44:11, <5 cm) | 55.8% (3 years) | 97.6% (3 years) | 10∼27% |
| Proton beam therapy | |||||
| Bush et al. (60) (LLUMC, 2004) | 51 CGE/10 fr/2 weeks (n = 22), 60 CGE/10 fr/2 weeks (n = 46) | 68 (29:39) | 44% (3 years) | 74% (3 years), T1: 87%, T2: 49% | None |
| Iwata et al. (61) (Hyogo, 2010) | 80.0 GyE/20 fr (n = 20) 60.0 GyE/10 fr (n = 37) | 57 (27:30) | 73% (3 years) | 81% (3 years) | 1.8% |
| Nihei et al. (62) (NCCE, 2006) | 70–94 GyE/20 fr | 37 (17:20) | 84% (2 years) | 80% (2 years) | 8.1% |
| Nakayama et al. (63) (Tsukuba, 2010) | Peripheral: 66.0 GyE/10 fr, central: 72.6 GyE/22 fr | 55 (Lesions 30:28) | 97.8% (2 years) | 97.0% (2 years) | 3.6% |
| Carbon ion therapy | |||||
| Miyamoto et al. (64) (NIRS, 2003) | 59.4–95.4 GyE/18 fr/6 weeks (n = 47), 68.4–79.2 GyE/9 fr/3 weeks (n = 34) | 81 (40:41) | 42% (5 years), T1: 64%, T2: 22% | 79% (5 years) | 3.7% |
| Miyamoto et al. (65) (NIRS, 2007) | 72 GyE/9 fr/3 weeks | 50 (29:21) | 50% (5 years), T1: 55%, T2: 43% | 95% (5 years) | 2.0% |
| Miyamoto et al. (66) (NIRS, 2007) | T1: 52.8 GyE/4fr/1 week, T2: 60.0 GyE/4 fr/1 week | 79 (42:37) | 45% (5 years), T1: 62%, T2: 25% | 90% (5 years), T1: 98%, T2: 80% | None |
| Yamamoto et al. (67) (NIRS, 2011) | Single fractionation (36–48 GyE/1 day) | 139 (83:56) | 76.9% (3 years) | 85% (3 years), T1: 87.6%, T2: 79.7% | None |
NSCLC, non-small cell lung cancer; fr, fractions.
In proton therapy, 3-year in-field control rates of 74 and 81% were observed in 68 and 57 patients, respectively, and overall survival rates for these two groups were 44 and 73%, respectively (60,61). Similar to experiences of SBRT, there are no data yet reported for proton therapy based on observation for 5-year or longer (60–63).
In carbon ion RT at NIRS, respiratory gating and image-guided RT have been integrated in order to allow for further sparing of normal lung tissues (22). Because of assumed difference in normal tissue tolerance, Stage I NSCLC was divided into two groups, peripheral type and central type. For peripheral tumors, the fraction number and treatment time have been reduced in gradual steps from 18 fractions/6 weeks through 9 fractions/3 weeks and 4 fractions/1 week and eventually to single-fraction treatment (64–67). In 129 patients treated at NIRS with 9- and 4-fraction regimens, there were no serious toxic reactions, and the 5-year LC rate was 95% for 9 fractions and 90% for 4 fractions (65,66). The 5-year overall survival rate for 9 and 4 fractions was 50.0 and 45.0%, respectively (corresponding cause-specific survival rates were 76 and 62%, respectively). Currently, a dose-escalation study for evaluating a single-fraction treatment with carbon ions is ongoing, in which high rates of LC and survival have been obtained with only minor toxicity (67).
For the treatment of the central type of NSCLC at NIRS, a larger fraction number has been used than for peripheral tumors, because it was felt that more careful observation was needed on the radiation-induced reactions of the main bronchus. This type of cancer is characterized by relatively superficial lesions and has been successfully controlled with 57.6 GyE in 9 fractions over 3 weeks. On the other hand, for a central-type tumor forming a bulky lesion, the higher dose has been employed.
Hepatocellular Cancer
Hepatocellular carcinoma (HCC) is one of the most common tumors in the world, causing 662 000 deaths per year with about half of them in China (68). HCC is one of the cancers with poor prognosis in China, where chronic hepatitis B is found in 90% of cases. In Japan, chronic hepatitis C is more common and is associated with 90% of HCC cases. The outcome of HCC is generally poor because only 10–20% of HCC can be removed completely with surgery, and the 5-year survival rate is ∼15% (69). HCC is associated with liver cirrhosis in 85% of all cases, which is itself a serious disorder of the liver. Although the tolerance of the liver to irradiation is generally small, proton therapy has been extensively applied for this disease. As shown in Table 5, the 2- or 3-year LC and overall survival rates obtained with proton therapy are 75–96 and 55–66%, respectively (70–73). The degree of liver dysfunction attributable to coexisting liver cirrhosis and the number of tumors in the liver significantly affected patient survival (72).
Table 5.
Hepatocellular carcinoma
| Author | Ions | No. of patients | Tumor diameter (range) (mm) | Dose/fractions | Local control |
Overall survival |
||
|---|---|---|---|---|---|---|---|---|
| 3 years | 5 years | 3 years | 5 years | |||||
| Bush et al. (70), LLUMC | Proton | 34 | 57 (T1–T4) | 63 GyE/15 fr | 75.0% (2 years) | — | 55.0% (2 years) | — |
| Kawashima et al. (71), NCCHE | Proton | 40 | 45 (25–82) | 76 GyE/20 fr | 96.0% (2 years) | — | 66.0% | — |
| Chiba et al. (72), Tsukuba | Proton | 162 | 38 (15–145) | 5–72 GyE/10–24fr | 90.0% | 86.9% | 45.0% | 23.5% |
| Fukumitsu et al. (73), Tsukuba | Proton | 51 | 28 (8–93) | 66 GyE/10 fr | 94.5% | 87.8% | 49.2% | 38.7% |
| Kato et al. (74,75), NIRS | C-ion | 69 | 40 (12–120) | 52.8 GyE/4 fr | 94.0% | 81.0% | 50.0% | 33.0% |
| Imada et al. (76), NIRS | C-ion | 64 | — | Porta hepatis group 52.8 GyE/4 fr | — | 87.8% | — | 22.2% |
| — | Non-porta hepatis group 52.8 GyE/4 fr | 95.7% | 34.8% | |||||
| Imada et al. (77), NIRS | C-ion | 40 | 38 (14–95) | High-dose group: 42.8–45.0 GyE/2 fr | 95.0% | — | 72.0% | — |
| 77 | 45 (15–140) | Low-dose group: 32.0–40.8 GyE/2 fr | 74.0% | — | 54.0% | — | ||
The eligibility criteria for enrollment for carbon ion RT at NIRS was that other therapies appeared to offer no potential of sufficient efficacy or other treatments had proved ineffective in local tumor control (74–77). In an attempt to develop a hypofractionated regimen, dose-escalation studies have been successively implemented from 15 fractions/5 weeks through 12 fractions/3 weeks, 8 fractions/2 weeks, 4 fractions/1 week and eventually 2 fractions in 2 days. It was possible to conduct all these fractionation regimens only with minor toxicities. In 69 patients treated with 52.8 GyE in 4 fractions, post-treatment impairment in hepatic function was minimal and the 5-year LC and survival rates were 81 and 33%, respectively (75). There were no significant differences in LC and survival rates and toxicities between the patients whose tumors were located within 2 cm from the main portal vein (porta hepatis group) and those who had tumors more than 2 cm from the main portal vein (non-porta hepatis group) (76). Since 2003, even a shorter irradiation schedule of 2 fractions in 2 days has been employed in a dose-escalation study, in which a total of 117 patients were treated with a total dose ranging from 32.0 to 45.0 GyE. There have been no therapy-related deaths and no severe adverse events (77). The patients who received a higher dose appeared to have better LC and survival rates than those who received a lower dose.
In conclusion, proton therapy has been documented as yielding similar tumor control probabilities when compared with carbon ion RT in HCC. However, there is a major difference in dose fractionation between the two regimens: the conventional regimen in proton therapy and the more hypofractionated regimen in carbon ion RT.
Prostate Cancer
In 1986, the US Food and Drug Administration approved the prostate specific antigen (PSA) test to monitor the disease status in patients with prostate cancer and in 1994 to aid in prostate cancer detection. Since then, the increased incidence of prostate cancer has led to remarkable improvement worldwide in diagnosis and treatment over the past century, and a variety of treatment data have been published including the use of IMRT and ion beam RT. In recent years, the major concern has been placed on ion beam RT permitting higher radiation doses to the prostate at the same toxicity level as photon IMRT. The effectiveness of proton therapy for prostate cancer was investigated in a prospective Phase III randomized trial on 202 patients with locally advanced Stage T3-4 N0-2 M0 tumors to receive 75.6 CGE with combined photons and proton boost (Arm I) or 67.2 Gy with photon RT alone (Arm II). A significant improvement in the LC rate by a conformal proton boost was identified in poorly differentiated tumors but was associated with increased late radiation sequelae (78). In proton therapy, the largest number of patients has been treated at the Loma Linda University, where the overall biochemical relapse-free (bNED) survival rate was 73%. It was 90% in patients with initial PSA ≤4.0 and 87% in patients with post-treatment PSA nadirs ≤0.50. The rates dropped with rises in the initial and nadir PSA values, and in particular, the long-term survival outcomes for the high-risk group that had a high Gleason score and/or high PSA values were very poor (79).
Regarding a radiation-induced morbidity, the incidence of late toxicity for photon therapy (80–84), proton therapy (85) and carbon ion RT (86–89) is compared in Table 6. The rationale of using carbon ions for prostate cancer lies on the expectation that high-LET carbon ion RT would offer distinct advantages in terms of its antitumor effects for prostate cancer, which is a slow-growing, relatively radioresistant tumor, as well as that the use of a hypofractionated regimen with carbon ions would be radiobiologically justified in this tumor. Based on this assumption, a hypofractionated regimen with 20 fractions in 5 weeks was initially employed at NIRS, which was then reduced to 16 fractions in 4 weeks. Currently, the safety and efficacy of 12 fractions in 3 weeks is being investigated at NIRS (88).
Table 6.
Incidence of late radiation toxicity in prostate cancer
| Institutes | Treatment | Dose/fractions | No. of patients | Late ≥G2 injury |
|
|---|---|---|---|---|---|
| Rectal | Urinary | ||||
| Christie H. (80) | IMRT | 60 Gy/20 fr | 60 | 9.5% | 4.0% |
| Princess Margaret H. (81) | IMRT | 60 Gy/20 fr | 92 | 6.3% | 10.0% |
| Cleveland CF. (82) | IMRT | 70 Gy/28 fr | 770 | 4.4% | 5.2% |
| Stanford U. (83) | SRT | 36.25 Gy/5 fr | 41 | 15.0% | 29.0% |
| RTOG 9406 (84) | 3DCRT | 68.4–79.2 Gy/38–41 fr | 275 | 7–16% | 18–29% |
| 3DCRT | 78.0 Gy/39 fr | 118 | 25–26% | 23–28% | |
| Loma Linda U. (85) | Proton | 75.0 GyE/39 fr | 901 | 3.5% | 5.4% |
| NIRS (88,89) | Carbon | 63.0 GyE/20 fr | 216 | 2.3% | 6.1% |
| Carbon | 57.6 GyE/16 fr | 539 | 0.6% | 1.9% | |
IMRT, intensity-modulated radiotherapy; 3DCRT, three-dimensional conformal radiotherapy; SRT, stereotactic radiotherapy.
It is reported that after carbon ion RT with 63.0 GyE/20 fractions/5 weeks, late complications of the lower urinary tract were about the same as in photon IMRT and proton therapy, while the late rectal toxicity was lower than with any other modality. When the schedule of 57.6 GyE/16 fractions/4 weeks was used, acute and late rectal toxicities were significantly reduced when compared with what had been observed with conventional three-dimensional conformal RT (3DCRT), IMRT or proton therapy. No serious toxic reactions have been observed to date in carbon ion RT (86–89). These results may be the proof for both the physical and biological advantage of carbon ions over other low-LET radiations.
Regarding the antitumor effect in carbon ion RT at NIRS, the bNED survival rates for a total of 1084 patients at 5 and 10 years were 95.4 and 90.6%, respectively, and high survival rates were obtained especially in the high-risk group (88,89). There were no differences in the relapse-free rate and the overall survival rate between 20 and 16 fractions, while the toxicity was even smaller in 16 fractions than 20 fractions. Table 7 shows a risk-grouped comparison of survival rates on the large-scale clinical studies of combined X-ray and endocrine therapy performed by RTOG prostate cancer trials in the USA (90) and the survival rates of carbon ion RT at NIRS (88). These results show that overall survival rates are higher in carbon ion RT in all groups, particularly in a high-risk group, most likely representing radiobiological benefit of carbon ions in slow-growing neoplasms like prostate cancer.
Table 7.
Overall survival rates of carbon ion RT compared with the results of RTOG meta-analysis in prostate cancer
| Studies | Total dose/fractions | Overall survival |
|||||
|---|---|---|---|---|---|---|---|
| Group 2 |
Group 3 |
Group 4 |
|||||
| No. of patients | 5-year OS (%) | No. of patients | 5-year OS (%) | No. of patients | 5-year OS (%) | ||
| RTOG meta-analysis (90) | |||||||
| RT alone | 65–70 Gy/30–35 fr | 443 | 82 | 338 | 68 | 324 | 52 |
| RT + hormone | 65–70 Gy/30–35 fr | 114 | 76 | 138 | 79 | 103 | 63 |
| Carbon + hormone (88,89) | 66–63 GyE/20 fr or 57.6 GyE/16 fr | 381 | 99 | 321 | 94 | 143 | 87 |
OS, overall survival.
Bone and Soft Tissue Tumors
Bone and soft tissue tumors comprise a variety of histological subtypes with different biological behavior and are generally photon-resistant. Advanced tumors originating from the trunk including the pelvis, para-spinal region and retroperitoneum are in many cases not suited for surgical resection with poor prognosis. When the tumors are unresectable, the prognosis is miserable. In the past, such patients used to be treated with fast neutron therapy based on the radiobiological property for the use of high-LET radiations (12,13). Despite the therapeutic advantage of fast neutrons, in many reports, the incidence of severe toxicities appeared unacceptably high (91).
Proton therapy has also been applied to these tumors, not as a sole treatment but mainly after the gross tumors are partially or totally resected (92). In contrast, carbon ion RT at NIRS has been applied as a sole treatment to advanced bone and soft tissue sarcomas of the trunk, which are primarily not suited to surgical resection or are entirely inoperable, offering a favorable prospect as a function-preserving modality (17,18,93). Clinical results of carbon ion RT based on a total of 514 lesions in 495 patients with a variety of histological types have shown that the 2- and 5-year LC rates were 85 and 69%, respectively, and the 2- and 5-year overall survival rates were 79 and 59%, respectively (94).
Osteosarcoma
Osteosarcoma is the most common type of primary bone cancer and usually develops in growing bones, particularly in the arm and leg. It is most commonly treated with surgery combined with chemotherapy. In contrast to extremity tumors, primary osteosarcomas of the axial skeleton or trunk more likely present with metastases at diagnosis, and local failure after surgical treatment is very common. As shown in Table 8, the prognosis of unresectable osteosarcoma is miserable with a survival rate of 10% or less (95–102). A total of 55 patients with a median age of 29 years (range, 2–76 years) were offered proton therapy at Massachusetts General Hospital (MGH) (96). The criteria for proton therapy were patients with unresected or partially resected osteosarcomas, positive postoperative margins, postoperative imaging studies with macroscopic disease or incomplete resection as defined by the surgeon. The mean dose was 68.4 Gy, of which 58.2% was delivered with protons. The LC rates after 3 and 5 years were 82 and 67%, respectively. Grade 3–4 late toxicity was seen in 30.1% of the patients. Osteosarcomas of the trunk constituted the next largest group at NIRS, for which carbon ion RT with or without chemotherapy appeared to provide a survival benefit. The 5-year LC and 5-year overall survival rates for the 78 patients with unresectable osteosarcoma of the trunk were 61 and 32%, respectively (94,103). The median diameter of the tumors was 9 cm. The tumor size was one of the most important prognostic factors. Of all 78 patients, 38 patients with a tumor volume of <500 cc showed a 5-year LC rate of 87%, while 40 patients with a volume of more than 500 cc had a rate of 21%. The 5-year survival rate of the 38 patients with smaller tumors was 46%, while that of the larger tumor group was 19%. There were no patients who developed Grade 3 or 4 late toxicities.
Table 8.
Osteosarcoma of the trunk
| Institutes | Treatment | No. of patients | Site | 5-year overall survival (%) |
||
|---|---|---|---|---|---|---|
| All case | Resectable | Unresectable | ||||
| MGH (95,96) | Surgery | 26 | S | 31 | — | — |
| Surgery + proton/photon | 55 | V | 67 | — | — | |
| Mayo Clinic (97) | Surgery | 43 | P | 38 | 38 | — |
| Inst Orthop Rizzoli (98) | Surgery | 60 | P | 15 | 30 | 0 |
| COSS (99,100) | Surgery | 67 | P | 27 | 34 | 0 |
| Surgery | 22 | S | 30 | 40 | 0 | |
| NCBT (101) | Surgery | 40 | P | 21 | 26 | — |
| MSKCC (102) | Surgery | 40 | P | 34 | 41 | 10 (1/10) |
| NIRS (94,103) | Carbon ions | 78 | Trunk | 32 | — | 32 |
S, spine; P, pelvis; V, various.
Sacral chordoma
Sacral chordoma is a rare tumor, for which surgery used to be the first choice of treatment, although it is not always possible. Sacral chordomas frequently occur among the elderly population, who are often contraindicated to surgery because of either associated diseases or overall frailty. They are often left undetected until they start to cause pain and other symptoms. The sacrum houses the sacral nerves, which innervate the excretory functions and ambulation. Depending on the level of tumor involvement to the sacral bone, excision of these nerves causes permanent gait, excretory and other disabilities, which impairs the patients' quality of life. Therefore, curative surgery for sacral chordoma (sacrectomy) is one of the most invasive treatments. As shown in Table 9, the LC rate of surgery alone or combined with proton/photon or helium ions ranges from 55 to 72% (104–106). Sacral chordomas usually grow slowly, are photon-resistant and are expected to benefit from carbon ion RT (107–109). They accounted for the largest proportion of sarcomas at NIRS, and between 1996 and 2007, 95 patients with sacral chordoma received carbon ion RT (94). The median age of these patients was 66 years, and the median tumor diameter was 9 cm. The 5-year LC rate was 88% and the 5-year overall survival rate was 86%. Of the 95 patients, 91% remained ambulatory with or without a supportive device. Two patients experienced severe skin and soft tissue reactions requiring a skin graft. Fifteen patients experienced severe sciatic nerve complications requiring continued medication.
Table 9.
Sacral chordoma
Chondrosarcoma
Chondrosarcoma is the second most common primary malignant bone tumor. Surgery has been the main form of treatment, and the definitive en bloc resection of the tumor is mandatory to obtain long-term disease-free survival. However, radical surgical intervention for chondrosarcoma of the trunk has sometimes been associated with substantial morbidities. From 1996 to 2009, 71 patients with chondrosarcoma received carbon ion RT at NIRS (88). The clinical target volumes ranged between 25 and 2900 cm3 (median 488 cm3). At 5 years, the actuarial overall LC rate and overall survival rate were 60 and 60%, respectively. Four patients experienced Grade 3 and/or 4 skin/soft tissue late reactions in this series.
Rectal Cancer (Postoperative Pelvic Recurrence)
Although postoperative recurrence of rectal cancer in the pelvis has decreased as a result of improvement in surgical techniques, its incidence is still in a range of 5–20%. For locally recurrent rectal cancer, surgical resection is the first choice, but a highly invasive procedure like a total pelvic exenteration is often required. The resection rate is reported to be in the range of 40–50% for liver metastases and 20–40% for lung metastases, whereas it was 10–40% for locally recurrent colorectal cancers (110,111). As shown in Table 10, the 5-year survival rate for surgically treated patients is around 35% (112–114). Many of the patients with local rectal recurrence are not eligible for surgical resection and are frequently referred to RT. Yet, the results of standard RT are still far from adequate, with many studies in the literature reporting a 50% survival period of 12 months and a 5-year survival rate of 0–16% (115–117). Therefore, the role of standard RT has been often described as mere pain control.
Table 10.
Post-operative pelvic recurrence of rectal cancer
| Author | Year | No. of patients | Treatment | Overall survival (%) |
Local control (%) | |
|---|---|---|---|---|---|---|
| 2 years | 5 years | |||||
| Wanebo et al. (112) | 1999 | 53 | Surgery | 62 | 31 | — |
| Saito et al. (113) | 2003 | 43 | Surgery | 78 | 39 | — |
| Moriya et al. (114) | 2004 | 48 | Surgery | 76 | 36 | — |
| O'Connel et al. (115) | 1982 | 17 | Photon 50 Gy | 45 | 0 | 24 (2 years) |
| Wong et al. (116) | 1991 | 22 | Photon 40–50 Gy | 27 | 16 | 9 (5 years) |
| Lybeert et al. (117) | 1992 | 76 | Photon 6–66 Gy | 61 (1 years) | 3 | 28 (3 years) |
| NIRS (118) | 2011 | 111 | Carbon 73.6 GyE | 86 | 42 | 95 (5 years) |
Postoperative pelvic recurrence of rectal cancer has been extensively treated with carbon ions at NIRS. The dose-escalation study showed that the LC and survival rates were dependent upon total doses delivered (118). The 5-year survival rates were only 24.0% for patients treated with 67.2 GyE/16 fractions (n = 10), 27.5% for patients treated with 70.4 GyE/16 fractions (n = 19) and 42.3% for those treated with 73.6 GyE/16 fractions (n = 111). A significant proportion of patients experienced rapid pain relief, and no particularly serious toxic reactions were observed. Furthermore, Mobaraki et al. (119) reported that when compared with conventional treatment including surgery, 3DCRT, chemotherapy and hyperthermia, carbon ion RT is a cost-effective treatment for locally recurrent rectal cancer.
Uterine Cervix Cancer
The incidence and mortality of uterine squamous cell carcinoma (SCC) is in a declining trend, and the treatment results have been relatively favorable through the introduction of concomitant chemoradiotherapy. The treatment results of uterine SCC in an advanced stage, however, are at present still unsatisfactory. This has led to an attempt to apply carbon ion RT to locally advanced lesions in order to achieve a new breakthrough in therapeutic results that have seen little or no progress. Although dose-escalation studies are still in progress, carbon ion RT has been considered to be effective for the treatment of Stage III and IVa cervical SCC (120).
In contrast to SCC, the incidence of adenocarcinoma of the uterine cervix has been increasing over the past few decades and accounts for 10–24% of all cervical carcinomas (121). Carbon ion RT for uterine adenocarcinoma has been targeted primarily at non-resectable tumors, and so far, a total of 57 patients with cervical adenocarcinoma have been treated at NIRS. Among them, 31 patients had Stage IIIB–IVA disease, whose LC and survival rates at 5 years were 53.3 and 50.0%, respectively. These figures were superior to the corresponding results of photon therapy reported by others (33–46 and 25–29%, respectively), suggesting that carbon ion RT provides favorable outcomes in the treatment of locally advanced cervical adenocarcinoma.
Pancreas Cancer
The prognosis of pancreas cancer is poor. Even if a curative resection is performed, the disease usually recurs and the 5-year survival rate is <20% (122). In the case of locally advanced unresectable pancreas cancer, the 2-year survival rate is even lower at ∼10% (123,124). To improve the treatment results for pancreas cancer, the critical factor lies in how effectively it is possible to prevent or control liver metastasis as well as retroperitoneal recurrence that accounts for 50% of all recurrences. At NIRS, carbon ion RT has been evaluated in a Phase I/II study to improve the LC rate and to establish therapeutic strategies, including the preoperative use of carbon ions for resectable cancer, as well as the concomitant use of chemotherapy and carbon ions for locally advanced pancreas cancer. In the 21 patients who had carbon ion RT (30.0–36.8 GyE/8 fractions/2 weeks) followed by curative resection, the 5-year LC and 5-year overall survival rates were 100 and 53%, respectively (125). A total of 60 patients with locally advanced cancer were treated with carbon ion RT over five dose levels (43.2–52.8 GyE/12 fractions/3 weeks) and concurrent weekly gemcitabine over three dose levels (400–1000 mg/m2). The LC and overall survival rate increased along with the dose escalation of carbon ions. In the high-dose group, for whom the total dose of ≥45.6 GyE/12 fractions with the concomitant use of gemcitabine (1000 mg/m2) was given, the 2-year LC and 2-year overall survival rates were 47 and 66%, respectively, with acceptable toxicities. These figures appear to be superior to those achieved in other trials.
Eye Tumors
Carbon ion RT has been applied to uveal melanoma at NIRS. The aspects in which carbon ion RT differs from proton therapy are, in particular, that carbon ion RT uses computed tomography (CT)-based treatment planning, that it is primarily applied to large tumors that are generally excluded from proton RT and that irradiation is performed from two portals to ensure the maximum possible prevention of cataract and neovascular glaucoma. The 3-year overall survival, disease-free survival and LC rates for 59 patients were 88.2, 84.8 and 97.4%, respectively (126). The incidence of post-treatment glaucoma was strongly related to the tumor size and location, and the enucleation rate of 5% at 3 years was lower than that for proton therapy in a similar patient group treated at Loma Linda University (127), although the number of patients might be insufficient for reliable comparison. The CT-based treatment planning employed at NIRS may offer the advantage of avoiding severe glaucoma requiring eye enucleation with the treatment of medium-to-large size tumors.
Malignant epithelial tumors originating from the lacrimal gland have a low incidence. Surgery offers poor results because of the difficulty in the total eradication of tumors. This has led to the use of carbon ion RT at NIRS using 12 fractions/3 weeks. So far, 12 patients have been treated with a total dose of 48 GyE in 5 patients and 52.8 GyE in 7 patients. It has been shown that the LC rate is excellent but determination of the target volume is vital for the prevention of marginal recurrence.
CONCLUSION
Large series of a review here have shown that carbon ion RT does indeed appear to have clinical advantages over other modalities such as photon IMRT and proton RT. The benefits of carbon ion RT have been demonstrated in non-squamous cell types of tumors including adenocarcinoma, ACC, malignant melanoma and various sarcomas arising in the head/neck and many other sites. In the treatment of chordomas of the skull base and sacrum, significant improvements have been achieved with proton and carbon ion RT, and after long-term observation (10 years), the difference in the LC rates became larger in favor of carbon ion therapy. The radiobiological advantage of carbon ions has been confirmed in the treatment of bone and soft tissue sarcomas including osteosarcoma, chordoma and many other types of sarcomas arising from the head/neck, pelvis, vertebra/paravertebral region and retroperitoneal region. These tumors are frequently found to be difficult to treat with surgical resection and are generally photon-resistant. The postoperative pelvic recurrence of rectal cancer has also been treated with the results comparable to or even better than those of surgery. For certain cancers such as malignant melanoma and pancreas cancer, carbon ion RT combined with chemotherapy has significantly prevented or delayed the development of distant metastasis with improved survival and LC. By taking advantage of the unique properties of carbon ions, it has been possible to complete the treatment in a short time and with small fractions. The experiences of the NIRS have provided the data in support of the single-fraction treatment for early-stage NSCLC, two-fraction treatment for HCC and 16- or 12-fraction treatment for prostate cancer. Even for other tumor sites, 16 or smaller fractions have been sufficient, less than half the number of fractions required in the standard RT. This means that the carbon therapy facility can be operated more efficiently, offering treatment for a larger number of patients than is possible with other modalities over the same period of time. In connection with this, carbon ion RT has been shown to be a cost-effective treatment in selected tumors.
The current status and anticipated future directions of the role of ion beam therapy in medicine may be a complex subject that involves an intimate interplay of radiobiology, accelerator physics and radiation oncology. In this context, together with highlighting the clinical results, the technical advances as well as future directions of carbon ion RT are promising.
Funding
Supported by Chang Yung-Fa Foundation and NIRS International Open Laboratory (IOL). These co-authors are proud to acknowledge the partial support of their work by these grants: Chang Yung-Fa Foundation, NIRS International Open Laboratory (IOL), and FNCA Radiation Oncology Project.
Conflict of interest statement
None declared.
Acknowledgements
The authors are pleased to thank Hiroshi Tsuji, Shigeru Yamada, Naoyoshi Yamamoto, Shigeo Yasuda, Reiko Imai, Azusa Hasegawa, Masashi Koto, Hiroshi Imada, Masaru Wakatsuki, Ryo Takagi and Makoto Shinoto for their assistance with the provision of reference material and data analysis. Also thanks are expressed to Takashi Fujita, Setsuko Fujimori, Youichi Kawamura, Yukari Nakamura, Shigeko Taki and Tomoko Takahashi for their important participation in the preparation of this manuscript.
References
- 1.Suit HD, Westgate SJ. Impact of improved local control on survival. Int J Radiat Oncol Biol Phys. 1986;12:453–8. doi: 10.1016/0360-3016(86)90052-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen GTY, Castro JR, Quivey JM. Heavy charged particle radiotherapy. Ann Rev Biophys Bioeng. 1981;10:499–529. doi: 10.1146/annurev.bb.10.060181.002435. [DOI] [PubMed] [Google Scholar]
- 3.Kanai T, Furusawa Y, Fukutsu K, et al. Irradiation of mixed beam and design of spread-out Bragg peak for heavy-ion radiotherapy. Rad Res. 1997;147:78–85. [PubMed] [Google Scholar]
- 4.Haberer T, Becher W, Schardt D, et al. Magnetic scanning system for heavy ion therapy. Nucl Instrum Methods. 1993;A330:295–305. [Google Scholar]
- 5.Kraft G. Tumor therapy with heavy charged particles. Prog Part Nucl Phys. 2000;45:S473–544. [Google Scholar]
- 6.Barendsen GW. RBE-LET relationships for lethal, potentially lethal and sublethal damage in mammalian cells: implications for fast neutron radiotherapy. Bull Cancer Radiother. 1996;83(Suppl):15s–8s. doi: 10.1016/0924-4212(96)84878-x. [DOI] [PubMed] [Google Scholar]
- 7.Skarsgard LD. Radiobiology with heavy charged particles: a historical review. Phys Med. 1998;14(Suppl 1):1–19. [PubMed] [Google Scholar]
- 8.Tepper J, Verhey L, Goitein M, et al. In vivo determination of RBE in a high energy modulated proton beam using normal tissue reactions and fractionated dose schedules. Int J Radiat Oncol Biol Phys. 1977;2:1115–22. doi: 10.1016/0360-3016(77)90118-3. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein LS, Phillips Ross GY. Biological effects of accelerated heavy ions. II. Fractionated irradiation of intestinal crypt cells. Rad Res. 1981;86:542–58. [PubMed] [Google Scholar]
- 10.Raju MR, Carpenter BA A heavy particle comparative study. Part IV: acute and late reactions. Br J Radiol. 1978;51:720–7. doi: 10.1259/0007-1285-51-609-720. [DOI] [PubMed] [Google Scholar]
- 11.Ando K, Kase Y. Biological characteristics of carbon-ion therapy. Int J Radiat Biol. 2009;85:715–28. doi: 10.1080/09553000903072470. [DOI] [PubMed] [Google Scholar]
- 12.Battermann JJ, Breur K, Hart GAM, et al. Observation on pulmonary metastases in patients after single doses and multiple fractions of fast neutrons and Cobalt-60 gamma rays. Eur J Cancer. 1981;17:539–48. doi: 10.1016/0014-2964(81)90056-6. [DOI] [PubMed] [Google Scholar]
- 13.Laramore GE. The use of neutrons in cancer therapy: a historical perspective through the modern era. Semin Oncol. 1997;24:672–85. [PubMed] [Google Scholar]
- 14.Denekamp J, Waites T, Fowler JF. Predicting realistic RBE values for clinically relevant radiotherapy schedules. Int J Radiat Biol. 1997;71:681–94. doi: 10.1080/095530097143699. [DOI] [PubMed] [Google Scholar]
- 15.Ando K, Koike S, Uzawa A, et al. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J Radiat Res. 2005;46:51–7. doi: 10.1269/jrr.46.51. [DOI] [PubMed] [Google Scholar]
- 16.Tsujii H, Mizoe J, Kamada T, et al. Overview of clinical experiences on carbon ion radiotherapy at NIRS. Radiother Oncol. 2004;73(Suppl 2):S41–9. doi: 10.1016/s0167-8140(04)80012-4. [DOI] [PubMed] [Google Scholar]
- 17.Tsujii H, Kamada T, Baba M, et al. Clinical advantages of carbon-ion radiotherapy. New J Phys. 2008;10:1367–2630. [Google Scholar]
- 18.Kamada T, Tsujii H. HIMAC: a new start for heavy ions. In: Linz U, editor. Ion Beam Therapy. Berlin, Heidelberg: Springer-Verlag; 2012. pp. 611–21. [Google Scholar]
- 19.Ogata T, Teshima T, Kagawa K, et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 2005;65:113–20. [PubMed] [Google Scholar]
- 20.Tamaki T, Iwakawa M, Ohno T, et al. Application of carbon-ion beams or gamma-rays on primary tumors does not change the expression profiles of metastatic tumors in an in vivo murine model. Int J Radiat Biol. 2009;74:210–8. doi: 10.1016/j.ijrobp.2008.12.078. [DOI] [PubMed] [Google Scholar]
- 21.Akino Y, Teshima T, Kihara A, et al. Carbon-ion beam irradiation effectively suppresses migration and invasion of human non-small-cell lung cancer cells. Int J Radiat Biol. 2009;75:475–81. doi: 10.1016/j.ijrobp.2008.12.090. [DOI] [PubMed] [Google Scholar]
- 22.Minohara S, Kanai T, Endo M, et al. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1097–103. doi: 10.1016/s0360-3016(00)00524-1. [DOI] [PubMed] [Google Scholar]
- 23.Scholz M, Kellerer AM, Kraft-Weyrather W, et al. Computation of cell survival in heavy ion beams for therapy- the model and its approximation. Rad Environ Biohys. 1997;36:59–66. doi: 10.1007/s004110050055. [DOI] [PubMed] [Google Scholar]
- 24.Endo M, Koyama-Ito H, Minohara S, et al. HIPLAN—a heavy ion treatment planning system at HIMAC. J Jpn Soc Ther Radiol Oncol. 1996;8:231–8. [Google Scholar]
- 25.Castro JR. Results of heavy ion radiotherapy. Radiat Environ Biophys. 1995;34:45–8. doi: 10.1007/BF01210545. [DOI] [PubMed] [Google Scholar]
- 26.Linstadt DE, Castro JR, Philips T. Neon ion radiotherapy: results of the phase I/II clinical trial. Int J Radiat Oncol Biol Phys. 1991;20:761–9. doi: 10.1016/0360-3016(91)90020-5. [DOI] [PubMed] [Google Scholar]
- 27.Combs S, Jaekel O, Haberer T, et al. Particle therapy at the Heidelberg Ion Therapy Center (HIT)—Integrated research-driven university-hospital-based radiation oncology service in Heidelberg, Germany. Radiother Oncol. 2010;95:41–4. doi: 10.1016/j.radonc.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Ohno T, Kanai T, Yamada S, et al. Carbon ion radiotherapy at the Gunma University Heavy Ion Medical Center: New Facility Set-up. Cancers. 2011;3:4046–60. doi: 10.3390/cancers3044046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slater JD, Yonemoto LT, Mantik DW, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62:494–500. doi: 10.1016/j.ijrobp.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 30.Chan AW, Pommier P, Deschler DG, et al. Change in patterns of relapse after combined proton and photon irradiation for locally advanced paranasal sinus cancer. Int J Radiat Oncol Biol Phys. 2004;60(Suppl):S320. [Google Scholar]
- 31.Mizoe J, Tsujii H, Kamada T, et al. Dose escalation study of carbon ion radiotherapy for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2004;60:358–64. doi: 10.1016/j.ijrobp.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa A, Koto M, Takagi R, et al. Carbon ion radiotherapy for head and neck tumors. Proceedings of NIRS-ETOILE 2nd Joint Symposium on Carbon Ion Radiotherapy; 2011. pp. 18–26. Centre ETOILE, Lyon, NIRS-M-243. [Google Scholar]
- 33.Schulz-Ertner D, Nikoghosyan A, Didinger B, et al. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer. 2005;104:338–44. doi: 10.1002/cncr.21158. [DOI] [PubMed] [Google Scholar]
- 34.Iseli TA, Karnell LH, Graham SM, et al. Role of radiotherapy in adenoid cystic carcinoma of the head and neck. J Laryngol Otol. 2009;123:1137–44. doi: 10.1017/S0022215109990338. [DOI] [PubMed] [Google Scholar]
- 35.Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–62. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 36.Pommier P, Liebsch NJ, Deschler DG, et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:1242–9. doi: 10.1001/archotol.132.11.1242. [DOI] [PubMed] [Google Scholar]
- 37.Huber PE, Debus J, Latz D, et al. Radiotherapy for advanced adenoid cystic carcinoma: neutrons, photons or mixed beam? Radiother Oncol. 2001;59:161–7. doi: 10.1016/s0167-8140(00)00273-5. [DOI] [PubMed] [Google Scholar]
- 38.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base Report on cutaneous and noncutaneous melanoma. Cancer. 1998;83:1664–78. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 39.Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247–57. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 40.Lund VJ, Howard DJ, Harding L, et al. Management options and survival in malignant melanoma of the sinonasal mucosa. Laryngoscope. 1999;109:208–11. doi: 10.1097/00005537-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Yanagi T, Mizoe J, Hasegawa A, et al. Mucosal malignant melanoma of the head and neck treated by carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:15–20. doi: 10.1016/j.ijrobp.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 42.Willers H, Hug EB, Spiro IJ, et al. Adult soft tissue sarcomas of the head and neck treated by radiation and surgery or radiation alone: patterns of failure and prognostic factors. Int J Radiat Oncol Biol Phys. 1995;33:585–93. doi: 10.1016/0360-3016(95)00256-X. [DOI] [PubMed] [Google Scholar]
- 43.Mendenhall WM, Mendenhall CM, Werning JW, et al. Adult head and neck soft tissue sarcomas. Head Neck. 2005;27:916–22. doi: 10.1002/hed.20249. [DOI] [PubMed] [Google Scholar]
- 44.Barker JL, Jr, Paulino AC, Feeney S, et al. Locoregional treatment for adult soft tissue sarcomas of the head and neck: an institutional review. Cancer J. 2003;9:49–57. doi: 10.1097/00130404-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Jungu K, Tsujii H, Mizoe J, et al. Carbon ion radiation therapy improves the prognosis of unresectable adult bone and soft tissue sarcoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;82:2125–31. doi: 10.1016/j.ijrobp.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 46.Schulz -Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:953–64. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 47.Hug EB, Loredo LN, Slater JD, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91:432–9. doi: 10.3171/jns.1999.91.3.0432. [DOI] [PubMed] [Google Scholar]
- 48.Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175:57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- 49.Noël G, Habrand JL, Jauffret E, et al. Radiation therapy for chordoma and chondrosarcoma of the skull base and the cervical spine. Prognostic factors and patterns of failure. Strahlenther Onkol. 2003;179:241–8. doi: 10.1007/s00066-003-1065-5. [DOI] [PubMed] [Google Scholar]
- 50.Igaki H, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys. 2004;60:1120–6. doi: 10.1016/j.ijrobp.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 51.Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111–8. doi: 10.1016/j.ijrobp.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 52.Castro JR, Linstadt DE, Bahary JP, et al. Experience in charged particle irradiation of tumors of the skull base: 1977–1992. Int J Radiat Oncol Biol Phys. 1994;29:647–55. doi: 10.1016/0360-3016(94)90550-9. [DOI] [PubMed] [Google Scholar]
- 53.Schulz-Ertner D, Karger CP, Feuerhake A, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68:449–57. doi: 10.1016/j.ijrobp.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 54.Mizoe J, Hasegawa A, Takagi R, et al. Carbon ion radiotherapy for skull base chordoma. Skull Base. 2009;19:219–24. doi: 10.1055/s-0028-1114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koto M, Hasegawa A, Takagi R, et al. Carbon ion radiotherapy for skull base and paracervical tumors. Proceedings of NIRS-ETOILE 2nd Joint Symposium on Carbon Ion Radiotherapy; 2011. pp. 12–7. Centre ETOILE, Lyon, NIRS-M-243. [Google Scholar]
- 56.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 57.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 58.Ricardi U, filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2009;68:72–7. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bush DA, Slater JD, Shin BB, et al. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest. 2004;126:1198–203. doi: 10.1378/chest.126.4.1198. [DOI] [PubMed] [Google Scholar]
- 61.Iwata H, Murakami M, Demizu Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer. 2010;116:2476–85. doi: 10.1002/cncr.24998. [DOI] [PubMed] [Google Scholar]
- 62.Nihei K, Ogino T, Ishikura S, et al. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:107–11. doi: 10.1016/j.ijrobp.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 63.Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for patients with medically inoperable stage I non-small cell lung cancer at the university of Tsukuba. Int J Radiat Oncol Biol Phys. 2010;78:467–71. doi: 10.1016/j.ijrobp.2009.07.1707. [DOI] [PubMed] [Google Scholar]
- 64.Miyamoto T, Yamamoto N, Nishimura H, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. 2003;66:127–40. doi: 10.1016/s0167-8140(02)00367-5. [DOI] [PubMed] [Google Scholar]
- 65.Miyamoto T, Baba M, Yamamoto N, et al. Curative treatment of stage I non-small cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007;67:750–8. doi: 10.1016/j.ijrobp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Miyamoto T, Baba M, Sugane T, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during one week. J Thorac Oncol. 2007;2:916–26. doi: 10.1097/JTO.0b013e3181560a68. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto N, Baba M, Nakajima M, et al. Carbon ion radiotherapy in a hypofraction regimen for Stage I non-small cell lung cancer. Proceedings of NIRS-ETOILE 2nd Joint Symposium on Carbon Ion Radiotherapy; 2011. pp. 27–37. Centre ETOILE, Lyon, NIRS-M-243. [Google Scholar]
- 68.Cancer ‘World Health Organization’. 2006. February retrieved 24 May 2007)
- 69.The Japan Society of Hepatology. A white paper for the liver cancer. Tokyo: Japan Society of Hepatology; 1999.
- 70.Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127(5 Suppl 1):S189–93. doi: 10.1053/j.gastro.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 71.Kawashima M, Furuse J, Nishio T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;20:1839–46. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 72.Chiba T, Tokuuye K, Matsuzaki Y, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799–805. doi: 10.1158/1078-0432.CCR-04-1350. [DOI] [PubMed] [Google Scholar]
- 73.Fukumitsu N, Sugahara S, Nakayama H, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831–6. doi: 10.1016/j.ijrobp.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 74.Kato H, Tsujii H, Miyamoto T, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59:1468–76. doi: 10.1016/j.ijrobp.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 75.Kato H, Yamada S, Yasuda S, et al. Four-fraction carbon ion radiotherapy for hepatocellular carcinoma. J Clin Oncol. 2004;22:4090. ASCO Annual Meeting Proceedings (Post-Meeting Edition) (14S) [Google Scholar]
- 76.Imada H, Kato H, Yasuda S, et al. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother Oncol. 2010;96:231–5. doi: 10.1016/j.radonc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 77.Imada H, Yasuda S, Shinoto M, et al. Carbon ion radiotherapy for liver cancer. Proceedings of NIRS-ETOILE 2nd Joint Symposium on Carbon Ion Radiotherapy; 2011. pp. 46–53. Centre ETOILE, Lyon, NIRS-M-243. [Google Scholar]
- 78.Shipley WU, Verhey LJ, Munzenrider JE, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys. 1995;32:3–12. doi: 10.1016/0360-3016(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 79.Slater JD, Rossi CJ, Jr, Yonemoto LT, et al. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–52. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 80.Coote JH, Wylie JH, Cowan RA, et al. Hypofractionated intensity-modulated radiotherapy for carcinoma of the prostate: analysis of toxicity. Int J Radiat Oncol Biol Phys. 2009;74:1121–7. doi: 10.1016/j.ijrobp.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 81.Martin JM, Rosewall T, Bayley R, et al. Phase II trial of hypofractionated image-guided intensity-modulated radiotherapy for localized prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2007;69:1084–9. doi: 10.1016/j.ijrobp.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 82.Kupelian PA, Wiloughby TR, Reddy C, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic Experience. Int J Radiat Oncol Biol Phys. 2007;68:1424–30. doi: 10.1016/j.ijrobp.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 83.King CR, Brooks JD, Gill H, et al. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009;73:1043–8. doi: 10.1016/j.ijrobp.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 84.Michalski JM, Bae K, Roach M, et al. Long-term toxicity following 3D conformal radiation therapy for prostate cancer from the RTOG 9406 phase I/II dose escalation study. Int J Radiat Oncol Biol Phys. 2010;76:14–22. doi: 10.1016/j.ijrobp.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schulte RW, Slater JD, Rossi C, et al. Value and perspectives of proton radiation therapy for limited stage prostate cancer. Strahlenther Onkol. 2000;176:3–8. doi: 10.1007/pl00002302. [DOI] [PubMed] [Google Scholar]
- 86.Tsuji H, Yanagi T, Ishikawa H, et al. Hypofractionated radiotherapy with carbon ion beams for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1153–60. doi: 10.1016/j.ijrobp.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 87.Ishikawa H, Tsuji H, Kamada T, et al. Carbon ion radiation therapy for prostate cancer: results of a prospective phase II study. Radiother Oncol. 2006;81:57–64. doi: 10.1016/j.radonc.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 88.Tsuji H, Mizoguchi N, Toyama S, et al. Carbon ion radiotherapy for prostate cancer. Proceedings of NIRS-ETOILE 2nd Joint Symposium on Carbon Ion Radiotherapy; 2011. pp. 66–72. Centre ETOILE, Lyon, NIRS-M-243. [Google Scholar]
- 89.Ishikawa H, Tsuji H, Kamada T, et al. Carbon ion radiotherapy for prostate cancer. Int J Urology. 2012;19:296–305. doi: 10.1111/j.1442-2042.2012.02961.x. [DOI] [PubMed] [Google Scholar]
- 90.Mack Roach III, Lu J, Pilepich MV, et al. Predicting long-term survival, and the need for hormonal therapy: a meta-analysis of RTOG prostate cancer trials. Int J Radiat Oncol Biol Phys. 2000;47:617–27. doi: 10.1016/s0360-3016(00)00577-0. [DOI] [PubMed] [Google Scholar]
- 91.Tsujii H, Kamada T, Deevus J. Overview of experience with heavier charged particle radiotherapy. In: Delaney TE, Kooy HM, editors. Proton and Charged Particle Therapy. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 115–24. [Google Scholar]
- 92.DeLaney TF, Kirsch DG. Bone and soft tissue. In: Delaney TE, Kooy HM, editors. Proton and Charged Particle Therapy. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 172–85. [Google Scholar]
- 93.Kamada T, Tsujii H, Tsuji H, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;22:4472–7. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 94.Imai R, Kamada T, Tsuji H, et al. Carbon Ion Radiotherapy for Bone and Soft Tissue Sarcomas. Proceedings of NIRS-ETOILE 2nd Joint Symposium on Carbon Ion Radiotherapy; 2011. pp. 38–45. Centre ETOILE, Lyon, NIRS-M-243. [Google Scholar]
- 95.Schoenfeld AJ, Hornicek FJ, Pedlow FX, et al. Osteosarcoma of the spine: experience in 26 patients treated at the Massachusetts General Hospital. Spine J. 2010;10:708–14. doi: 10.1016/j.spinee.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 96.Ciernik IF, Niemierko A, Harmon DC, et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer. 2011;117:4522–30. doi: 10.1002/cncr.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuchs B, Hoekzema N, Larson DR, et al. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin Orthop Relat Res. 2009;467:510–8. doi: 10.1007/s11999-008-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Donati D, Giacomini S, Gozzi E, et al. Osteosarcoma of the pelvis. Eur J Surg Oncol. 2004;30:332–40. doi: 10.1016/j.ejso.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study group. J Clin Oncol. 2003;21:334–41. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- 100.Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study group. Cancer. 2002;94:1069–77. [PubMed] [Google Scholar]
- 101.Ham SJ, Kroon HM, Kroops HS, Hoekstra HJ. Osteosarcoma of the pelvis-oncological results of 40 patients registered by the Netherlands Committee on Bone Tumors. Eur J Surg Oncol. 2000;26:53–60. doi: 10.1053/ejso.1999.0741. [DOI] [PubMed] [Google Scholar]
- 102.Kawai A, Huvos AG, Meyers PA, Healey JH. Osteosarcoma of the pelvis-oncologic results of 40 patients. Clin Orthop. 1998;348:196–207. [PubMed] [Google Scholar]
- 103.Matsunobu A, Imai R, Kamada T, et al. Impact of Carbon Ion Radiotherapy for Unresectable Osteosarcoma of the Trunk. Cancer. 2012 doi: 10.1002/cncr.27451. ; published online: 22 February 2012. doi:10.1002/cncr.27451. [DOI] [PubMed] [Google Scholar]
- 104.Park L, DeLaney TF, Liebsch NJ, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65:1514–21. doi: 10.1016/j.ijrobp.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 105.Schoenthaler R, Castro JR, Petti PL, et al. Charged particle irradiation of sacral chordoma. Int J Radiat Oncol Biol Phys. 1993;26:291–8. doi: 10.1016/0360-3016(93)90209-e. [DOI] [PubMed] [Google Scholar]
- 106.Fuchs B, Dickey ID, Yaszemski MJ, et al. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–6. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 107.Imai R, Kamada T, Tsuji H, et al. Carbon ion radiotherapy for unresectable sacral chordomas. Clin Cancer Res. 2004;10:5741–6. doi: 10.1158/1078-0432.CCR-04-0301. [DOI] [PubMed] [Google Scholar]
- 108.Imai R, Kamada T, Tsuji H, et al. Effect of carbon ion radiotherapy for sacral chordoma: results of Phase I-II and Phase II clinical trials. Int J Radiat Oncol Biol Phys. 2010;77:1470–6. doi: 10.1016/j.ijrobp.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 109.Imai R, Kamada T, Sugahara S, et al. Carbon ion radiotherapy for sacral chordoma. Br J Radiol. 2011;84:548–54. doi: 10.1259/bjr/13783281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kobayashi H, Hashiguchi Y, Ueno H. Follow-up for recurrent colorectal cancer. J Ppn Soci Coloproctology. 2006;59:851–6. [Google Scholar]
- 111.Sugihara K. Guidelines for Treatment of Recurrent Rectal Cancer. Tokyo: Nankodo Co. Ltd; 2003. pp. 89–149. [Google Scholar]
- 112.Wanebo HJ, Antoniuk P, Koness RJ, et al. Pelvic resection of recurrent rectal cancer. Dis Colon Rectum. 1999;42:1438–48. doi: 10.1007/BF02235044. [DOI] [PubMed] [Google Scholar]
- 113.Saito N, Koda K, Takiguchi N, et al. Curative surgery for local pelvic recurrence of rectal cancer. Dig Surg. 2002;20:192–200. doi: 10.1159/000070385. [DOI] [PubMed] [Google Scholar]
- 114.Moriya Y, Akasu T, Fujita S, et al. Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Dis Colon Rectum. 2004;47:2047–54. doi: 10.1007/s10350-004-0714-9. [DOI] [PubMed] [Google Scholar]
- 115.O'Connel MJ, Child DS, Moertel CG. A prospective controlled evaluation of combined pelvic radiotherapy and methanol extraction residue of BCG for locally unresectable or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1982;8:1115–9. doi: 10.1016/0360-3016(82)90057-8. [DOI] [PubMed] [Google Scholar]
- 116.Wong CS, Cummings BJ, Keane TJ. Combined radiation therapy, mitomycin C, and 5-Fluorouracil for locally recurrent rectal carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:1291–6. doi: 10.1016/0360-3016(91)90288-f. [DOI] [PubMed] [Google Scholar]
- 117.Lybeert MLM, Martijin H, De Neve W. Radiotherapy for locoregional relapses of rectal carcinoma after initial radical surgery. Int J Radiat Oncol Biol Phys. 1992;24:241–6. doi: 10.1016/0360-3016(92)90678-b. [DOI] [PubMed] [Google Scholar]
- 118.Yamada S, Shinoto M, Endo S. Carbon ion radiotherapy for patients with locally recurrent rectal cancer. Proceedings of NIRS-ETOILE 2nd Joint Symposium on Carbon Ion Radiotherapy; 2011. pp. 54–9. Centre ETOILE, Lyon, NIRS-M-243. [Google Scholar]
- 119.Mobaraki A, Ohno T, Yamada S, et al. Cost-effectiveness of carbon ion radiation therapy for locally recurrent rectal cancer. Cancer Sci. 2010;101:1834–9. doi: 10.1111/j.1349-7006.2010.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kato S, Ohno T, Tsujii H, et al. Dose escalation study of carbon ion radiation for locality advanced carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2006;65:388–97. doi: 10.1016/j.ijrobp.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 121.Smith HO, Tiffany MF, Qualls CR, Keys CR. The rising incidence of adenocarcinomas relative to squamous cell carcinomas of the uterine cervix–a 24-year population based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 122.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 123.Willett CG, Czito BG, Bendell JC, Ryan DP. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538–44. doi: 10.1200/JCO.2005.23.911. [DOI] [PubMed] [Google Scholar]
- 124.Koong AC, Christofferson E, Le Q-T, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–3. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 125.Shinoto M, Yamada S, Yasuda S, et al. Carbon ion radiotherapy for pancreatic cancer. Proceedings of NIRS-ETOILE 2nd Joint Symposium on Carbon Ion Radiotherapy; 2011. pp. 60–5. Centre ETOILE, Lyon, NIRS-M-243. [Google Scholar]
- 126.Tsuji H, Ishikawa H, Hirasawa H, et al. Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: a Phase I/II Dose Escalation Study. Int J Radiat Oncol Biol Phys. 2007;67:857–62. doi: 10.1016/j.ijrobp.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 127.Fuss M, Loredo LN, Blacharski PA, et al. Proton radiation therapy for medium and large choroidal melanoma: preservation of the eye and its functionality. Int J Radiat Oncol Biol Phys. 2001;49:1053–9. doi: 10.1016/s0360-3016(00)01430-9. [DOI] [PubMed] [Google Scholar]
- 128.Hirasawa N, Tsuji H, Ishikawa H, et al. Risk factors for neovascular glaucoma after carbon ion radiotherapy of choroidal melanoma using dose–volume histogram Analysis. Int J Radiat Oncol Biol Phys. 2007;67:538–43. doi: 10.1016/j.ijrobp.2006.08.080. [DOI] [PubMed] [Google Scholar]