Abstract
Background
CuO is one of the most important transition metal oxides due to its captivating properties. It is used in various technological applications such as high critical temperature superconductors, gas sensors, in photoconductive applications, and so on. Recently, it has been used as an antimicrobial agent against various bacterial species. Here we synthesized different sized CuO nanoparticles and explored the size-dependent antibacterial activity of each CuO nanoparticles preparation.
Methods
CuO nanoparticles were synthesized using a gel combustion method. In this approach, cupric nitrate trihydrate and citric acid were dissolved in distilled water with a molar ratio of 1:1. The resulting solution was stirred at 100°C, until gel was formed. The gel was allowed to burn at 200°C to obtain amorphous powder, which was further annealed at different temperatures to obtain different size CuO nanoparticles. We then tested the antibacterial properties using well diffusion, minimum inhibitory concentration, and minimum bactericidal concentration methods.
Results
XRD spectra confirmed the formation of single phase CuO nanoparticles. Crystallite size was found to increase with an increase in annealing temperature due to atomic diffusion. A minimum crystallite size of 20 nm was observed in the case of CuO nanoparticles annealed at 400°C. Transmission electron microscopy results corroborate well with XRD results. All CuO nanoparticles exhibited inhibitory effects against both Gram-positive and -negative bacteria. The size of the particles was correlated with its antibacterial activity.
Conclusion
The antibacterial activity of CuO nanoparticles was found to be size-dependent. In addition, the highly stable minimum-sized monodispersed copper oxide nanoparticles synthesized during this study demonstrated a significant increase in antibacterial activities against both Gram-positive and -negative bacterial strains.
Keywords: CuO, nanoparticles, X-ray diffraction, FTIR, antimicrobial activity
Introduction
The unique, unusual and interesting physical, chemical, and biological properties of nanometer-sized materials have recently attracted a great deal of interest in the scientific community. As the size of materials is reduced to the nanometer regime the resulting properties change noticeably. Considerable efforts have been made to characterize and describe the physical and chemical properties of metal oxide nanomaterials because of their significant applications in numerous technological fields.1–4 The oxides of transition metals are an important class of semiconductors that have wider applications in magnetic storage media, solar energy transformation, electronics, and catalysis.5–12 Among various transition metal oxides, copper oxide (CuO) has attracted greater attention due to its fascinating properties such as the basis of high critical temperature (Tc) superconductors. CuO is a semiconducting compound with a narrow band gap and is used for photoconductive and photothermal applications.13 Reports on the preparation and characterization of nanocrystalline CuO are few compared to those on some other transition metal oxides, such as zinc oxide, titanium dioxide, tin dioxide, and iron oxide. Some methods for the preparation of nanocrystalline CuO have been reported such as the sonochemical method,14 sol-gel technique,15 one-step solid state reaction method at room temperature,16 electrochemical method,17 and thermal decomposition of precursors.18 Copper can also be used as an antimicrobial agent, and CuO nanoparticles have been investigated previously for enhancing antibacterial properties.19–22 Here we report a novel gel-combustion method, controlling the size of the synthesized nanoparticles and its effect on antimicrobial characteristics. The bactericidal property of such nanoparticles depends on their size, stability, and concentration added to the growth medium, since this provides greater retention time for bacterium nanoparticles interaction. In general, bacterial cells are in the micron-sized range. Most bacterial cells have cellular membranes that contain pores in the nanometer range. A unique property of crossing the cell membrane can potentially be attributed to synthesized nanoparticles through such bacterial pores. However, to make this possible, it is important to overcome challenges and prepare/design nanoparticles which are stable enough to significantly restrict bacterial growth while crossing the cell membrane.
To realize the potential of CuO nanoparticles to act as antimicrobial agents, we synthesized different sized CuO nanoparticles by controlling the annealing temperature during the gel-combustion synthesis. Furthermore, the antibacterial activities of CuO nanoparticles against two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) and two Gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli) were investigated.
Materials and methods
Synthesis of CuO nanoparticles
In a typical synthesis procedure, Cu(NO3)2 · 3H2O and citric acid were dissolved in distilled water with a molar ratio of 1:1. The solution was stirred with a magnetic stirrer at 100°C. Stirring continued until gel formation (approximately 1 hour). Afterwards, the gel was allowed to burn at 200°C. A light fluffy mass was obtained as a result of combustion, which was further annealed for 2 hours at varying temperatures, 400°C, 500°C, 600°C, and 700°C, to obtain the highly crystalline CuO nanoparticles.
Characterization of CuO nanoparticles
Synthesized CuO nanoparticles were characterized by X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), Raman spectroscopy, and transmission electron microscopy (TEM). Crystallinity, structure, and crystallite size of CuO nanoparticles were determined by XRD technique using a Rigaku-Miniflex X-ray diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu-Kα radiations (λ = 0.15406 nm) in 2θ range from 20° to 80°. TEM analysis was carried out using a 200 kV JEOL transmission electron microscope (JEOL Ltd, Tokyo, Japan). FTIR spectra of the samples were obtained using a PerkinElmer FTIR spectrophotometer (PerkinElmer Inc, Waltham, MA).
Determination of antimicrobial activity by the well-diffusion method
Antimicrobial activities of the synthesized CuO nanoparticles of different sizes were determined using Gram-negative bacteria (E. coli and P. aeruginosa) and Gram-positive bacteria (B. subtilis and S. aureus) following a modified Kirby Bauer disc diffusion method.23 In brief, the bacteria were cultured in Müller–Hinton broth at 35°C ± 2°C on an orbital shaking incubator (Remi, India) at 160 rpm. A lawn of bacterial culture was prepared by spreading 100 μL culture broth, having 106 CFU/mL of each test organism on solid nutrient agar plates. The plates were allowed to stand for 10–15 minutes. to allow for culture absorption. The 8 mm size wells were punched into the agar with the head of sterile micropipette tips. Wells were sealed with one drop of molten agar (0.8% nutrient agar) to prevent leakage from the bottom of the plate. Using a micropipette, 100 μL (50 μg) of the nanoparticles solution sample was poured into each of five wells on all plates. After incubation at 35°C ± 2°C for 24 hours, the size of the zone of inhibition was measured. A solvent blank was run as a negative control whereas the antibiotic (tetracycline) was used as a positive control.
Determination of minimal inhibitory/bactericidal concentrations (MIC/MBC)
Minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) of CuO nanoparticles were determined by the broth dilution method which conformed to the recommended standards of the National Committee for Clinical Laboratory Standards (NCCLS; now renamed the Clinical and Laboratory Standards Institute, CLSI, 2000). Tetracycline was used as a positive control. A dilution series with 10 mL nutrient broth medium containing 10–100 μg/mL copper oxide nanoparticles was prepared. Each set was inoculated aseptically with 50 μL of respective bacterial suspension (approximately 106 CFU/mL). The bacteria were plated onto solid nutrient agar plates. The lowest concentration inhibiting bacterial growth was defined as the MIC. In contrast the minimal concentration which completely inhibited the bacterial growth was defined as the minimum bactericidal concentration (MBC).24 Each experiment was repeated three times, and the resulting bacterial growth on three plates corresponding to a particular sample were averaged and reported.
Results
Structural analysis
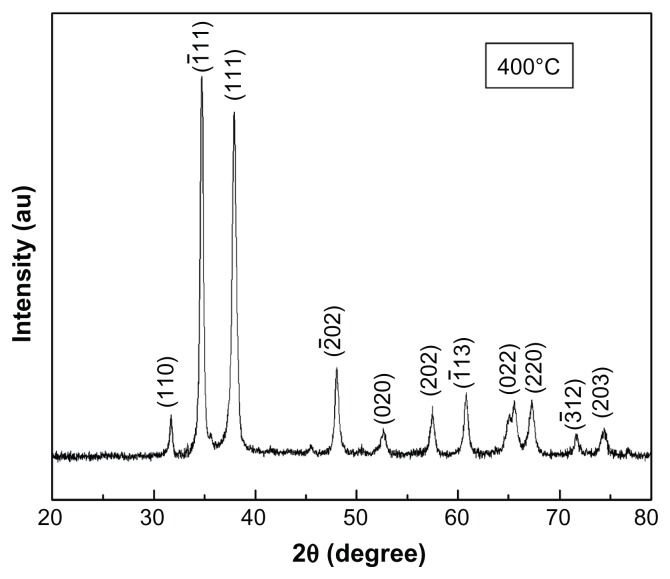
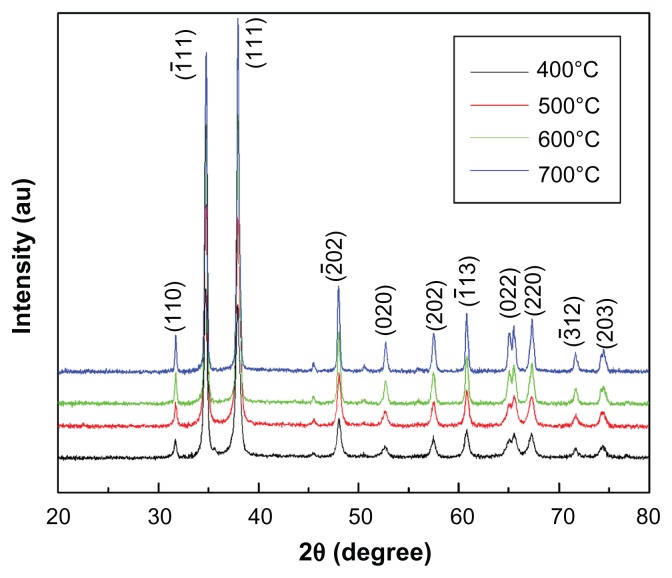
The typical XRD pattern of the CuO nanoparticles annealed at 400°C is shown in Figure 1. The peak positions of the sample exhibited the monoclinic structure of CuO which was confirmed from the International Centre for Diffraction Data (ICDD) card No 801916. Further, no other impurity peak was observed in the XRD pattern, showing the single phase sample formation. The crystalline size was calculated using the Scherrer formula, D = 0.9 λ/β cosθ, where λ is the wavelength of X-ray radiation, β is the full width at half maximum (FWHM) of the peaks at the diffracting angle θ. Crystallite size calculated by the Scherrer formula was found to be 20 nm. Lattice parameters calculated by powder X software (Cheng Dong, Institute of Physics, Chinese Academy of Sciences, Beijing, China) were found to be a = 4.688 Å, b = 3.427 Å, c = 5.132 Å. These values are in good agreement with the standard values reported by the ICDD Card No 801916. In order to investigate the effect of temperature on CuO nanoparticles, samples were further annealed at 500°C, 600°C, and 700°C. Figure 2 exhibits the XRD spectra of CuO nanoparticles annealed at different temperatures. It is clear from Figure 2 that the intensity of crystalline peaks increases with temperature, indicating an improvement in the samples crystallinity. Simultaneously, the peaks become narrower as the temperature increases resulting in the increase of crystallite size. The variation of crystallite size and lattice parameters with temperature was calculated and the results are presented in Table 1. It can be seen from Table 1 that crystallite size and lattice parameters increase with the increase in annealing temperature. The increase in crystallite size with temperature can be attributed to atomic diffusion. From an atomic perspective, diffusion is the stepwise migration of atoms from lattice site to lattice site. In fact, the atoms in solid materials are in constant motion, rapidly changing positions. For an atom to make such a move, the atom must have sufficient energy to break bonds with its neighbor atoms and then cause some lattice distortion during the displacement. As the temperature increases, the atoms gain sufficient energy for diffusive motion and hence increase in size takes place.
Figure 1.
XRD spectra of CuO nanoparticles annealed at 400°C.
Abbreviations: XRD, X-ray diffraction; CuO, copper oxide; AU, units of intensity.
Figure 2.
XRD spectra of CuO nanoparticles annealed at different temperatures.
Abbreviations: XRD, X-ray diffraction; CuO, copper oxide; AU, units of intensity.
Table 1.
Variation of crystallite size and lattice parameters with annealing temperature
| Temperature (°C) | a (Å) | b (Å) | c (Å) | Crystallite size (nm) |
|---|---|---|---|---|
| 400 | 4.688 | 3.427 | 5.132 | 20 |
| 500 | 4.711 | 3.429 | 5.133 | 21 |
| 600 | 4.715 | 3.430 | 5.135 | 25 |
| 700 | 4.719 | 3.432 | 5.136 | 27 |
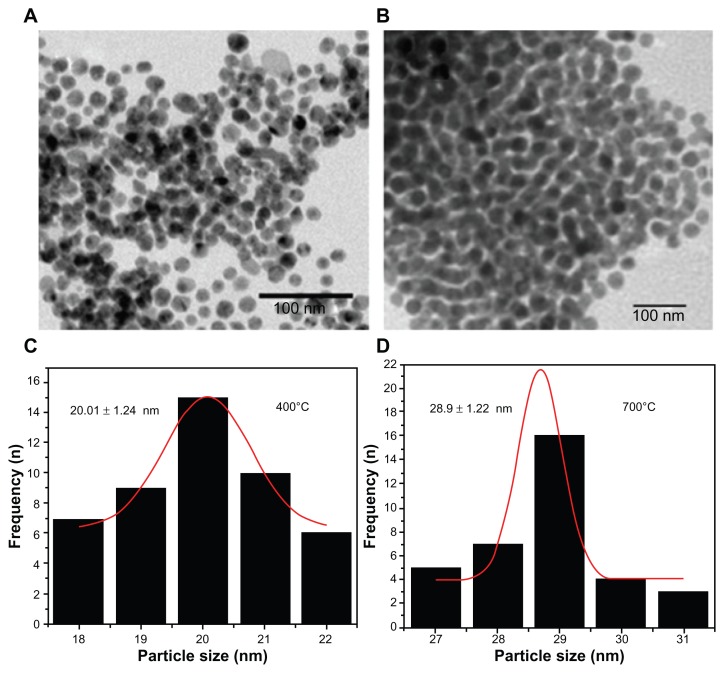
Figure 3A and B show the TEM micrographs of CuO nanoparticles sintered at 400°C and 700°C, respectively, while Figure 3C and D exhibit the size distribution of CuO nanoparticles sintered at 400°C and 700°C, respectively. Average particle sizes obtained from TEM images were found to be 20 ± 1.24 nm and 28.9 ± 1.22 nm for the samples sintered at 400°C and 700°C, respectively. The average particle sizes determined by TEM are closely matched to the crystallite size calculated from XRD results. TEM results also confirm the increase in the particle size with sintering temperature which corroborates well with the XRD results.
Figure 3.
TEM image of CuO nanoparticles.
Notes: TEM image of CuO nanoparticles annealed at (a) 400°C (b) 700°C.
Abbreviations: TEM, transmission electron microscopy; CuO, copper oxide; n, number.
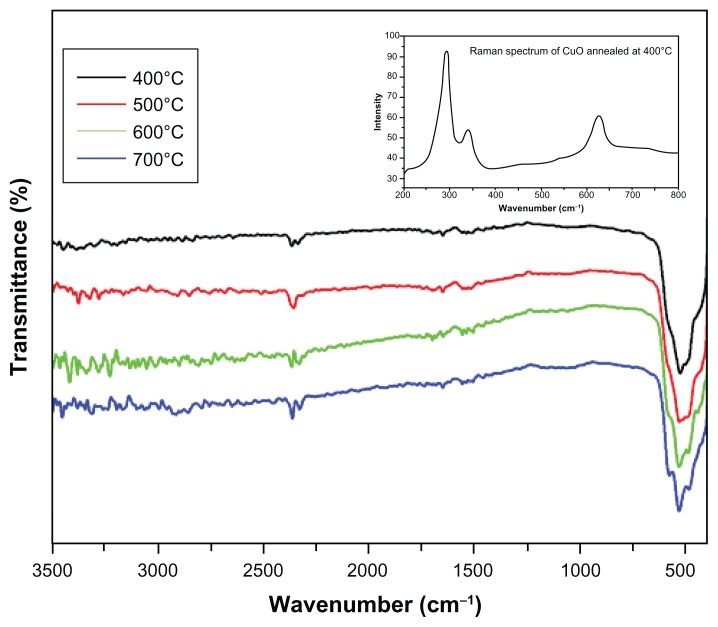
FTIR spectra were recorded in solid phase using the KBr pellets technique in the range of 3500–400 cm−1. FTIR spectra of CuO nanoparticles treated at 400°C, 500°C, 600°C, and 700°C are shown in Figure 4. FTIR spectra exhibit only three vibrations: occurring at approximately 480 cm−1, 530 cm−1, and 580 cm−1 for all the samples, which can be attributed to the vibrations of Cu-O, confirming the formation of highly pure CuO nanoparticles. A weak band at around 2300 cm−1 may be attributed to the vibrations of atmospheric CO2. These assignments are in agreement with the values available in literature.25–27
Figure 4.
FTIR spectra of CuO nanoparticles annealed at different temperatures.
Note: Inset shows Raman spectrum of CuO nanoparticles annealed at 400°C.
Abbreviations: FTIR, Fourier-transform infrared spectroscopy; CuO, copper oxide.
The Raman spectrum of CuO nanocrystals sintered at 400°C is shown in Figure 4, which exhibits three one-phonon Raman scattering bands at approximately 277.5 cm−1 (Ag), 329.9 cm−1 (Bg), and 610.8 cm−1 (Bg), respectively.28 The Raman spectra showed the presence of all three Raman active phonons (Ag + 2Bg) of CuO confirming the formation of CuO nanoparticles. The high crystalline nature of CuO nanoparticles is reflected by the significant intensity of Raman bands.
Antimicrobial properties
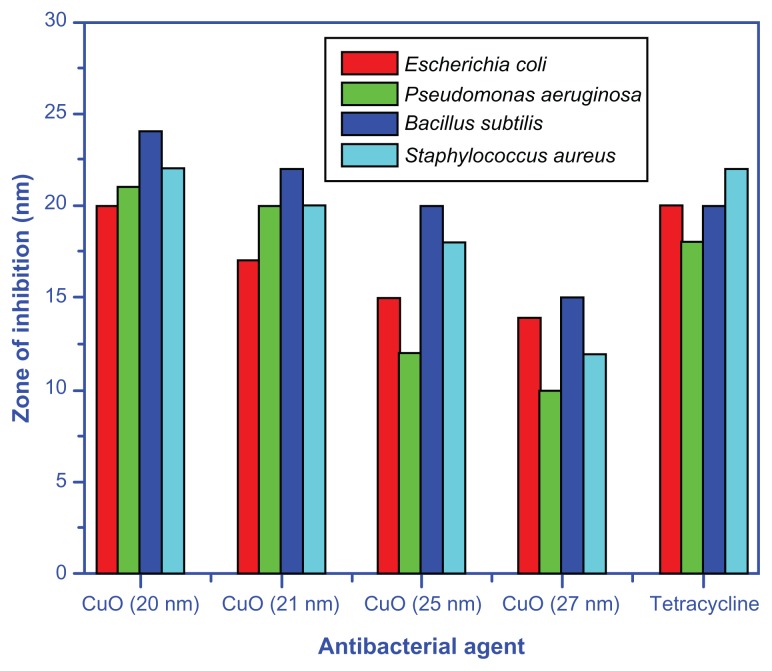
In this study, the copper oxide nanoparticles showed remarkable antibacterial activity against both Gram-positive (B. subtilis and S. aureus) and Gram-negative (E. coli and P. aeruginosa) bacteria (Table 2). The extent of inhibition of bacterial growth observed in this study was found to be variable and size-dependant. The smallest CuO nanoparticles (particle size 20 ± 1.24 nm) synthesized at the lowest temperature of 400°C, showed a significant inhibitory effect against both Gram-negative and -positive bacteria as compared to the CuO samples sintered at higher temperatures (Tables 3 and 4). One unique observation was that CuO nanoparticles synthesized at 400°C with the smallest particle size demonstrated the maximum zone of inhibition in the case of B. subtilis, which was 20% more than the zone of inhibition observed for tetracycline (Table 2). While comparing the effect of nanoparticles synthesized at varying temperature ranges, the greatest inhibitory effect was recorded for those CuO particles, which were synthesized at 400°C against P. aeruginosa strain. Furthermore, the smaller particles synthesized at 400°C had a zone of inhibition radii twice that of particles produced at 700°C. While comparing the effect of nanoparticles on bacterial strains, CuO nanoparticles were more toxic to E. coli regardless of the particle size except in one case (Tables 3 and 4). In all cases, the smaller the CuO nanoparticles, the lower the MIC and MBC values. In the case of the MIC, the most pronounced difference amongst all strains except S. aureus was between 400°C and 500°C nanoparticles. In case of S. aureus, the largest increase in MIC was observed for nanoparticles synthesized at temperatures between 500°C and 600°C (Table 3). For E. coli and S. aureus, the MIC values for particles synthesized at 700°C were threefold higher than those recorded for the nanoparticles synthesized at a comparatively smaller temperature (400°C). In general, the CuO nanoparticles had a less pronounced effect on other bacterial strains, but these effects were still twofold higher than those determined for 700°C synthesized particles. As can be seen in Table 4, the MBC values were even more drastically dependent on the particle size. Again we observed that E. coli and S. aureus had threefold lower values for the 400°C MBC than those synthesized at 700°C. As mentioned above, unlike the MIC values, each temperature dependent synthesis had more variable MBC values (Table 4); however, for the S. aureus, the largest MBC impact was from the 400°C to 500°C transition, unlike the MIC.
Table 2.
Antimicrobial activity of copper oxide (CuO) nanoparticles against two Gram-positive and two Gram-negative bacteria
| Treatment (100 μL) | Zone of inhibition (mm) | |||
|---|---|---|---|---|
|
| ||||
| Escherichia coli | Pseudomonas aeruginosa | Bacillus subtilis | Staphylococcus aureus | |
| CuO (400°C)a | 20 | 21 | 24 | 22 |
| CuO (500°C)b | 17 | 20 | 22 | 20 |
| CuO (600°C)c | 15 | 12 | 20 | 18 |
| CuO (700°C)d | 14 | 10 | 15 | 12 |
| Tetracyclinee | 20 | 18 | 20 | 22 |
Note:
indicate the copper oxide nanoparticles synthesized at different temperatures was used in an antimicrobial experiment on nutrient agar plates in Figure 5.
Table 3.
MIC of copper oxide nanoparticles (annealed at different temperatures) against different laboratory bacterial strains
| Microorganisms | MIC of copper nanoparticles (μg/mL) | |||
|---|---|---|---|---|
|
|
||||
| 400°C | 500°C | 600°C | 700°C | |
| E. coli | 20 ± 3 | 35 ± 5 | 45 ± 4 | 65 ± 6 |
| P. aeruginosa | 28 ± 4 | 40 ± 3 | 50 ± 5 | 55 ± 5 |
| B. subtilis | 30 ± 5 | 50 ± 4 | 65 ± 4 | 70 ± 5 |
| S. aureus | 25 ± 4 | 42 ± 2 | 70 ± 5 | 75 ± 4 |
Note: Each value in mean ± standard deviation.
Abbreviation: MIC, minimum inhibitory concentration.
Table 4.
MBC of copper oxide nanoparticles (annealed at different temperatures) against different laboratory bacterial strains
| Microorganisms | MBC of copper nanoparticles (μg/mL) | |||
|---|---|---|---|---|
|
|
||||
| 400°C | 500°C | 600°C | 700°C | |
| E. coli | 30 ± 2 | 45 ± 5 | 60 ± 3 | 95 ± 4 |
| P. aeruginosa | 35 ± 2 | 50 ± 3 | 75 ± 5 | 85 ± 5 |
| B. subtilis | 45 ± 3 | 62 ± 4 | 85 ± 4 | 95 ± 5 |
| S. aureus | 32 ± 5 | 75 ± 2 | 90 ± 5 | 100 ± 4 |
Note: Each value in mean ± standard deviation.
Abbreviation: MBC, minimum bactericidal concentration.
Discussion
A few studies have been performed to elucidate the mechanism of bactericidal action of nanoparticles. For example, Tsao et al suggested that the exposure of Gram-positive bacteria to carboxyfullerene nanoparticles resulted in the puncturing of the bacteria leading to cell death.29 Another proposed way in which the membrane can be compromised is the alteration of membrane lipid components.30 It is, however, difficult to distinguish between the bactericidal activity of nanoparticles from the ions released by the nanoparticles themselves.31 Previously, Ruparelia et al estimated the concentration of released ions for 10 mg of copper nanoparticles suspended in 100 mL nutrient media and distilled water.32 They found that the concentration of Cu2+ ions released in nutrient media was 17 mgL−1 after 24 hours of incubation in a rotary shaker, while in distilled water under the same conditions over a period of 24 hours, the concentration of ions released was 0.5 mgL−1. These results indicate that the nutrient media can facilitate the release of Cu2+ ions. The considerably greater release of Cu2+ ions in the nutrient media is possibly due to the interaction of the media chloride ions with the oxide layer of the nanoparticles.32 For oxidized copper particles embedded in an inert, Teflon-like matrix, Cioffi et al demonstrated significant antimicrobial activity due to the release of ions.33 Consequently, the bactericidal effects observed in this study might have been influenced by the release of Cu2+ ions in solution. The presence of nanoparticles in suspension would ensure continuous release of ions into the nutrient media.33 However, the preceding studies did not correlate their findings with the size of the nanoparticles and hence, the precise mechanisms of how nanoparticles act as biocidal agent needs to be explained. There are, however, a few mechanisms of nanoparticle toxicity suggested by other works. For example, copper ions released by the nanoparticles may attach to the negatively charged bacterial cell wall and rupture it, thereby leading to protein denaturation and cell death.34 Copper ions inside the bacterial cells may bind to deoxyribonucleic acid molecules and become involved in cross-linking within and between the nucleic acid strands, resulting in the disorganized helical structure. In addition, copper ion uptake by the bacterial cells has also been found to damage important biochemical processes.35,36
It is clear from Table 2 (maximum zone of inhibition against B. subtilis and S. aureus) that CuO nanoparticles have shown greater antimicrobial activity against B. subtilis and S. aureus. The variation in the sensitivity or resistance to both Gram-positive and -negative bacteria populations could be due to the differences in the cell structure, physiology, metabolism, or degree of contact of organisms with nanoparticles. For example, greater sensitivity among Gram-positive bacteria such as B. subtilis and S. aureus to the CuO nanoparticles has been attributed to the greater abundance of amines and carboxyl groups on their cell surface and greater affinity of copper towards these groups.37 Alternatively, Gram-negative bacteria like E. coli have a special cell membrane structure which possesses an important ability to resist antimicrobial agents.38 Furthermore, other factors such as nanoparticle diffusion rates may also affect bacterial strain differently. Nevertheless, further studies are required to confirm this and it is beyond the scope of this manuscript.
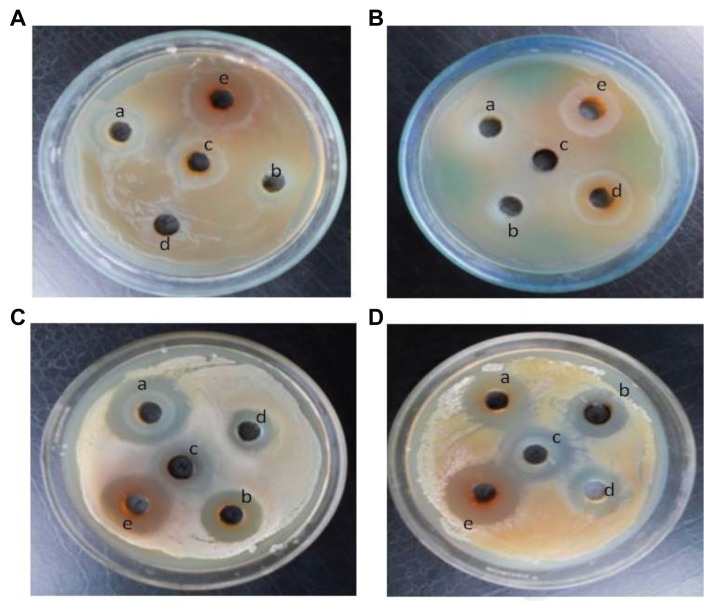
Figures 5 and 6 exhibit the zone of inhibition of CuO nanoparticles synthesized at different temperatures (a–d) and positive control, a known antibiotic tetracycline (e), against two Gram-negative bacteria [(A) E. coli (B) P. aeruginosa], and two Gram-positive bacteria [(C) B. subtilis (D) S. aureus]. Figure 5 clearly indicates that the copper oxide nanoparticles inhibit the growth of both Gram- negative and -positive bacteria and the zone of inhibition decreases with the increase in annealing temperature from 400°C–700°C. Figure 6 shows that the zone of inhibition is maximum when the particle size is minimum (20 ± 1.24 nm). These results demonstrate the excellent antimicrobial behavior of CuO nanoparticles synthesized at low temperature. Broadly, interactions between the negative charges of microorganisms and the positive charge of nanoparticles produces an electromagnetic attraction between the microbe and effective levels of active nanoparticles. Such interactions lead to oxidation of surface molecules of microbes resulting in their death. Biodestructive effects such as degradation of deoxyribonucleic acid was observed following exposure of Gram-positive bacteria to silver and copper nanoparticles by other works,39,40 and are in agreement with present findings.
Figure 5.
Zone of inhibition of copper oxide nanoparticles.
Notes: Zone of inhibition of copper oxide nanoparticles synthesized at different temperatures (a–d) and positive control, a known antibiotic tetracycline (e) against two Gram-negative bacteria (A) Escherichia coli and (B) Pseudomonas aeruginosa, and two Gram-positive bacteria (C) Bacillus subtilis and (D) Staphylococcus aureus.
Figure 6.
Bar graph representing the zone of inhibition for CuO nanoparticles and tetracycline against Gram-positive and -negative bacteria.
Abbreviation: CuO, copper oxide.
Conclusion
We have successfully synthesized CuO nanoparticles using a gel combustion route. XRD spectra confirmed the formation of single phase CuO nanoparticles. Crystallite size was found to increase with the increase in annealing temperature. Minimum crystallite size of 20 ± 1.24 nm was observed in the case of CuO nanoparticles annealed at 400°C. TEM results corroborate well with XRD results. FTIR and Raman spectra also validated the purity of CuO nanoparticles. Antibacterial activity experiments performed on various microorganisms clearly demonstrated the higher effectiveness of CuO nanoparticles annealed at 400°C against bacterial growth due to smaller particle size of this sample compared to other samples. Zone of inhibition for all the microorganisms reached a maximum point using CuO nanoparticles annealed at 400°C. Moreover, minimum inhibitory concentration and minimum bactericidal concentration of CuO nanoparticles annealed at 400°C was lowest for all the bacterial strains.
Acknowledgments
Mr Arham S Ahmed and Mr M Oves are thankful to CSIR, New Delhi for providing financial support in the form of SRF. Dr Adnan Memic would like to thank the Strategic Technologies Program by King Abdulaziz City for Science and Technology, grant number 10-NAN1081-3 for their partial support and funding of this project.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ueda N, Maeda H, Hosono H, Kawazoe H. Band-gap widening of CdO thin films. J Appl Phys. 1998;84(11):6174–6177. [Google Scholar]
- 2.Liu H, Zhang X, Li L, et al. Role of point defects in room-temperature ferromagnetism of Cr-doped ZnO. Appl Phys Lett. 2007;91(7):072511–072513. [Google Scholar]
- 3.Zhu H, Zhao F, Pan L, et al. Structural and magnetic properties of Mn-doped CuO thin films. J Appl Phys. 2007;101(9):09H111–09H111-113. [Google Scholar]
- 4.Ferreira FF, Tabacniks MH, Fantinia MCA, Fariab IC, Gorensteinb A. Electrochromic nickel oxide thin films deposited under different sputtering conditions. Solid State Ionics. 1996;(2):86–88. 971–976. [Google Scholar]
- 5.Bradley FN. Chapter 2. In: Bradley FN, editor. Materials for Magnetic Functions. New York, NY: Hayden; 1976. [Google Scholar]
- 6.Mitsuyu T, Yamazaki O, Ohji K, Wasa K. Piezoelectric thin films of zinc oxide for saw devices. Ferroelectrics. 1982;42(1):233–240. [Google Scholar]
- 7.O’Regan B, Gratzel M. A low-cost, high-efficiency solar cell based on dye- sensitized colloidal TiO2 films. Nature. 1991;353(6346):737–740. [Google Scholar]
- 8.Wang Y, Cheng H, Zhang L, et al. The preparation, characterization, photoelectrochemical and photocatalytic properties of lanthanide metal-ion-doped TiO2 nanoparticles. J Mol Catal A Chem. 2000;151(1–2):205–216. [Google Scholar]
- 9.Bjoerksten U, Moser J, Graetzel M. Photoelectrochemical studies on nanocrystalline hematite films. Chem Mater. 1994;6(6):858–863. [Google Scholar]
- 10.Dow WP, Huang TJ. Yttria-stabilized zirconia supported copper oxide catalyst: II. Effect of oxygen vacancy of support on catalytic activity for CO oxidation. Journal of Catalysts. 1996;160(2):171–182. [Google Scholar]
- 11.Larsson PO, Andersson A, Wallenberg RL, Svensson B. Combustion of CO and Toluene; Characterisation of copper oxide supported on Titania and activity comparisons with supported cobalt, iron, and manganese oxide. Journal of Catalysts. 1996;163(2):279–293. [Google Scholar]
- 12.Jiang Y, Decker S, Mohs C, Klabunde KJ. Catalytic solid state reactions on the surface of nanoscale metal oxide particles. Journal of Catalysts. 1998;180(1):24–35. [Google Scholar]
- 13.Rakhshani AE. Preparation, characteristics and photovoltaic properties of cuprous oxide – a review. Solid State Electron. 1986;29(1):7–17. [Google Scholar]
- 14.Kumar RV, Diamant Y, Gedanken A. Sonochemical synthesis and characterization of nanometer-size transition metal oxides from metal acetates. Chem Mater. 2000;12(8):2301–2305. [Google Scholar]
- 15.Eliseev AA, Lukashin AV, Vertegel AA, Heifets LI, Zhirov AI, Tretyakov YD. Complexes of Cu(II) with polyvinyl alcohol as precursors for the preparation of CuO/SiO2 nanocomposites. Materials Research Innovations. 2000;3(5):308–312. [Google Scholar]
- 16.Xu JF, Ji W, Shen ZX, et al. Preparation and characterization of CuO nanocrystals. J Solid State Chem. 2000;147(2):516–519. [Google Scholar]
- 17.Borgohain K, Singh JB, Rama Rao MV, Shripathi T, Mahamuni S. Quantum size effects in CuO nanoparticles. Phys Rev B. 2000;61(16):11093–11096. [Google Scholar]
- 18.Salavati-Niasari M, Davar F. Synthesis of copper and copper (I) oxide nanoparticles by thermal decomposition of a new precursor. Mater Lett. 2009;63(3–4):441–443. [Google Scholar]
- 19.Horiguchi H. Chemistry of Antimicrobial Agents. Tokyo, Japan: Sankyo Press; 1980. p. 46. [Google Scholar]
- 20.Ojas M, Bhagat M, Gopalakrishnan C, Arunachalam KD. Ultrafine dispersed CuO nanoparticles and their antibacterial activity. Journal of Experimental Nanoscience. 2008;3(3):185–193. [Google Scholar]
- 21.Li B, Yu S, Hwang JY, Shi S. Antibacterial vermiculite nano-material. Journal of Minerals and Materials Characterization and Engineering. 2002;1(1):61–68. [Google Scholar]
- 22.Condorelli GG, Costanzo IL, Fragala IL, Giuffrida S, Ventimiglia G. A single photochemical route for the formation of both copper nanoparticles and patterned nanostructured films. J Mater Chem. 2003;13(10):2409–2411. [Google Scholar]
- 23.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 24.Tarpay MM, Welch DF, Marks MI. Antimicrobial susceptibility testing of Streptococcus pneumonia by micro-broth dilution. Antimicrob Agents Chemother. 1980;18(4):579–581. doi: 10.1128/aac.18.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagminas A, Kuzmarskyt J, Niaura G. Electrochemical formation and characterization of copper oxygenous compounds in alumina template from ethanolamine solutions. Appl Surf Sci. 2002;201(1–4):129–137. [Google Scholar]
- 26.Jagminas A, Niaura G, Kuzmarskyt J, Butkiene R. Surface-enhanced Raman scattering effect for copper oxygenous compounds array within the alumina template pores synthesized by ac deposition from Cu(II) acetate solution. Appl Surf Sci. 2004;225(1–4):302–308. [Google Scholar]
- 27.Zhang YC, Tang JY, Wang GL, Zhang M, Hu XY. Facile synthesis of submicron Cu2O and CuO crystallites from a solid metallorganic molecular precursor. J Crys Growth. 2006;294(2):278–282. [Google Scholar]
- 28.Irwin JC, Chrzanowski J, Wei T, Lockwood DJ, Wold A. Raman scattering from single crystals of cupric oxide. Physica C Supercond. 1990;166(5–6):456–464. [Google Scholar]
- 29.Tsao N, Luh TY, Chou CK, et al. In vitro action of carboxyfullerene. J Antimicrob Chemother. 2002;49(4):641–649. doi: 10.1093/jac/49.4.641. [DOI] [PubMed] [Google Scholar]
- 30.Koch AM, Reynolds F, Merkle HP, Weissleder R, Josephson L. Transport of surface- modified nanoparticles through cellmonolayers. Chem bio chem. 2005;6(2):337–345. doi: 10.1002/cbic.200400174. [DOI] [PubMed] [Google Scholar]
- 31.Yoon K, Hoon Byeon J, Park JH, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ. 2007;373(2–3):572–575. doi: 10.1016/j.scitotenv.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4(3):707–716. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Cioffi N, Ditaranto N, Torsi L, et al. Analytical characterization of bioactive fluoropolymer ultra-thin coatings modified by copper nanoparticles. Anal Bioanal Chem. 2005;381(3):607–616. doi: 10.1007/s00216-004-2761-4. [DOI] [PubMed] [Google Scholar]
- 34.Lin YE, Vidic RD, Stout JE, McCartney CA, Yu VL. Inactivation of Mycobacterium avium by copper and silver ions. Water Res. 1998;32(7):1997–2000. [Google Scholar]
- 35.Kim JH, Cho H, Ryu SE, Choi MU. Effects of metal ions on the activity of protein tyrosine phosphatase VHR: highly potent and reversible oxidative inactivation by Cu2+ ion. Arch Biochem Biophys. 2000;382(1):72–80. doi: 10.1006/abbi.2000.1996. [DOI] [PubMed] [Google Scholar]
- 36.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18(2):321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 37.Beveridge TJ, Murray RG. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141(2):876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang X, Sun M, Li L, et al. Preparation and antibacterial activities of polyaniline/Cu0.05Zn0.95O nanocomposites. Dalton Trans. 2012;41(9):2804–2811. doi: 10.1039/c2dt11823h. [DOI] [PubMed] [Google Scholar]
- 39.Rezaei-Zarchi S, Javed A, Ghani MJ, et al. Comparative study of antimicrobial activities of TiO2 and CdO nanoparticles against the pathogenic strain of Escherichia coli. Iran J Pathol. 2010;5(2):83–89. [Google Scholar]
- 40.Hosseinkhani AM, Zand AM, Imani S, Rezayi M, Rezaei-Zarchi S. Determining the antibacterial effect of ZnO nanoparticle against the pathogenic bacterium, Shigella dysenteriae (type 1) International Journal of Nano Dimension. 2010;1(4):279–285. [Google Scholar]