Abstract
The present study evaluated the acute effects of radiation dose, dose rate and fractionation as well as the energy of protons in hematopoietic cells of irradiated mice. The mice were irradiated with a single dose of 51.24 MeV protons at a dose of 2 Gy and a dose rate of 0.05–0.07 Gy/min or 1 GeV protons at doses of 0.1, 0.2, 0.5, 1, 1.5 and 2 Gy delivered in a single dose at dose rates of 0.05 or 0.5 Gy/min or in five daily dose fractions at a dose rate of 0.05 Gy/min. Sham-irradiated animals were used as controls. The results demonstrate a dose-dependent loss of white blood cells (WBCs) and lymphocytes by up to 61% and 72%, respectively, in mice irradiated with protons at doses up to 2 Gy. The results also demonstrate that the dose rate, fractionation pattern and energy of the proton radiation did not have significant effects on WBC and lymphocyte counts in the irradiated animals. These results suggest that the acute effects of proton radiation on WBC and lymphocyte counts are determined mainly by the radiation dose, with very little contribution from the dose rate (over the range of dose rates evaluated), fractionation and energy of the protons.
INTRODUCTION
As reviewed previously (1), the primary components of radiation in interplanetary space are galactic cosmic radiation (GCR) and solar cosmic radiation (SCR). GCR originates from outside of our Solar System and consists of 98% baryons and 2% electrons. The baryonic component consists of 87% protons (hydrogen nuclei), 12% α particles (helium nuclei) and approximately 1% of heavier nuclei with atomic numbers (Z) up to 92 (uranium). SCR is composed predominately of protons, with a minor contribution from helium ions (~10%) and an even smaller contribution from heavy ions and electrons (~1%) (2, 3). Exposure to space radiation may place astronauts at significant risk for acute radiation sickness and significant skin injury as well as death from a major solar particle event (SPE) or combined SPE and GCR. The biological effects of ionizing radiation are influenced by three parameters of the radiation exposure: the total dose absorbed, the rate at which the dose is delivered, and the quality of the radiation (4, 5). Exposure to radiation during a space mission may immediately affect the probability for successful mission completion (mission critical) or may result in late radiation effects in individual astronauts (1).
Acute radiation sickness has a sequence of a phased syndrome that varies with radiation dose, dose rate and quality and individual radiation sensitivity (1). The acute effects are manifested at approximately 4 to 24 h after radiation exposures at sublethal doses, with a latent time that is inversely correlated with dose. There is also a reasonable concern about a compromised immune system because high skin doses from an SPE can lead to burns. While avoidance of the radiation exposure is the best protective strategy, it is nearly impossible to avoid the radiation risk completely for astronauts. Thus health risks are a concern for astronauts exposed to GCR and SCR, of which protons are an important component, particularly during an SPE. One of the vital tissues/organs that can be suppressed after total-body irradiation (TBI) is the hematopoietic system. The fate of hematopoietic cells after TBI may determine whether the irradiated subjects survive or die (4–7). The present study was undertaken to determine the acute effects of dose, dose rate and dose fractionation as well as the energy of the proton radiation on hematopoietic cells of mice irradiated with protons.
Several previous studies have been performed to evaluate the effects of radiation, including simulated space radiation, on blood cells in mice [e.g. refs. (8, 9)]. In studies evaluating mouse blood cell counts, the blood samples were processed by several different methods, including manual cell counts performed in the Kennedy laboratory and instrument-based automated cell counts from various clinical facilities. A major difference between the methods used involves the time between the collection of blood samples from the irradiated animals in the laboratory and the time at which the automated analyses were performed by the clinical facilities (which could be up to 18 h after the blood was collected).
In preparation for these studies in which the blood cell counts of mice exposed to SPE radiations would be evaluated, a pilot study was performed to determine the most appropriate methods to use for quantitative comparisons of blood cell samples taken from mice at different radiation facilities in different cities. Blood cell samples were taken from mice exposed to 0.25, 0.5, 1 and 2 Gy of 6 MeV electrons for the analysis of white blood cell (WBC) differential counts by manual cell counts as well as automated cell counts (performed by Antech Diagnostics). The results obtained for lymphocytes and neutrophils, the most abundant WBCs, were compared with the two different methods of preparation by the Student’s t test and linear regression analysis, and the agreement between the two techniques was determined by Bland-Altman plotting. The results of these studies demonstrated a close correlation and a high degree of agreement between manual counts performed shortly after the radiation exposures and those determined from automated cell counts produced by Antech Diagnostics (Drs. Ana Romero-Weaver and Ann R. Kennedy, unpublished data). Because Antech Diagnostics (Irvine, CA) has clinical laboratories near the radiation facilities that are being used by the Kennedy laboratory for research projects involving blood cell counts [i.e., the University of Pennsylvania, the NASA Space Radiation Laboratory (NSRL) and the Loma Linda University Medical Center], it was determined that Antech Diagnostics would be used for all of the mouse blood cell samples taken for analysis of WBC differential counts in future studies, including those described in this report.
There is particular concern about the acute effects of SPE radiation on blood cell numbers shortly after the radiation exposure. In this article, the focus is on the effects of SPE radiation on blood cell numbers from blood cell samples taken from animals at 24 h after the radiation exposure. Thus the results presented here do not reflect the effects of SPE radiation on hematopoietic stem cell renewal systems.
MATERIALS AND METHODS
Animals
Male ICR mice aged 4–5 weeks were purchased from Taconic Farms, Inc. (Germantown, NY) and used in the present study. This outbred animal model was chosen for the present study so that results can be compared with results of a recently completed X-ray study that also used 4–5-week-old male ICR mice (8). Upon arrival, the animals were acclimated for 7 days in the Brookhaven National Laboratory (BNL) Animal Facility. Four animals were housed per cage with ad libitum access to water and food pellets.
The animals were maintained on a control AIN-93G diet, which was supplied by Bio-Serv (Frenchtown, NJ). Based on information from Taconic Farms Inc., the reference values for WBC and lymphocyte counts of male ICR mice are 6.66 ± 2.05 (mean ± SD, × 103 cells/μl) and 5.52 ± 1.71 (× 103 cells/μl), respectively (n = 10).
The animal use and the procedures for the animal care and treatment were approved by the Institutional Animal Care and Use Committees (IACUCs) of the University of Pennsylvania and BNL.
Proton Radiation Exposure
All proton irradiations performed as part of the studies described here were performed at the NASA Space Radiation Laboratory (NSRL) at BNL. After 7 days of acclimation, the mice were exposed to TBI with 51.24 MeV protons at a dose of 2 Gy or 1 GeV protons at doses of 0.1, 0.2, 0.5, 1, 1.5 and 2 Gy. Sham-irradiated animals were used as controls. The 51.24 MeV proton radiation was delivered in a single dose at a dose rate of 0.05–0.07 Gy/min. The 1 GeV proton radiation was delivered in a single dose at a low (0.05 Gy/min) or high (0.5 Gy/min) dose rate or in five daily dose fractions with a fixed dose rate of 0.05 Gy/min.
For a 51.24 MeV proton beam, the uniformity is ±5% (σ). The LET and range in water are 1.23 keV/μm and 2.3 cm, respectively. The beam was delivered from the booster such that, after emerging from the vacuum window and traversing the few meters of air in the target room, it reached the target volume at 51.24 MeV, without the use of degraders in the target room.
For a 1 GeV proton beam, the uniformity is ±1.3% (σ). The LET and range in water are 0.22 keV/μm and 3.22 m, respectively.
The dosimetry is done at NSRL as follows:
The ion chamber that is used for beam cutoff and that is positioned just upstream of the target volume is calibrated against a small thimble chamber positioned at the center of the target volume. The thimble chamber is calibrated annually by the manufacturer (FarWest).
The dose to the target volume is now measured by the dose on the calibrated cutoff chamber, which affects the beam cutoff when the desired dose, entered by the user at the dosimetry computer is reached, with cutoff-related overshoot errors typically kept to sub percent levels.
The thimble chamber used at NSRL is of the thin-wall variety (0.5-mm tissue-equivalent plastic), making it sufficiently reliable for 51.24 MeV protons. Much lower energy or a thicker wall would make the proton track variation throughout the volume of the chamber too large to allow straightforward use of the manufacturer’s calibration number.
The holder used for the animals was a 50-ml tube for each animal, all held together in a slotted foam holder that positions up to 10 animals at a time in the beam. The tube walls are about 1-mm-thick polyethylene, in which 51.24 MeV protons would lose about 1 MeV before reaching the animal. The animals are free to move around in the tube and in fact do, causing randomization of beam direction in the body. The tubes are oriented with their long axis perpendicular to the beam direction, so the mice spend almost all of the time with their width along the beam direction. Thus, for the 1 GeV proton beam, the protons are not expected to stop in the animal. For the 51.24 MeV protons, the protons are expected to stop in the animal. During the irradiation, the incident dose and dose rate were measured. In this case, if the animal movement has effectively created an isotropic source, the dose at the center of the animal would be about 2.5 times higher than the total incident dose as measured by the dosimetry ion chamber. This value was obtained from a simulation in which the animal was modeled by a 3-cm-diameter cylinder of density ρ = 1 and the proton beam was made to come in from four directions.
Hematopoietic Cell Count Analyses
At 24 h after the last radiation exposure, six mice irradiated at each dose and dose rate were killed by CO2 asphyxiation followed by cardiac puncture to collect blood. The blood from each animal was collected into lavender top blood collection tubes containing EDTA and kept at ambient temperature. The blood samples were sent to Antech Diagnostics (the company headquarters are in Irvine, CA; however, the Antech Diagnostics blood cell analysis facility used for these particular studies was located in New York) and complete blood count (CBC) analyses were performed by a Bayer Advia 120 Hematology Analyzer (detailed methods are available).
Data and Statistical Analyses
The white blood cell and lymphocyte count data for different treatment groups were analyzed and compared by one-way ANOVA followed by Tukey’s test using GraphPad InStat statistical software (version 3.00, GraphPad Software, San Diego, CA). The data are presented in the figures as means ± SE.
RESULTS
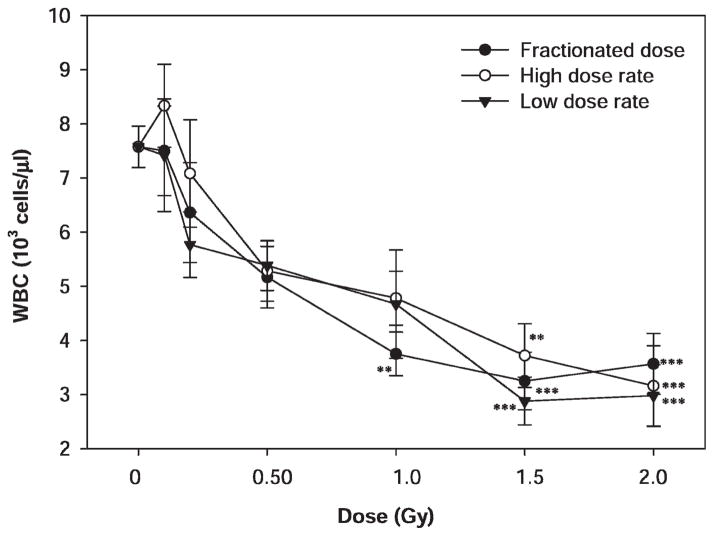
In the present study, the blood cell count was performed on blood samples collected from mice by cardiac puncture 24 h after irradiation with 1 GeV protons. The WBC and lymphocyte count data were analyzed using a general linear model followed by one-way ANOVA and Tukey’s test to compare the means among different treatment groups. The mean WBC and lymphocyte counts for the sham-irradiated animals were 7.6 ± 1.3 and 3.7 ± 1.7 (× 103 cells/μl), respectively, which were not significantly different from the reference WBC and lymphocytes counts for male ICR mice. Exposure to 1 GeV proton radiation in five daily dose fractions or a single dose with a low or high dose rate resulted in dose-dependent decreases WBCs (Fig. 1), and the dose responses were statistically significant (P < 0.001). Due to relatively large variations in individual radiation dose groups, the differences in WBC counts between the sham-irradiated and irradiated groups reached statistical significance (P < 0.05) only at a dose of 1 Gy or higher for animals irradiated with protons in five daily dose fractions and at a dose of 1.5 Gy or higher for animals irradiated with protons in a single dose with a low or high dose rate. The difference among animals irradiated with multiple fractions or a single dose at low or high dose rate did not reach statistical significance at any of the doses evaluated (P > 0.05).
FIG. 1.
Dose–response effects of proton radiation on WBC counts in irradiated mice. The mice were irradiated with 1 GeV protons in five daily dose fractions at a dose rate of 0.05–0.07 Gy/min or in a single dose at a high (0.5 Gy/min) or low (0.05 Gy/min) dose rate (5–10 mice per group). The WBC counts were determined 24 h after the last radiation exposure. The results were compared among different treatment groups by one-way ANOVA followed by Tukey’s test. The statistical significance of the difference between the sham-irradiated control and each of the irradiated groups is indicated by asterisks (**P < 0.01 and ***P < 0.001 by Tukey’s test). The error bars represent standard errors.
At 24 h after radiation exposure, the mean WBC counts were 3.6 ± 1.4, 3.2 ± 1.7, 3.0 ± 1.3 and 3.1 ± 2.3 (× 103 cells/μl), respectively, for the animals irradiated with 2 Gy of 1 GeV protons in dose fractions or a single dose with a high or low dose rate or irradiated with 2 Gy of 51.24 MeV protons. These mean WBC counts were not significantly different from one another, but they were all significantly below the WBC count of the sham-irradiated control group (P < 0.001).
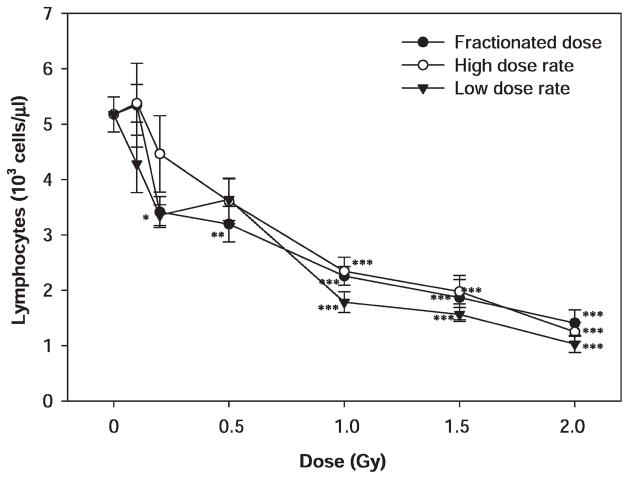
Dose-dependent decreases were also observed for lymphocytes after the proton exposure (Fig. 2), and the dose responses were statistically significant (P < 0.001). The differences in lymphocyte counts between the sham and irradiated groups reached statistical significance (P < 0.05) at a dose of 0.5 Gy or higher for animals irradiated with protons in five daily dose fractions and at a dose of 1 Gy or higher for animals irradiated with protons with a single dose at a low or high dose rate. The differences among animals irradiated with multiple fractions or a single dose with a low or high dose rate did not reach statistical significance at any of the radiation doses evaluated (P > 0.05).
FIG. 2.
Dose–response effects of proton radiation on lymphocyte counts in irradiated mice. The mice were irradiated with 1 GeV protons in five daily dose fractions at a dose rate of 0.05–0.07 Gy/min or in a single dose at a high (0.5 Gy/min) or low (0.05 Gy/min) dose rate (5–10 mice per group). The lymphocyte counts were determined 24 h after the last radiation exposure. The results were compared among different treatment groups by one-way ANOVA followed by Tukey’s test. The statistical significance for the difference between the sham-irradiated control and each of the irradiated groups is indicated by asterisks (*P < 0.05, **P < 0.01 and ***P < 0.001 by Tukey’s test). The error bars represent standard errors.
For the animals irradiated with 2 Gy of 1 GeV protons in multiple fractions or a single dose with a high or low dose rate or irradiated with 2 Gy of 51.24 MeV protons, the mean lymphocyte counts were 1.4 ± 0.6, 1.3 ± 0.4, 1.0 ± 0.4 and 1.2 ± 0.6 (× 103 cells/μl), respectively, at 24 h after irradiation. These mean lymphocyte counts were not significantly different from one another, but they were all significantly below the mean lymphocyte count of the sham-irradiated control.
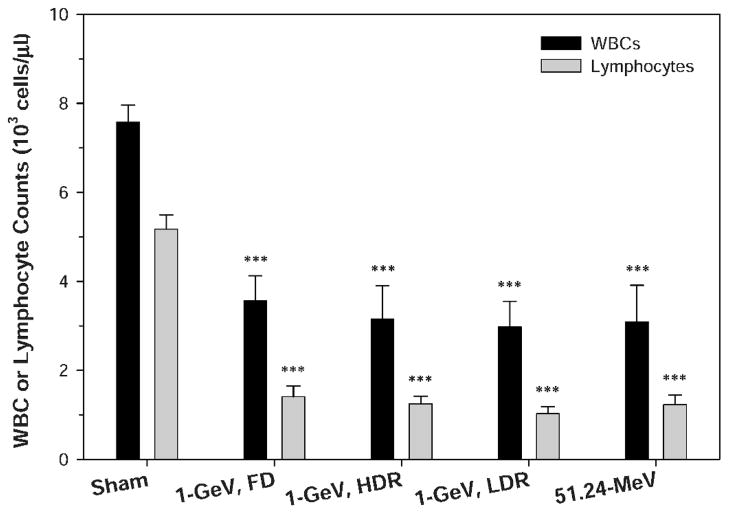
The results for the different treatment regimens used in these studies described above compared directly in terms of WBC and lymphocyte counts among mice irradiated with 1 GeV and 51.24 MeV protons at a dose of 2 Gy in Figure 3. The mice were irradiated with 1 GeV protons in five daily fractions at a dose rate of 0.05–0.07 Gy/min or in a single dose at a high or low dose rate or with 51.24 MeV protons in a single dose at a dose rate of 0.05–0.07 Gy/min (5–10 mice per group). The WBC and lymphocyte counts were determined 24 h after the last radiation exposure. The results were compared among different treatment groups by one-way ANOVA followed by Tukey’s test. There were no significant differences in the WBC or lymphocyte counts in the mice exposed to a dose of 2 Gy according to the different treatment conditions.
FIG. 3.
Comparison of WBC and lymphocyte counts among mice irradiated with 1 GeV and 51.24 MeV protons at a dose of 2 Gy. The mice were irradiated with 1 GeV protons in five daily dose fractions at a dose rate of 0.05–0.07 Gy/min (1-GeV, FD) or in a single dose at a high (1-GeV, HDR) or low (1-GeV, LDR) dose rate or with 51.24 MeV protons in a single dose at a dose rate of 0.05–0.07 Gy/min (51.24–MeV) (5–10 mice per group). The WBC and lymphocyte counts were determined 24 h after the last radiation exposure. The results were compared among different treatment groups by one-way ANOVA followed by Tukey’s test. The statistical significance for the difference between the sham-irradiated control and each of the irradiated groups is indicated by asterisks (***P < 0.001 by Tukey’s test). The error bars represent standard errors.
DISCUSSION
The present study was undertaken to determine the acute effects of proton radiation dose, dose rate and fractionation pattern as well as the energy of proton radiation on hematopoietic cells after total-body irradiation of mice. WBC and lymphocyte counts exhibited significant and dose-dependent declines of up to 61% and 72%, respectively, within 24 h after exposure to 1 GeV or 51.24 MeV protons at doses up to 2 Gy. Thus the acute adverse biological effects of proton radiation in hematopoietic cells affect cells involved in immune responses. The results presented here indicate that doses of proton radiation in the range expected for an SPE (up to 2 Gy deep dose, or a dose expected to irradiate the blood-forming tissues) and a proton energy (51.24 MeV) like that of a major fraction of protons in an SPE can result in losses of white blood cells that are statistically significant in mice and could lead to adverse biological effects. It is not known, however, whether lower dose rates than used here, such as the dose rate expected during an SPE (up to 0.5 Gy/h), would have the same effects on blood cell losses as those observed at the higher dose rates we used. The hematopoietic system is very important in determining the final outcome of radiation exposure. Thus the development of methods to mitigate the adverse biological effects of proton radiation on hematopoietic cells and/or to enable the recovery of the hematopoietic system after radiation exposure will remain an important goal of space radiation research.
In the present study, the proton radiation dose rate (at the doses used) and fractionation pattern had no significant effects on the dose responses of WBC and lymphocyte counts in the irradiated animals. These results are consistent with a previous report by Evans et al. (10), who found that the survival response of bone marrow stem cells is independent of dose rate in the range of 0.08 to 0.48 Gy/min. A similar lack of dose-rate effects for protons was also observed for some hematopoietic cell types (11, 12). When the results of the studies reported here are compared to those of other studies performed at comparable dose rates, the results are also consistent (11–13), even though the other studies used animals of a different strain, gender and age. The present results are not consistent with one report on hematopoietic stem cells/tissues showing a significant impact of dose rate and/or fractionation (14) on hematopoietic cell survival and/or recovery after irradiation. The studies described in ref. (14) were designed to evaluate the long-term effects of radiation exposure on hematopoietic cells, whereas the studies reported here were intended to determine the acute effects with WBC and lymphocyte counts taken at 24 h after the irradiation. Thus it is possible that the radiation dose rate and dose fractionation pattern may affect the long-term survival and recovery of hematopoietic cells, whereas the acute effects of radiation on hematopoietic stem cells are mainly dependent on dose with very little contribution from the dose rate, fractionation pattern and energy/LET of the protons.
The present results showing a lack of effect of dose fractionation on WBC and lymphocyte counts could have been due to the fractionation schedule used, which involved irradiation of the animals once daily over 5 days. If the interval between fractions had been increased, it is possible that a different effect could have been observed, particularly if the interval was sufficient for stem cell repopulation between doses. The observed results could also have been influenced by protective effects from adaptive responses induced by the earlier fractions; adaptive responses have been observed previously in ICR mice (15).
A major difficulty that underlies meaningful comparisons of relative biological effectiveness (RBE) values for light ions/heavy particles (e.g., protons, neutrons, HZE particles) and γ rays is that the dose distributions (i.e., the relative dose received by different organ systems during total-body irradiation) produced by these forms of radiation are different. While this problem has been recognized for a long time (16), it remains a significant problem and may help to explain the widely differing RBE values for neutrons and HZE particles. This is less of a problem for protons at high energies (1 GeV) that produce a relatively homogeneous dose distribution in tissue because the dose deposited is from the relatively flat, “plateau” portion of the depth–dose curve. Since a large fraction of the protons during an SPE are in the range of 50 MeV (17, 18), 51.24 MeV protons were evaluated in the present study at a dose that is relevant for an SPE. The present results indicate that mice irradiated with 51.24 MeV protons at a dose of 2 Gy had significantly reduced WBC counts at 24 h after exposure. Although the dose rate of the 51.24 MeV protons used in this study (0.05–0.07 Gy/min) was higher than that expected for an SPE (<0.5 Gy/h), the results do suggest that the acute effects of 51.24 MeV protons on WBC and lymphocyte counts are comparable in mice to those of 1 GeV protons at a comparable dose (2 Gy) and dose rate (0.05 Gy/min).
Acknowledgments
This work was supported by the National Space Biomedical Research Institute (NSBRI) through NASA NCC 9-58 and NIH Training Grant 2T32CA09677. We would like to thank the staff of the NASA Space Radiation Laboratory and the Brookhaven National Laboratory for help with the animal proton irradiations, with particular thanks to Drs. I-Hung Chiang and Peter Guida. We also acknowledge the expert technical assistance of Corinne Reizel, Brookhaven National Laboratory, who helped us to obtain blood samples from the mice in the studies reported here.
References
- 1.Hellweg CE, Baumstark-Khan C. Getting ready for the manned mission to Mars: the astronauts’ risk from space radiation. Naturwissenschaften. 2007;94:517–526. doi: 10.1007/s00114-006-0204-0. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JW, Cucinotta FA, Shinn JL, Simonsen LC, Dubey RR, Jordan WR, Jones TD, Chang CK, Kim MY. Shielding from solar particle event exposures in deep space. Radiat Res. 1999;30:361–382. doi: 10.1016/s1350-4487(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 3.Smart DF, Shea MA. The local time dependence of the anisotropic solar cosmic ray flux. Adv Space Res. 2003;32:109–114. doi: 10.1016/s0273-1177(03)90377-2. [DOI] [PubMed] [Google Scholar]
- 4.Koenig KL, Goans RE, Hatchett RJ, Mettler FA, Jr, Schumacher TA, Noji EK, Jarrett DG. Medical treatment of radiological casualties: current concepts. Ann Emerg Med. 2005;45:643–652. doi: 10.1016/j.annemergmed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, Moulder JE, Preston RJ, Seed TM, Wong RS. Radiat Res; Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop; Bethesda, Maryland. December 17–18, 2001; 2003. pp. 812–834. [DOI] [PubMed] [Google Scholar]
- 6.Mettler FA, Jr, Voelz GL. Major radiation exposure—what to expect and how to respond. N Engl J Med. 2002;346:1554–1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 7.Herodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp Hematol. 2005;33:1071–1080. doi: 10.1016/j.exphem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Wambi C, Sanzari J, Nuth M, Davis J, Ko Y-H, Sayers CM, Baran M, Ware JH, Kennedy AR. Dietary antioxidants protect hematopoietic cells and improve animal survival following total-body irradiation. Radiat Res. 2008;169:384–396. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wambi CO, Sanzari JK, Sayers CM, Nuth M, Zhou Z, Davis J, Finnberg N, Lewis-Wambi JS, Ware JH, Kennedy AR. Protective effects of dietary antioxidants on proton total-body irradiation-mediated hematopoietic cell and animal survival. Radiat Res. 2009;172:175–186. doi: 10.1667/RR1708.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans RG, Wheatley CL, Nielsen JR. Modification of radiation-induced damage to bone marrow stem cells by dose rate, dose fractionation, and prior exposure to cytoxan as judged by the survival of CFUs: application to bone marrow transplantation (BMT) Int J Radiat Oncol Biol Phys. 1988;14:491–495. doi: 10.1016/0360-3016(88)90265-9. [DOI] [PubMed] [Google Scholar]
- 11.Pecaut MJ, Gridley DS, Smith AL, Nelson GA. Dose and dose rate effects of whole-body proton-irradiation on lymphocyte blastogenesis and hematological variables: part II. Immunol Lett. 2002;80:67–73. doi: 10.1016/s0165-2478(01)00307-8. [DOI] [PubMed] [Google Scholar]
- 12.Gridley DS, Pecaut MJ, Dutta-Roy R, Nelson GA. Dose and dose rate effects of whole-body proton irradiation on leukocyte populations and lymphoid organs: part I. Immunol Lett. 2002;80:55–66. doi: 10.1016/s0165-2478(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 13.van Os R, Thames HD, Konings AW, Down JD. Radiation dose-fractionation and dose-rate relationships for long-term repopulating hemopoietic stem cells in a murine bone marrow transplant model. Radiat Res. 1993;136:118–125. [PubMed] [Google Scholar]
- 14.Ainsworth EJ, Afzal SM, Crouse DA, Hanson WR, Fry RJM. Tissue responses to low protracted doses of high LET radiations or photons: early and late damage relevant to radio-protective countermeasures. Adv Space Res. 1989;9:299–313. doi: 10.1016/0273-1177(89)90453-5. [DOI] [PubMed] [Google Scholar]
- 15.Yonezawa M. Induction of radio-resistance by low dose X-irradiation. Yakugaku Zasshi J Pharm Soc Jpn. 2006;126:833–840. doi: 10.1248/yakushi.126.833. [DOI] [PubMed] [Google Scholar]
- 16.Storer JB, Harris PS, Furchner JE, Langham WH. The relative biological effectiveness of various ionizing radiations in mammalian systems. Radiat Res. 1952;6:188–288. [PubMed] [Google Scholar]
- 17.NCRP. Guidance on Radiation Received in Space Activities Report No 98. National Council on Radiation Protection and Measurements; Bethesda, MD: 1989. [Google Scholar]
- 18.NCRP. Information Needed to Make Radiation Protection Recommendations for Space Missions beyond Low-Earth Orbit Report No 153. National Council on Radiation Protection and Measurements; Bethesda, MD: 2006. [Google Scholar]