Abstract
Our study objective was to compare light exposure and sleep parameters between adolescents with delayed sleep phase disorder (n=16, 15.3 ± 1.8 years) and unaffected controls (n=22, 13.7 ± 2.4 years) using a prospective cohort design. Participants wore wrist actigraphs with photosensors for 14 days. Mean hourly lux levels from 20:00-05:00 h and 05:00-14:00 h were examined, in addition to the 9-hour intervals prior to sleep onset and after sleep offset. Sleep parameters were compared separately, and were also included as covariates within models that analyzed associations with specified light intervals. Additional covariates included group and school night status. Adolescent subjects with delayed sleep phase disorder received more evening (p<0.02, 22:00-02:00 h) and less morning light (p<0.05, 08:00-09:00 h and 10:00-12:00 h) than controls, but had less pre-sleep exposure with adjustments for the time of sleep onset (p<0.03, fifth-seventh hours prior to onset hour). No differences were identified with respect to the sleep offset interval. Increased total sleep time and later sleep offset times were associated with decreased evening (p<0.001 and p=0.02, respectively) and morning (p=0.01 and p<0.001, respectively) exposure, and later sleep onset times were associated with increased evening exposure (p<0.001). Increased total sleep time also correlated with increased exposure during the 9 hours before sleep-onset (p=0.01), and a later sleep onset time corresponded with decreased exposure during the same interval (p<0.001). Outcomes persisted regardless of school night status. In conclusion, light exposure interpretation requires adjustments for sleep timing among adolescents with delayed sleep phase disorder. Pre- and post-sleep exposure do not appear to contribute directly to phase delays. Sensitivity to morning light may be reduced among adolescents with delayed sleep phase disorder.
Keywords: adolescents, circadian, delayed sleep phase disorder, light, sleep
INTRODUCTION
A delay in the preferred timing of sleep and wakefulness in association with pubertal development has been described within numerous societies worldwide and contributes to the significant reductions in total sleep time associated with adolescence (Andrade et al., 1993; Carskadon and Acebo 2002; Gau and Soong 2003; Giannotti et al., 2002; Laberge et al., 2001; National Sleep Foundation 2006; National Sleep Foundation 2000; Ouyang et al., 2009; Sadeh et al., 2009; Wolfson and Carskadon 1998). Survey data show that only 15% of United States adolescents report sleeping ≥8.5 hours on school nights, and an alarming 26% report typically sleeping 6.5 hours or less (Wolfson and Carskadon 1998). One group demonstrated an objective degree of sleepiness (during the early periods of school) equivalent to that of narcoleptics (Carskadon et al., 1998).
Delayed sleep phase disorder (DSPD), characterized by an extreme “eveningness” circadian preference, is common among adolescents (American Academy of Sleep Medicine 2005; Dagan et al., 1998; Thorpy et al., 1998) and further increases students’ susceptibility to chronic sleep restriction and associated deleterious outcomes (Fernandez-Mendoza 2010; National Sleep Foundation 2000) In a series investigating 22 adolescents with DSPD (hereafter referred to as DSPD-A), 59% demonstrated poor scholastic performance, and 45% displayed a variety of behavioral problems (Thorpy et al., 1998).
Data regarding the epidemiology and comorbidities of DSPD have not yet been combined with a discrete elucidation of the underlying etiology, and adolescent data is particularly scarce (reviewed by Sack and colleagues (Sack et al., 2007)). Some believe that the condition represents an extreme manifestation of the delayed circadian preference observed in concert with pubertal development (Carskadon et al., 1997; Carskadon et al., 1993; Jenni et al., 2005). Longer intervals from the core body temperature minimum to sleep offset have been reported among DSPD subjects in comparison to controls, suggesting a contribution from decreased exposure to the phase advance portion of the light phase response curve (PRC) as an etiological factor (Ozaki et al., 1996; Uchiyama et al., 2000; Watanabe et al., 2003). More recent studies (one of which included adolescents (Chang et al., 2009) have not replicated these findings, however (Chang et al., 2009; Mundey et al., 2005; Wyatt et al., 2006). As two (Chang et al., 2009; Mundey et al., 2005) describe comparatively reduced total sleep times in comparison to the previous investigations, reported alterations in phase relationships may simply be a consequence of longer habitual sleep times. In the study by Mundey and colleagues (Mundey et al., 2005), participants related reduced sleep duration directly to restrictions imposed by work and/or school obligations, similar to the scenario of adolescents suffering from this condition.
The lack of research regarding external contributors to the induction or perpetuation of DSPD-A is conspicuous. Numerous exogenous factors, such as increased autonomy with respect to sleep time, employment, and involvement in extracurricular activities have been identified as factors contributing to the general changes in the sleep patterns of adolescents, but have not been studied specifically among DSPD-A cohorts (Carskadon 1990; Wyatt 2004). The failure to address these variables systematically may contribute to poor treatment outcomes, which emphasize presumed innate underpinnings (Okawa et al., 1998; Sack et al., 2007; Szeinberg et al., 2006) and ignore external factors.
Particularly pertinent to this contention are the findings of an investigation by Burgess and Eastman (Burgess and Eastman 2004) that assessed circadian parameters in two adult groups with differing bedtimes but enforced wake times, similar to the scenario of school-attending adolescents. Those with a late bedtime demonstrated circadian phase delays compared with those with an early bedtime, proposed to be due to the greater exposure to indoor light during late evening/early morning hours (Burgess and Eastman 2004). An investigation by Aoki and colleagues (involving predominantly adult patients) supports the possibility of hypersensitivity to nocturnal photic stimulation among DSPD patients compared with unaffected counterparts (Aoki et al., 2001), resulting in excessive stimulation of the phase delay portion of the light PRC. Both findings suggest that DSPD-A patients may unwittingly induce or perpetuate their condition by exposing themselves to nighttime light of sufficient intensity to stimulate the delay portion of the light PRC.
In response to the mandates of various circadian rhythm sleep disorders research agendas (Morgenthaler et al., 2007; Okawa and Uchiyama 2007; Sack et al., 2007), the aim of the current study was to prospectively compare light exposure patterns during select intervals among DSPD-A and unaffected adolescents in the field setting. Given the fixed wake times enforced by school attendance, we hypothesized that those with DSPD-A would receive significantly more light exposure during time periods correlating with the phase delay portion of the light PRC. A secondary aim was to explore associations between various light intervals and sleep parameters, in an effort to examine the prospect of increased evening light sensitivity among DSPD-A patients.
METHODS
Inclusion/Exclusion Criteria
Adolescents (10-18 years, inclusive) were recruited through local advertisements (Rochester, Minnesota, USA) to participate in a study to investigate the “sleep habits” of both “night owls” (cases with DSPD-A) and those without sleep complaints (controls). Prospective subjects initially responded to questions (by telephone or electronically) to determine whether he/she was in a school setting with enforced wake times, to determine whether an exclusionary medication was prescribed (see further below), and to ascertain the baseline sleep schedule. As there are no discrete clock times associated with the International Classification of Sleep Disorders, second edition, diagnostic criteria for DSPD (American Academy of Sleep Medicine 2005) (and sparse normative data regarding adolescent sleep-wake times in general), we initially mandated that DSPD-A participants document sleep onset timing of midnight or later for at least 7 of the 14 days preceding the initial assessment. As it became apparent that it was exceedingly difficult to identify participants among the younger age range that endorsed such a sleep schedule, however, we ultimately modified the criterion for those ≤14 years of age, such that 23:00 h was used as the threshold clock time. This cutoff was in part based upon the average bedtime reported by sixth-through eighth-graders in the 2006 Sleep in America Poll (National Sleep Foundation 2006), which ranged from 21:25-21:51 h. Wake times were enforced due to school attendance 5 days of the week. Within the Rochester Public School District, elementary schools start at an average time of 09:06 h (bussing commences at 08:00 h), middle schools start at an average time of 07:50 h (bussing commences at 06:45 h), and high schools commence at 07:40 h (bussing commences at 06:30 h) [data updated August 12, 2009].
Responses to the above determined whether further screening occurred. All DSPD-A participants met the remaining International Classification of Sleep Disorders, second edition, diagnostic criteria (American Academy of Sleep Medicine 2005) as determined by questionnaire. In an effort to enhance diagnostic rigor, eligibility additionally required a Morningness-Eveningness Questionnaire score of ≤41 (consistent with an “evening type”) (Horne and Ostberg 1976). To eliminate the confounding effect of psychiatric disturbances on sleep complaints, the parent-administered Mood Disorder Questionnaire-Adolescent Version and the self-administered Children’s Depression Inventory were also completed (Brooks and Kutcher 2001; Finch et al., 1985; Wagner et al., 2006). The production of scores beyond established thresholds resulted in exclusion from the study, with appropriate handling of related concerns. Patients receiving hypnotics, antidepressants, stimulant-containing or other psychotropic medications were not eligible for participation. Non-steroidal anti-inflammatory drugs and/or beta-blockers were also prohibited during the active portion of the protocol due to their ability to suppress melatonin (Murphy et al., 1996; Stoschitzky et al., 1999) and potentially affect circadian phase. Eligible subjects did not work night shifts, nor did they report travel across more than 2 time zones within 2 months prior to study entry. All participants were free from recreational drug use, as confirmed by a urine toxicology screen, and none reported tobacco use or habitual use of caffeine after noon.
Inclusion criteria for controls required that he/she enter the study without subjective sleep complaints, supported by a Pittsburgh Sleep Quality Index score of ≤5 (Buysee et al., 1989). Exclusion criteria for this group were identical to those described above. Controls also completed the Morningness-Eveningness questionnaire, but their scores did not affect eligibility. Similarly, DSPD-A subjects completed the Pittsburgh Sleep Quality Index but, since subjective sleep complaints were established with previous assessments (and the instrument is not designed to detect circadian dysrhythmias), the results had no bearing on their participation.
The study was approved by the Mayo Foundation Institutional Review Board, and meets the ethical standards of the journal (Portaluppi et al., 2010). All subjects and their parents/guardians provided consent/assent, and individuals were compensated for their efforts. Prospective participants ultimately deemed ineligible for study entry were provided with referrals to the Mayo Center for Sleep Medicine for clinical consultation, if desired.
Sleep-Wake & Illuminance Assessments
Participants wore actigraphs [Actiwatch-L®, MiniMitter/Respironics] continually on the non-dominant dorsal wrist for 14 days (range 13-15 for both groups), except for during periods of water exposure, or when damage to the instrument was likely. Studies that have compared sleep/wake parameters obtained in this fashion with those of polysomnography (gold standard) have shown excellent correlations (Sadeh et al., 1995). The sampling rate for both motion and light parameters was 1/minute. Although subjects were aware of the necessity of maintaining the device unencumbered by clothing, they were not aware of the specific ability of the apparatus to detect illumination, or the particular focus of the investigation with respect to light exposure.
The period of assessment spanned 386 days, beginning on May 12, 2008, and ending on June 2, 2009, during periods of school attendance. All of the participants attended school within the Mayo Clinic Rochester catchment area (~44.0 N, 92.5 W) on a typical schedule, with an extended summer break. As the length of daylight varied throughout this time period, the following definitions were used to characterize the season during which subjects were assessed: Winter (December 21-March 21), Spring (March 22-June 21), Summer (June 22-September 22), and Fall (September 23-December 20).
Data Collection
The same individual (NLS) reviewed and tabulated all actigraphy and illuminance output. Information was downloaded in the presence of each subject, and attempts were made to resolve any discrepancies with sleep logs. Invalid light data (e.g., instances where the device was removed or covered) were discarded, and the remaining information was summarized into individual hourly average lux calculations. These averages formed the raw lux data used for analyses. There were no intergroup differences with respect to the proportion of usable data (p=0.60, repeated measures model where the outcome was hourly percent invalid time). Other extracted information included total sleep time, time of sleep onset and offset, and percent wakefulness after sleep onset. Although we intended to also calculate initial sleep latency and sleep efficiency, the inconsistent completion of sleep diaries made this impossible.
Determination of Illuminance Intervals for Analyses
The primary goal of the study was to assess intergroup differences in light exposure during specified time intervals as measured in lux (group hourly mean values) by the Actiwatch-L®. “Evening hours” were defined as those occurring between 20:00-05:00 h, and “morning hours” as those encompassing 05:00-14:00 h. The use of these particular intervals was intended to coincide with the light PRC described in association with a conventional adult 00:00-08:00 h sleep schedule (reviewed by Burgess and colleagues (Burgess et al., 2002)), in order to contrast the findings that arise when considering phase relationships as anchored entities, rather than ones that require adjustments according to one’s preferred sleep period (Burgess and Eastman 2005).
The dim light melatonin onset typically occurs 2-3 hours prior to sleep initiation (Burgess and Fogg 2008; Burgess et al., 2003), and the core body temperature minimum occurs approximately 7 hours thereafter (Lee et al., 2006). In an individual normally entrained to the light/dark cycle, bright light exposure prior to the core body temperature minimum phase delays circadian rhythms and light after the core body temperature minimum advances rhythms (Khalsa et al., 2003). Comparison studies involving young adults with and without DSPD have demonstrated equivalent and persistent phase relationships in settings where sleep times are not inordinately prolonged (thereby masking the advance portion of the light PRC) (Chang et al., 2009; Shibui et al., 1999; Wyatt et al., 2006). As such, we used the comparison of 9-hour illuminance patterns both prior to sleep onset and subsequent to sleep offset to assess for intergroup differences during the phase delay and advance portions of the light PRC, respectively. As the sleep period itself occurred in a typical darkened environment, photic input was deemed negligible with respect to circadian-based effects (Robinson et al., 1991).
Statistical Analysis
Demographic and baseline characteristics were compared between the two subject groups using Pearson’s chi-square test or Fisher’s exact test for categorical variables, and two-sample t-tests or rank sum tests for continuous variables, as appropriate. Summaries (means and standard deviations) of the nightly sleep variables (onset, offset, wakefulness after sleep onset, and total sleep time) for the duration of the study are presented. Means were computed by first averaging the daily values for each subject, with subsequent compilation of group averages. Similarly, the standard deviations were determined by first calculating the daily values for each subject and then averaging the group standard deviations. Light exposure means are presented graphically over time, as per the method described above. In situations where illuminance was plotted relative to the sleep episode, the onset/offset times were associated with the clock time hourly interval during which they occurred.
Repeated measures linear models were utilized in all modeling situations. A within-subject covariance (i.e., an exchangeable covariance structure) was estimated to account for the possible correlation between outcome measurements from the same subject. Robust standard errors of the model parameters were computed using the methods of Diggle and colleagues (Diggle et al., 1994), which give consistent estimates even when the within-subject covariance matrix is misspecified. The situations that follow are all multivariable models, such that all covariates listed are included in the model at the same time for each outcome. Thus, all reported effects are adjusted for the covariates.
Modeling Situations
Outcomes of log(1 + hourly average lux)* from 20:00-05:00 h and 05:00-14:00 h, with covariates for subject group (case vs. control), age, gender, season, school night (yes/no), clock time hour, and a group by clock time interaction.
Outcomes of log(1 + hourly average lux)* relative to sleep onset/offset hour with covariates for group, age, gender, season, school night, and hour relative to onset/offset hour [-8, -7, …,0 (onset/offset hour), …, +7, +8], and a group by hour interaction.
Outcomes of log(1 + entire time block average lux)* from 20:00-05:00 h, 05:00-14:00 h, from the 9-hour interval preceding and including the sleep onset hour (-8 to 0), and from the 9-hour interval containing and subsequent to the hour of sleep offset (0 to +8), with covariates for subject group, age, gender, season, school night, wakefulness after sleep onset (%),total sleep time (minutes), and 24-hour sleep onset and offset times. † The latter three sleep variables did not appear in models simultaneously, as total sleep time is linearly related to the difference between the offset and onset times. Thus, total sleep time was considered in isolation, with onset and offset times considered as a separate pair. These models utilized one observation per night per subject, and were used solely to determine associations between the nightly sleep summary variables and light exposure. The other covariates were included for purposes of adjustment only.
Outcomes of total sleep time, 24-hour sleep onset and offset times,† and wakefulness after sleep onset, with covariates for subject group, age, gender, season, and school night. These models produced one observation per night per subject.
Interactions between subject group and school night status were considered in every model. Since none of these were statistically significant, these interactions were removed to make inferences.
RESULTS
Demographics
Demographics and other participant characteristics are summarized in Table 1. Cases were slightly older than controls (15.3 ± 1.8 vs. 13.7 ± 2.4, respectively, p=0.03). There were no significant intergroup differences with respect to gender (p=0.44), ethnicity (p=0.68), or season of assessment (p=0.15).
TABLE 1.
Subject demographics
| DSPD-A (n=16) | Controls (n=22) | p-value | |
|---|---|---|---|
| Male/female, number | 10/6 | 11/11 | 0.444 |
| Age, years* | 15.3 ± 1.8 | [11.2-18.3] | 13.7 ± 2.4 |
| [10.3-18.2] | 0.034 | ||
| MEQ score | ** 32.3 ± 4.9 | 52.9 ± 9.3 | <0.001 |
| PSQI score | 6.6 ± 1.9 | *** 2.8 ± 1.3 | <0.001 |
Data are presented as mean ± SD [range]
A score of ≤41 was an inclusion criterion for this arm
A score of ≤5 was an inclusion criterion for this arm
DSPD-A = Adolescent delayed sleep phase disorder
MEQ = Morningness-Eveningness Questionnaire
PSQI = Pittsburgh Sleep Quality Index
Sleep & Illuminance Data
Sleep Parameters
Group sleep data summaries are displayed in Table 2. Mean school night total sleep time was reduced by 35.3 minutes (p<0.001, CI: 19.1-51.6) compared with non-school nights due to a disproportionate advance in the time of awakening [1.7 hours earlier (p<0.001, CI: 1.4-2.1)] vs. the time of sleep onset [1.1 hours earlier (p<0.001, CI: 0.7-1.4)]. DSPD-A subjects initiated sleep 1.2 hours later than controls [p<0.001, CI: 0.6-1.8], but also delayed sleep offset by 1.0 hours (p=0.01, CI: 0.3-1.7), regardless of school night status, such that there were no statistically significant group differences with respect to total sleep time (p=0.36). There were similarly no group or school night status differences with respect to wakefulness after sleep onset (p=0.99 and p=0.59, respectively).
TABLE 2.
Sleep parameter summaries for DSPD-A subjects and controls by school/non-school night status (mean ± s.d.)
| Subject group |
School night status |
Onset (24-hour clock time) |
Offset (24-hour clock time) |
Efficiencyψ (%) |
Total sleep time (min) |
|---|---|---|---|---|---|
| Control | Non-school night |
23.7±1.0 | 8.2±1.0 | 87.3±2.7 | 446.2±62.0 |
| Control | School night | 22.7±0.6 | 6.6±0.6 | 87.7±3.0 | 414.5±43.9 |
| DSPD-A | Non-school night |
1.4±1.0 | 9.4±1.3 | 88.0±2.7 | 427.8±80.5 |
| DSPD-A | School night | 0.2±1.1 | 7.5±1.4 | 87.8±3.0 | 382.5±86.3 |
Refers to % time asleep subsequent to sleep onset, until final time of awakening.
Group Differences in Light Exposure
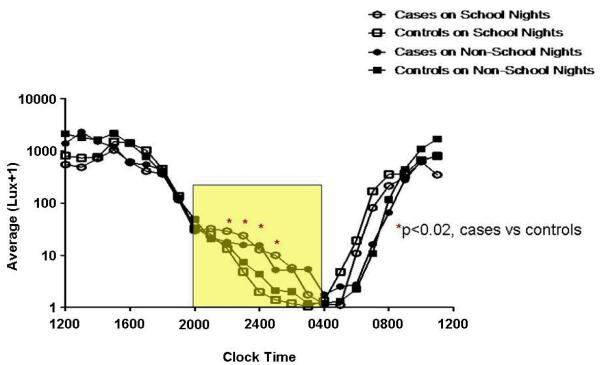
Hourly Evening Light Exposure (20:00-05:00 h) (Figure 1)
Figure 1. Light Exposure Relative to Clock Time (20:00-05:00 h).
The y-axes depict hourly average lux values on a logarithmic scale. Outcomes were transformed to the log(lux+1) scale to better approximate model assumptions and to bound values away from 0 (or logged values away from negative infinity). The x-axes depict hourly time intervals. Clock times correlate with an hourly interval that begins at the corresponding time and ends 1-hour later (e.g., the 0400 data point corresponds to the lux interval encompassing 0400-0500 h). In the figures that describe time in relation to the time of sleep onset or offset, the intervals 0-8 are calculated in an identical fashion. The intervals -1 to -8 encompass the preceding hour (e.g. interval -8 refers to the lux recorded between 7 and 8 hours prior to sleep onset or offset. The rectangles encapsulate the illuminance intervals of interest. Asterisks denote hours during which statistically significant group differences were identified (regardless of school night/morning status), with affiliated p-values. Circles represent DSPD-A subjects (cases), and squares controls. Open and closed shapes are representative of school night and non-school night status, respectively.
DSPD-A subjects received significantly more light exposure during the hours spanning 22:00-02:00 h (p<0.02 for all 4 hourly intervals). Less exposure was found on school vs. non-school nights (p=0.001). School night exposures (mean lux ± S.D.) over the entire interval were 15.0 ± 11.5 and 7.9 ± 5.3 for cases and controls, respectively. On non-school nights, average values for cases and controls were 12.8 ± 8.5 and 9.6 ± 9.4 lux, respectively.
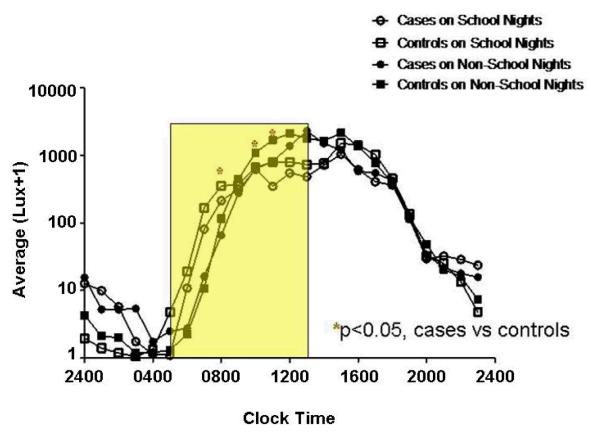
Hourly Morning Light Exposure (05:00-14:00 h) (Figure 2)
Figure 2. Light Exposure Relative to Clock Time (05:00-14:00 h).
The y-axes depict hourly average lux values on a logarithmic scale. Outcomes were transformed to the log(lux+1) scale to better approximate model assumptions and to bound values away from 0 (or logged values away from negative infinity). The x-axes depict hourly time intervals. Clock times correlate with an hourly interval that begins at the corresponding time and ends 1-hour later (e.g., the 0400 data point corresponds to the lux interval encompassing 0400-0500 h). In the figures that describe time in relation to the time of sleep onset or offset, the intervals 0-8 are calculated in an identical fashion. The intervals -1 to -8 encompass the preceding hour (e.g. interval -8 refers to the lux recorded between 7 and 8 hours prior to sleep onset or offset. The rectangles encapsulate the illuminance intervals of interest. Asterisks denote hours during which statistically significant group differences were identified (regardless of school night/morning status), with affiliated p-values. Circles represent DSPD-A subjects (cases), and squares controls. Open and closed shapes are representative of school night and non-school night status, respectively.
DSPD-A subjects received significantly less light exposure during the hours spanning 08:00-09:00 h and 10:00-12:00 h (p<0.05 for all 3 hourly intervals). More exposure was found on school vs. non-school mornings (p<0.001). School morning exposures (mean lux ± S.D.) over the entire interval were 350.6 ± 428.0 and 419.2 ± 425.6 for cases and controls, respectively. On non-school mornings, average values for cases and controls were 575.2 ± 785.5 and 711.5 ± 851.3 lux, respectively.
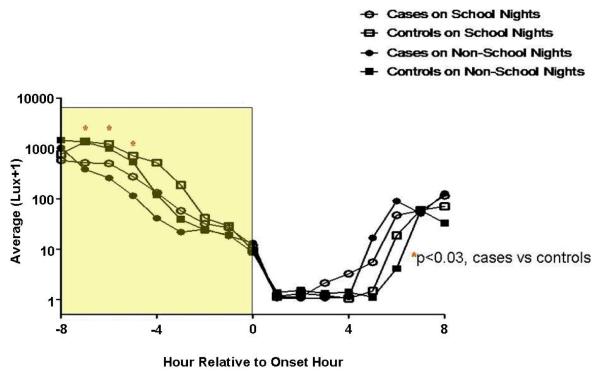
Hourly Light Exposure Relative to Time of Sleep Onset (Figure 3)
Figure 3. Light Exposure Relative to Time of Sleep Onset.
The y-axes depict hourly average lux values on a logarithmic scale. Outcomes were transformed to the log(lux+1) scale to better approximate model assumptions and to bound values away from 0 (or logged values away from negative infinity). The x-axes depict hourly time intervals. Clock times correlate with an hourly interval that begins at the corresponding time and ends 1-hour later (e.g., the 0400 data point corresponds to the lux interval encompassing 0400-0500 h). In the figures that describe time in relation to the time of sleep onset or offset, the intervals 0-8 are calculated in an identical fashion. The intervals -1 to -8 encompass the preceding hour (e.g. interval -8 refers to the lux recorded between 7 and 8 hours prior to sleep onset or offset. The rectangles encapsulate the illuminance intervals of interest. Asterisks denote hours during which statistically significant group differences were identified (regardless of school night/morning status), with affiliated p-values. Circles represent DSPD-A subjects (cases), and squares controls. Open and closed shapes are representative of school night and non-school night status, respectively.
DSPD-A subjects received significantly less light exposure during the fifth, sixth, and seventh hours prior to sleep onset (p<0.04 for all 3 hourly intervals). More exposure was found on school vs. non-school nights (p<0.001). School night exposures (mean lux ± S.D.) over the entire interval were 193.8 ± 218.5 and 519.8 ± 547.7 for cases and controls, respectively. On non-school nights, average values for cases and controls were 196.8 ± 211.0 and 433.7 ± 429.5 lux, respectively.
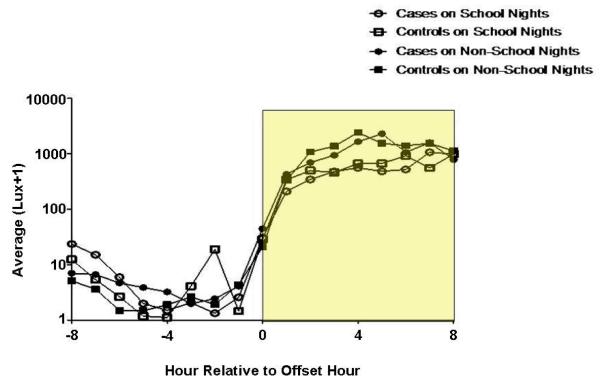
Hourly Light Exposure Relative to Time of Sleep Offset (Figure 4)
Figure 4. Light Exposure Relative to Time of Sleep Offset.
The y-axes depict hourly average lux values on a logarithmic scale. Outcomes were transformed to the log(lux+1) scale to better approximate model assumptions and to bound values away from 0 (or logged values away from negative infinity). The x-axes depict hourly time intervals. Clock times correlate with an hourly interval that begins at the corresponding time and ends 1-hour later (e.g., the 0400 data point corresponds to the lux interval encompassing 0400-0500 h). In the figures that describe time in relation to the time of sleep onset or offset, the intervals 0-8 are calculated in an identical fashion. The intervals -1 to -8 encompass the preceding hour (e.g. interval -8 refers to the lux recorded between 7 and 8 hours prior to sleep onset or offset. The rectangles encapsulate the illuminance intervals of interest. Asterisks denote hours during which statistically significant group differences were identified (regardless of school night/morning status), with affiliated p-values. Circles represent DSPD-A subjects (cases), and squares controls. Open and closed shapes are representative of school night and non-school night status, respectively.
There were no significant group differences at any of the 9 hourly intervals encompassing and subsequent to sleep offset (all p-values larger than 0.13), regardless of school night status (p=0.68).
Sleep Parameter Associations with Illuminance Values (Table 3)
TABLE 3.
Sleep parameter associations with illuminance intervals
| Effect | 20:00-05:00 h {%change (lux + 1)} |
05:00-14:00 h {%change (lux + 1)} |
Pre-Onset (−8 to 0) {%change (lux + 1)} |
Post-Offset (0 to +8) {%change (lux + 1)} |
|---|---|---|---|---|
| TST (min) | p<0.001 {−0.2%} | p=0.01 {−0.3%} | p=0.01 {+0.2%} | p=0.86 |
| Sleep Onset Time (Hour) | p<0.001 {+23.2%} | p=0.60 | p<0.001 {−25.9%} | p=0.98 |
| Sleep Offset Time (Hour) | p=0.02 {−5.7%) | p<0.001 {−23.4%} | p=0.17 | p=0.84 |
The leftmost column depicts various actigraphically-derived sleep parameters (TST=total sleep time, min=minutes), and associated units of measurement are displayed in parentheses. Wakefulness after sleep onset is not shown, as no significant associations were detected. The second through fifth columns correspond to the illuminance intervals of interest, with the associated p-values representative of statistical associations with the sleep parameters. The fourth and fifth columns represent the entire 9-hour interval lux average in relation to sleep onset and offset (“pre-onset” and “post-offset,” respectively). Values within {} represent the % change in lux+1 associated with a one-unit increase in the sleep parameter. For example, for every 1-minute increase in total sleep time, pre-onset lux exposure increased by 0.2%. For every 1-hour delay in the time of sleep onset, lux intensity decreased by 25.9% during the same interval.
All lux model outcomes were transformed to the log(lux+1) scale to better approximate model assumptions and to bound values away from 0 (or logged values away from negative infinity).
Increased total sleep time and later sleep offset times were associated with decreased evening (p<0.001 and p=0.02, respectively) and morning (p=0.01 and p<0.001, respectively) light exposure, and later sleep onset times were associated with increased evening exposure (p<0.001). No associations were detected with respect to sleep onset times and morning exposure (p=0.60). Increased total sleep time also correlated with augmented light intensity during the 9-hour pre-sleep-onset period (p=0.01), and a later time of sleep initiation corresponded with decreased light exposure during the same interval (p<0.001). No associations were detected with respect to sleep offset and either the pre-sleep-onset or post-sleep-offset intervals (p=0.17 and p=0.84, respectively), with the latter demonstrating no statistically significant sleep time-related associations whatsoever (p=0.84-0.98). Wakefulness after sleep onset exhibited no statistically significant associations (p=0.07-0.91) with any of the illuminance intervals.
DISCUSSION
To our knowledge, this is the first cohort comparison study to prospectively examine light and sleep parameters among adolescents with DSPD in a field setting. The goal of the study in particular was to determine whether differential patterns of light exposure were evident between groups. Since all participants slept in darkened environments, the analyses according to defined clock times simply mirrored differences in sleep timing. As such, cases exhibited increased evening (20:00-05:00 h) and decreased morning (05:00-14:00 h) light exposure. It seems likely that if an older group of subjects were chosen that the latter comparison would have been more similar, as school start times would have been sufficiently early so as to diminish intergroup sleep offset disparities. Both groups received comparatively less evening and more morning light exposure on school vs. non-school mornings.
All participants were exposed to significantly dim levels of light from the 20:00-05:00 h interval (hourly average values were consistently <48 lux), regardless of school night status and, accordingly, the mean group difference during this time period was very small. Differences in morning (05:00-14:00 h) light levels were more dependent on school attendance with maximum hourly values <800 lux and <2300 lux on school and non-school mornings, respectively. It is noteworthy that morning lux levels were lower for cases at all assessed intervals during school days (although not all differences were statistically significant), despite the fact that both groups spent time in presumably similar lighting environments.
While clinicians in particular tend to think of the PRC in terms of fixed clock times, proper interpretation requires adjustments of various phase markers, which in turn can be predicted based upon the habitual sleep/wake schedule (Burgess and Eastman 2005; Cagnacci et al., 1992; Lee et al., 2006). As the sleep period itself was spent in conditions of minimal illuminance, and transmission of light to the retina is further reduced with closed eyelids (Robinson et al., 1991), the portions of exposure during subjects’ periods of wakefulness seem solely relevant with respect to analyses of circadian effects of light. In contrast to the clock time findings, cases received comparatively less light exposure during the 9-hour pre-sleep interval, and no significant group differences were detected with respect to exposure during the 9-hour post-sleep interval. The former finding is contrary to our initial hypothesis. As with the previous analysis, the pre-sleep interval was spent in relatively dim light, particularly on school nights, with a maximal hourly mean case value of 575.0 lux, during the furthest assessed hour from sleep onset. The maximal hourly post-sleep school morning light exposure value was 1065.5 lux. While not statistically significant at all data points, hourly mean values were increased for controls during the majority of all pre- and post-sleep assessments.
Some interpretations are required. First, the findings appear to refute the role of relatively increased evening light intensity in the induction or perpetuation of DSPD-A. Additionally (in keeping with the findings associated with morning clock times), the post-sleep illumination data suggest the possibility of differential school day behavior patterns between groups, possibly impacting discrepancies in light exposure. Indeed, a study by Harada and colleagues demonstrated a negative correlation between high school students’ propensity to seek outdoor light and a delayed circadian preference (Harada et al., 2002). These findings should be explored in greater detail with larger numbers of subjects.
Relationships between exposure patterns and sleep parameters were also explored and did not conform to a pattern indicative of circadian-based effects of light. Associations were detected between increased total sleep time and augmented pre-sleep exposure, as well as later sleep onset times and diminished light intensity during the same interval. It may be conjectured that subjects with increased pre-sleep exposure were attempting to initiate sleep closer to their circadian preference, while those initiating sleep at later hours were lying in darkness “trying to sleep,” subsequently promoting a conditioned insomnia, a potential comorbidity with DSPD (Auger 2008; Marchetti et al., 2006). Our lack of sleep latency information renders this contention entirely speculative.
These data additionally appear to counter the notion of increased sensitivity to light among DSPD-A patients, which has been described among adults (with 2-hour 1000 lux exposure (Aoki et al., 2001)), and suggested as a possible explanation for phase delays among adolescents (Hagenauer et al., 2009). As the group of subjects described herein received relatively low levels of evening light exposure, however, it is possible that such a variable contributes to other DSPD phenotypes and/or that different subtypes of this condition exist, comprised of varying amounts of exogenous and endogenous contributors. Salivary dim light melatonin onset assessments may serve to discriminate such subgroups (Rahman et al., 2009), but are not yet used routinely in the clinical setting. More widespread use of actigraphs that provide light data is also clearly warranted within both the clinical and research arenas.
Data from this study provide avenues for novel areas of light therapy research. While strong associations between sleep parameters and post-sleep light exposure were not demonstrated, the relatively low levels of school day illuminance may have created a floor effect such that higher intensities lacked the capacity to exert circadian benefits. Given the combined prospect of a blunted phase advance response among pubertal humans (Hagenauer et al., 2009), it would seem that therapies aimed at augmenting exposure to this PRC region would be most fruitful. Treatments that either deliver light stimuli during sleep (e.g., as described by Cole and colleagues (Cole et al., 2002)) and/or are administered post-awakening with a more flexible schedule need to be further developed and refined for those with DSPD-A, particularly taking into account dismal compliance with daily morning light therapy (Bjorvatn and Pallesen 2009). Equally relevant, school lighting environments should be optimized for maximal circadian benefits, to account for the proportion of time adolescents spend within this setting (Figueiro and Rea 2010). Finally, while the need for protection from high-intensity evening light was negated among these select patients, it does not detract from the potential role of blocking blue wavelengths. In a recent proof-of-concept study involving insomnia patients, subjects who wore “blue-blocker” glasses during the 3 hours prior to habitual bedtimes demonstrated improved subjective sleep quality compared with the placebo intervention (Burkhart and Phelps 2009). The eventual identification of various DSPD subtypes will likely require multicomponent treatment modalities.
Although not the primary foci of our investigations, various results warrant separate mention, as they appear to objectively support large-scale survey studies (Eaton et al., 2010). Data reported from the Centers for Disease Control and Prevention Youth Risk Behavior Survey described insufficient school night sleep (defined as <8hours nightly) among nearly 69% of respondents and optimal sleep (≥9 hours nightly) among less than 8%. On average, all subjects within our study slept <7 hours on school nights and maintained <8 hours sleep on weekends. Given the minimal reported sleep requirement of 8.5 hours among adolescents (National Sleep Foundation 2000), the reduced sleep demonstrated among both cases and controls is quite alarming, taking into account reports of associated adverse consequences (Carskadon 1990; National Sleep Foundation 2000; Pack et al., 1995; Wolfson and Carskadon 1998). Our findings of a reduction in school night vs. weekend total sleep time, and delays in sleep onset and offset during weekend sleep are in accordance with the large survey-based study reported by Wolfson and Carskadon (Wolfson and Carskadon 1998).
Surprisingly, there were no group differences with respect to total sleep time, as DSPD-A subjects routinely arose 1.0 hours later than controls. Taking into account the average wake time of these individuals on school mornings (07:30 h), in combination with the school and transportation schedules described above, it seems likely that a significant decrement in sleep time would have been detected if we were to have selected solely those of middle school age or older (school start time 07:50 h or earlier). Given this equal amount of sleep between groups, it is curious that controls did not endorse sleep-related complaints. Group differences in sleep quality may be implicated. Although there were no differences in actigraphically-derived wakefulness after sleep onset, an adult case-control DSPD study demonstrated objective decrements in sleep quality measured polysomnographically (Campbell and Murphy 2007).
Various limitations to our study need to be addressed. Since physiologic phase markers were not used, we cannot be certain about the extent that described pre-sleep and post-sleep intervals corresponded to the proposed portions of the light PRC. As the habitual sleep/wake schedule can accurately predict an individual’s dim light melatonin onset (Burgess and Eastman 2005), however, and other studies have demonstrated comparable phase relationships between those with DSPD and controls (Chang et al., 2009; Shibui et al., 1999; Wyatt et al., 2006), we believe that our estimates are reasonable. An additional consideration is to what degree light recorded by the Actiwatch-L® reflected ambient light, particularly when accounting for the current widespread use of media with screens held close to the eyes. Although we did not account for the use of such devices with the daily sleep logs, their contributions to phase shifts would likely have been minimal in the instance they were used, considering the unexpected favorable influence of pre-sleep light on total sleep and sleep onset times.
With respect to intergroup differences, cases were slightly older than controls, which may have served to amplify their relative phase delays. This discrepancy should otherwise have not affected our primary findings, however, because group comparisons in light exposure were age-adjusted, thereby removing its effect on the outcomes when making inferences about the other covariates. The two groups were otherwise comparable, and DSPD-A patients were selected with criteria much more stringent than those required by the International Classification of Sleep Disorders, second edition (American Academy of Sleep Medicine 2005). Our selections of cutoff times for entry into the DSPD-A arm were rooted in both clinical experience and the results of recent survey data (National Sleep Foundation 2006), but remain arbitrary in the face of sparse normative information. Further studies are needed in this regard. With respect to other inclusion criteria, the Morningness-Eveningness questionnaire and the Pittsburgh Sleep Quality Index are not validated within pediatric populations, but similar scales with defined cutoffs are not otherwise available, and our subjects had no difficulties in their completion. Finally, the fact that our subjects were culled from a recruited sample and predominantly of Caucasian origin may minimize the generalizability of our findings.
In conclusion, this study appears to refute the role of increased evening light exposure in the induction or perpetuation of the symptoms of DSPD-A, to detract from the notion of increased sensitivity to the delaying effects of light, and to suggest the possibility of insensitivity to the advancing effects of light. If these data are confirmed with subsequent investigations, they may further contribute to the research regarding the underlying etiology of DSPD-A, and may also serve to guide or refine treatment interventions. The identification of successful treatments for DSPD-A is an essential “first step” in establishing whether improvement of sleep complaints can directly address other comorbidities, and may serve to further characterize directional relationships.
ACKNOWLEDGMENTS
The authors wish to thank the Rochester Minnesota Public School District for their support of this project (with particular thanks to Jeffrey Lunde), Christina Suh for assistance with creation of figures, and Lori Solmonson for administrative and technical assistance.
Sources of Support This publication was made possible by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the USA National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
Footnotes
All lux model outcomes were transformed to the log(lux+1) scale to better approximate model assumptions and to bound values away from 0 (or logged values away from negative infinity). Associations between the covariates and lux outcomes are presented as percent changes of lux+1 with 95% confidence intervals (CI).
When the nightly sleep summary variables were used as model outcomes, they were kept on their original scales since the model assumptions were approximately true. Thus, linear slopes with 95% CI’s are presented as summaries of the associations between the covariates and sleep parameters. Sleep onsets were shifted by -12 hours to avoid the issue of values occurring on both sides of midnight, where the 24-hour clock time resets to 0. Shifting the onset times by -12 hours gives the correct mathematical distance between the onset hours (e.g., values of 23.5 and 1.5 were shifted to 11.5 and 13.5, respectively). When the onset/offset time variables were used as model covariates, both the 24-hour onset and offset times were shifted by -12 hours to give correct onset time distances around midnight and to preserve the distances between the onset and offset times relative to each other.
There are no other financial disclosures or conflicts of interest.
REFERENCES
- American Academy of Sleep Medicine . Diagnostic and Coding Manual. American Academy of Sleep Medicine; Westchester, IL: 2005. International Classification of Sleep Disorders. [Google Scholar]
- Andrade MM, Benedito-Silva AA, Domenice S, Arnhold IJ, Menna-Barreto L. Sleep characteristics of adolescents: a longitudinal study. J Adolesc Health. 1993;14:401–406. doi: 10.1016/s1054-139x(08)80016-x. [DOI] [PubMed] [Google Scholar]
- Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18:263–271. doi: 10.1081/cbi-100103190. [DOI] [PubMed] [Google Scholar]
- Auger RR. Circadian Rhythm Sleep Disorder, Delayed Sleep Phase Type (Pediatric Case) American Academy of Sleep Medicine; Westchester, Illinois: 2008. pp. 195–199. [Google Scholar]
- Bjorvatn B, Pallesen S. A practical approach to circadian rhythm sleep disorders. Sleep Med Rev. 2009;13:47–60. doi: 10.1016/j.smrv.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Kutcher S. Diagnosis and measurement of adolescent depression: a review of commonly utilized instruments. J Child Adolesc Psychopharmacol. 2001;11:341–376. doi: 10.1089/104454601317261546. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14:229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci Lett. 2004;356:115–118. doi: 10.1016/j.neulet.2003.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE. 2008;3:e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med. 2003;1:102–114. doi: 10.1207/S15402010BSM0102_3. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6:407–420. [PubMed] [Google Scholar]
- Burkhart K, Phelps JR. Amber lenses to block blue light and improve sleep: a randomized trial. Chronobiol Int. 2009;26:1602–1612. doi: 10.3109/07420520903523719. [DOI] [PubMed] [Google Scholar]
- Buysee D, Reynolds C, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: A new instrument for psychaitric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Elliott JA, Yen SS. Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab. 1992;75:447–452. doi: 10.1210/jcem.75.2.1639946. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Murphy PJ. Delayed sleep phase disorder in temporal isolation. Sleep. 2007;30:1225–1228. doi: 10.1093/sleep/30.9.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25:606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Chang AM, Reid KJ, Gourineni R, Zee PC. Sleep timing and circadian phase in delayed sleep phase syndrome. J Biol Rhythms. 2009;24:313–321. doi: 10.1177/0748730409339611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R, Smith J, Alcala Y, Elliott J, Kripke D. Bright-light mask treatment of delayed sleep phase syndrome. Journal of Biological Rhythms. 2002;17:89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- Dagan Y, Stein D, Steinbock M, Yovel I, Hallis D. Frequency of delayed sleep phase syndrome among hospitalized adolescent psychiatric patients. J Psychosom Res. 1998;45:15–20. doi: 10.1016/s0022-3999(97)00299-7. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford University Press; 1994. [Google Scholar]
- Eaton DK, McKnight-Eily LR, Lowry R, Perry GS, Presley-Cantrell L, Croft JB. Prevalence of insufficient, borderline, and optimal hours of sleep among high school students - United States, 2007. J Adolesc Health. 2010;46:399–401. doi: 10.1016/j.jadohealth.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Iliuodi C, Montes MI, Olavarrieta-Bernardino S, Aguirre-Berrocal A, De La Cruz-Troca JJ, Vela-Bueno A. Circadian preference, nighttime sleep and daytime functioning in young adulthood. Sleep and Biological Rhythms. 2010;8:52–62. [Google Scholar]
- Figueiro MG, Rea MS. Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuro Endocrinol Lett. 2010;31:92–96. [PMC free article] [PubMed] [Google Scholar]
- Finch AJ, Jr., Saylor CF, Edwards GL. Children’s depression inventory: sex and grade norms for normal children. J Consult Clin Psychol. 1985;53:424–425. doi: 10.1037//0022-006x.53.3.424. [DOI] [PubMed] [Google Scholar]
- Gau SF, Soong WT. The transition of sleep-wake patterns in early adolescence. Sleep. 2003;26:449–454. doi: 10.1093/sleep/26.4.449. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Morisane H, Takeuchi H. Effect of daytime light conditions on sleep habits and morningness-eveningness preference of Japanese students aged 12-15 years. Psychiatry Clin Neurosci. 2002;56:225–226. doi: 10.1046/j.1440-1819.2002.00983.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. J Sleep Res. 2001;10:59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Lee C, Smith MR, Eastman CI. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23:859–875. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- Marchetti LM, Biello SM, Broomfield NM, Macmahon KM, Espie CA. Who is pre-occupied with sleep? A comparison of attention bias in people with psychophysiological insomnia, delayed sleep phase syndrome and good sleepers using the induced change blindness paradigm. J Sleep Res. 2006;15:212–221. doi: 10.1111/j.1365-2869.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, Brown T, Chesson AL, Jr., Kapur V, Maganti R, Owens J, Pancer J, Swick TJ, Zak R. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28:1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation [Retrieved Accessed July 3, 2008];Sleep In America Poll Summary Findings. 2006 2006. at http://www.sleepfoundation.org/sites/default/files/2006_summary_of_findings.pdf, 2008.
- National Sleep Foundation . Adolescent Sleep Needs and Patterns: Research Report and Resource Guide. Washington, D.C.: 2000. pp. 1–30. [Google Scholar]
- Okawa M, Uchiyama M. Circadian rhythm sleep disorders: characteristics and entrainment pathology in delayed sleep phase and non-24-h sleep-wake syndrome. Sleep Med Rev. 2007;11:485–496. doi: 10.1016/j.smrv.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Okawa M, Uchiyama M, Ozaki S, Shibui K, Ichikawa H. Circadian rhythm sleep disorders in adolescents: clinical trials of combined treatments based on chronobiology. Psychiatry Clin Neurosci. 1998;52:483–490. doi: 10.1046/j.1440-1819.1998.00449.x. [DOI] [PubMed] [Google Scholar]
- Ouyang F, Lu BS, Wang B, Yang J, Li Z, Wang L, Tang G, Xing H, Xu X, Chervin RD, Zee PC, Wang X. Sleep patterns among rural Chinese twin adolescents. Sleep Med. 2009;10:479–489. doi: 10.1016/j.sleep.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S, Uchiyama M, Shirakawa S, Okawa M. Prolonged interval from body temperature nadir to sleep offset in patients with delayed sleep phase syndrome. Sleep. 1996;19:36–40. [PubMed] [Google Scholar]
- Pack AI, Pack AM, Rodgman E, Cucchiara A, Dinges DF, Schwab CW. Characteristics of crashes attributed to the driver having fallen asleep. Accid Anal Prev. 1995;27:769–775. doi: 10.1016/0001-4575(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–1929. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Rahman SA, Kayumov L, Tchmoutina EA, Shapiro CM. Clinical efficacy of dim light melatonin onset testing in diagnosing delayed sleep phase syndrome. Sleep Med. 2009;10:549–555. doi: 10.1016/j.sleep.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Robinson J, Bayliss SC, Fielder AR. Transmission of light across the adult and neonatal eyelid in vivo. Vision Res. 1991;31:1837–1840. doi: 10.1016/0042-6989(91)90031-y. [DOI] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr., Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30:1484–1501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32:1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Hauri P, Kripke D, Lavie P. The Role of Actigraphy in the Evaluation of Sleep Disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- Shibui K, Uchiyama M, Okawa M. Melatonin rhythms in delayed sleep phase syndrome. J Biol Rhythms. 1999;14:72–76. doi: 10.1177/074873049901400110. [DOI] [PubMed] [Google Scholar]
- Stoschitzky K, Sakotnik A, Lercher P, Zweiker R, Maier R, Liebmann P, Lindner W. Influence of beta-blockers on melatonin release. Eur J Clin Pharmacol. 1999;55:111–115. doi: 10.1007/s002280050604. [DOI] [PubMed] [Google Scholar]
- Szeinberg A, Borodkin K, Dagan Y. Melatonin treatment in adolescents with delayed sleep phase syndrome. Clin Pediatr (Phila) 2006;45:809–818. doi: 10.1177/0009922806294218. [DOI] [PubMed] [Google Scholar]
- Thorpy MJ, Korman E, Spielman AJ, Glovinsky PB. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1998;9:22–27. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Okawa M, Shibui K, Kim K, Tagaya H, Kudo Y, Kamei Y, Hayakawa T, Urata J, Takahashi K. Altered phase relation between sleep timing and core body temperature rhythm in delayed sleep phase syndrome and non-24-hour sleep-wake syndrome in humans. Neurosci Lett. 2000;294:101–104. doi: 10.1016/s0304-3940(00)01551-2. [DOI] [PubMed] [Google Scholar]
- Wagner KD, Hirschfeld RM, Emslie GJ, Findling RL, Gracious BL, Reed ML. Validation of the Mood Disorder Questionnaire for bipolar disorders in adolescents. J Clin Psychiatry. 2006;67:827–830. doi: 10.4088/jcp.v67n0518. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kajimura N, Kato M, Sekimoto M, Nakajima T, Hori T, Takahashi K. Sleep and circadian rhythm disturbances in patients with delayed sleep phase syndrome. Sleep. 2003;26:657–661. doi: 10.1093/sleep/26.6.657. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment options. Sleep. 2004;27:1195–1203. doi: 10.1093/sleep/27.6.1195. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29:1075–1080. doi: 10.1093/sleep/29.8.1075. [DOI] [PubMed] [Google Scholar]