Abstract
Attention-deficit/Hyperactivity Disorder (ADHD) is a developmental disorder which, by current definition, has onset prior to age 7 years. MRI studies have provided some insight into brain differences associated with ADHD, but thus far have almost exclusively focused on children ages 7 years and older. To better understand the neurobiological development of ADHD, cortical and subcortical brain development should be systematically examined in younger children presenting with symptoms of the disorder. High resolution anatomical (MPRAGE) images, acquired on a 3.0T scanner, were analyzed in a total of 26 preschoolers, ages 4–5 years (13 with ADHD, 13 controls, matched on age and sex). The ADHD sample was diagnosed using DSM-IV criteria, and screened for language disorders. Cortical regions were delineated and measured using automated methods in Freesurfer; basal ganglia structures were manually delineated. Children with ADHD showed significantly reduced caudate volumes bilaterally; in contrast, there were no significant group differences in cortical volume or thickness in this age range. After controlling for age and total cerebral volume, left caudate volume was a significant predictor of hyperactive/impulsive, but not inattentive symptom severity. Anomalous basal ganglia, particularly caudate, development appears to play an important role among children presenting with early onset symptoms of ADHD.
Keywords: preschool, MRI, caudate, cortical, hyperactivity, cognitive, ADHD, striatum, basal ganglia
A Developmental Perspective in ADHD
By the age of 4 years, as many as 40% of children have sufficient problems with attention to be of concern to parents and preschool teachers. Attention-deficit/Hyperactivity Disorder (ADHD) has become the most commonly diagnosed form of psychopathology in the preschool years (Armstrong & Nettleton, 2004), with prevalence estimates in preschoolers ranging from 2% to 8% for community studies using strict DSM-IV criteria (Connor, 2002; Egger, Kondo, & Angold, 2006) to as high as 59% to 86% of preschoolers referred to psychiatry clinics (Wilens et al., 2002). Of concern is the observation that preschoolers with ADHD are found to have similar patterns of comorbid psychopathology and functional impairment as school age children with ADHD (Wilens et al., 2002), and that few are described as well-adjusted by adolescence (Lee, Lahey, Owens, & Hinshaw, 2007). In addition, preschoolers with ADHD have increased rates of emergency room visits compared to children without ADHD (Greenhill, Posner, Vaughan, & Kratochvil, 2008). Subthreshold ADHD in early childhood is also significantly associated with academic failure and grade retention (Bussing, 2010). Thus, preschool children presenting with symptoms of ADHD (even those without formal DSM-IV diagnosis) are at significant risk for social and academic difficulties, relative to typically developing children (American Academy of Pediatrics, 2000). Earlier identification and treatment of children presenting with attention problems in the preschool years may minimize the harmful impact of ADHD (Sonuga-Barke, 2010; Wilens et al., 2002).
From a developmental perspective, brain systems affected in ADHD, including the cortex, subcortical white matter, basal ganglia, and cerebellum share a pattern of reciprocal influence (“crossed trophic effect”), such that early injury to subcortical structures impairs not only the cerebral cortical development, but also the development of the more remote-developing cerebellum, and vice versa (Inder, 1999; Limperopoulos et al., 2005). A developmental perspective is therefore crucial in understanding the early growth patterns underlying the development of (and mitigation of risk for) ADHD. In particular, since ADHD is a disorder that by definition has its onset prior to age 7 years, it is critical to examine children prior to that age to better understand the neurobiological course of the disorder. However, virtually all of the neuroimaging studies of children with ADHD have included only children of school age (i.e., age 6 years and older) (Bush, 2008; Valera, Faraone, Murray, & Seidman, 2006).
Studies have shown that by school age, ADHD is associated with reduced cortical and subcortical (basal ganglia, cerebellum) volumes (Bush, 2008); however, it remains unclear whether behaviors associated with emergence of ADHD are associated with anomalous development of the basal ganglia and/or cerebellum, with subsequent reduction in growth of the cerebral cortex or vice versa. Preliminary studies (Qiu et al., 2009) suggest that structures of the basal ganglia, which mature earlier than the cerebellum and cortex (Lenroot, 2007), may play a crucial role in this process. Furthermore, animal models of ADHD (using spontaneously hypertensive rats) similarly highlight the importance of early striatal anomalies (reductions) that stabilize by age 6 weeks (human equivalent = 7–9 years) (Hsu et al., 2010). In humans, evidence suggests that normalization of reduced caudate in ADHD occurs by puberty (Castellanos et al., 2002); thus, the early developmental trajectory of caudate anomalies in ADHD appears to parallel the reduction of hyperactive/impulsive symptoms (Biederman, 2000).
Characterization of ADHD in Preschoolers
Recent research has supported the validity of the diagnosis of ADHD for younger children (Lahey, 1998) and the predictive validity of ADHD diagnoses made in preschool age children (Lahey, 2004; Massetti et al., 2007; McGee, Partridge, Williams, & Silva, 1991). Despite the increased interest in assessing ADHD in preschoolers, the NIH Consensus Statement (Health, 1998) called for additional research on ADHD, particularly in the areas of age- and sex-specific diagnostic criteria, and in the development of reliable and valid assessment procedures. The NIMH clinical trial examining the effects of methylphenidate in preschoolers (ages 3–6 years) with ADHD, i.e., the Preschool ADHD Treatment Study (PATS), has shed light on diagnostic methods and treatments for ADHD in the preschool years (Abikoff et al., 2007; Greenhill, 2006; Swanson et al., 2006; Vitiello et al., 2007). In the present study, ADHD diagnostic methods were adapted from PATS, with age 4 years as the earliest entry point, because prior to age 4, it is more difficult to characterize the behavioral features associated with ADHD or distinguish them from behaviors that occur in typically developing children (Smidts & Oosterlaan, 2007).
Summary
There remains a dearth of knowledge about the effects of early brain development and associated behavior in children with symptoms of ADHD, as well as how these developmental issues contribute to presentation in the school years (DuPaul, McGoey, Eckert, & VanBrakle, 2001). For a disorder (presently) requiring symptom onset by age 7, there have been few studies of brain development in preschoolers with ADHD, and none involving neuroimaging in preschoolers with ADHD. The present study examined cortical and basal ganglia volumes in preschool children (ages 4–5 years) with and without symptoms of ADHD, and explored the relationship between brain volumes and ADHD symptom severity.
Methods
Study Procedures
Approval was granted for this study from the Johns Hopkins Medicine Institution Review Board. After description of the study, parents of participants signed written consent, and participants provided verbal assent. Participants were initially screened via telephone interview with a parent to determine eligibility (outlined below). Once enrolled, all participants received MRI scans and completed a neuropsychological assessment battery that included measures of attention, memory, language, visual, and motor skills. Parents of the participants also completed behavior rating scales at the time of neuropsychological testing. Parent ratings (emphasizing ADHD symptomatology) were analyzed for the present study. MRI scans were obtained within six weeks of the child’s neuropsychological assessment (mean time between testing and scan = 12.2 ± 11.5 days; range = 1–42 days).
Participants
Participants were recruited from advertisements in the community, pediatricians’ offices, and local daycare centers. For entry into the study, children were required to be between ages 4 years, 0 months and 5 years, 11 months.
Inclusion and Exclusion Procedures
Participants were excluded if they had any of the following, established via review of medical/developmental history, and/or by study screening assessment: 1) diagnosis of Intellectual Disability or Pervasive Developmental Disorder; 2) known visual impairment; 3) treatment of any psychiatric disorder (other than ADHD) with psychotropic medications [for those with diagnosis of ADHD, treatment with stimulants was allowed, whereas children treated with other psychotropic medications were excluded]; 4) any history of DSM-IV Axis I diagnosis (Note: history of Oppositional Defiant Disorder, Adjustment Disorder, or mild anxiety symptoms—i.e., not meeting DSM-IV diagnosis nor treated pharmacologically—was allowed); 5) neurological disorder (e.g., epilepsy, cerebral palsy, traumatic brain injury, Tourette syndrome); 6) documented hearing loss ≥ 25 dB loss in either ear; 7) evidence of physical, sexual, or emotional abuse; 8) medical contraindication to MRI procedures (e.g., implanted electrical devices, dental braces); or, 9) Full Scale IQ scores (either by previous assessment or by study screening assessment) less than 80. In addition, children were excluded if there was a history of a Developmental Language Disorder (DLD), either determined during the initial phone screen, based on prior assessment (completed within one year of the current assessment), or determined during screening visit. Developmental Language Disorder exclusion is in deference to literature suggesting that language impairments may, developmentally, influence development of inhibitory control, response preparation, and working memory—core features of ADHD (Hagberg, Miniscalco, & Gillberg, 2010). Further, DLD represent a significant diagnostic confound in the brain basis of ADHD (Posner et al., 2007). Children who were unable to cooperate with brain imaging also were excluded. A total of 4 children initially recruited (3 ADHD, 1 control) were unable to cooperate with scanning procedures, and were excluded.
Diagnostic methods for the ADHD and control groups were adapted from the NIH Preschoolers with Attention-deficit/Hyperactivity Disorder Treatment (PATS) Study (Kollins et al., 2006; Posner et al., 2007). Diagnosis of ADHD was made using parent report on the Diagnostic Interview Schedule for Children-Young Child (YC-DISC) (Lucas, Fisher, & Luby, 1998; Lucas, Fisher, & Luby, 2008). The YC-DISC is a highly structured diagnostic instrument that includes computer-assisted administration, and assesses common psychiatric disorders, as defined by DSM-IV, that present in young children. The following modules were administered: ADHD, Social Phobia, Generalized Anxiety Disorder, Separation Anxiety, Depression, Oppositional Defiant Disorder, and Conduct Disorder. To be included in the ADHD group, symptoms must have been present for at least 6 months, and cross-situational impairment (defined as parent report of problems at home and with peers) was required. Additionally, children in the ADHD group were required to have T-scores ≥ 70 on one or both of the DSM-IV ADHD Scales (Scales L and M) of the Conners’ Parent Scales-Revised.
Once children met general entry/exclusion criteria above, they were included in the control group only if they did not meet categorical diagnostic criteria for ADHD on the YC-DISC. Additionally, children in the control group were required to have T-scores < 65 on the DSM-IV ADHD Scales (Scales L and M) of the Conners’ Parent Scales-Revised. A total of 26 preschool-aged children participated, including 13 controls (5 boys, 8 girls) and 13 children with ADHD (8 boys, 5 girls).
Assessment Methods
Wechsler Preschool and Primary Scale of Intelligence-Third Edition (WPPSI-III) (Wechsler, 2002). The WPPSI-III was used to assess IQ in our sample for each participant. Children with WPPSI-III FSIQ < 80 were excluded.
Clinical Evaluation of Language Functions-Preschool-2 (CELF-P-2) (Wiig, Secord, & Semel, 2004). The CELF-P-2 is an individually administered, norm-referenced test developed to identify and diagnose language and communication disorders in preschool children. Children who scored less than −1.5 sd on either the Receptive Language or Expressive Language Index of the CELF-P-2, or below -1.0 sd on both indices, were excluded.
Conners’ Parent Rating Scales-Revised-Long Form (CPRS-R) (Conners, 1997). Dimensional ratings of ADHD symptom severity were obtained using the DSM-IV oriented scales from the CPRS-R, including Scale L (DSM-IV Inattentive) and Scale M (DSM-IV Hyperactive/Impulsive).
MRI Scanning in Preschoolers
Preparation of children for scans
MRI scanning in children is challenging and particularly so for young children with behaviors associated with ADHD. Two problems must be overcome: first, getting children to enter the MRI environment willingly, and second, keeping their heads sufficiently still to acquire good data. Head motion is one of the most vexing problems in MRI scanning generally, and is a special challenge in young children with neurodevelopmental disorders. For the present study, we used a behavioral protocol, designed for use with young children and those with developmental disabilities. The protocol was conducted during one or two brief (10–15 minutes) practice scans using an inactive (mock) scanner that matched the active study scanner. The behavioral training protocol was provided to both ADHD and control group participants by a trained, master’s level behavioral therapist with oversight by a senior behavior analyst. The initial interaction with the child involved having him or her select a movie to watch during the mock scan and a prize (small toy) to be awarded for cooperative participation in the practice session. The training employed verbal and gestural instructions presented one step at a time based on a task analysis of the behavior required for a successful, unsedated anatomic MRI. While following the task analysis, the therapist gradually exposed the child to the mock scanner, positioning on the patient support, movement of the support into the scanner bore, and increasing volume of prerecorded scanner noises. After verbal instruction and placement in the mock scanner, the child was instructed to watch the selected movie and to remain in position without talking or head movement unless prompted by the therapist. Movement detected by the therapist resulted in brief verbal feedback (e.g. “Don’t forget to keep your head still”), sometimes combined with a tactile prompt (e.g. touching the child’s chin or arm for 1–3 sec with the index finger). Cooperation and inhibition of head movement were positively reinforced with intermittent verbal praise (about every 3 minutes) and with the small toy that the child had selected presented at the end of the mock scan. A movie, contingent praise and another prize were similarly provided for the child’s actual scan (Slifer, Cataldo, Gerson, & Tucker, 1994).
Imaging methods
All scanning was completed, using a 3.0T Philips GyroscanNT scanner in the F.M. Kirby Center at the Kennedy Krieger Institute. Magnetization Prepared Rapid Gradient Recalled Echo (MPRAGE) images were used for volumetric assessment and for detailed analysis of macroscopic features of the cortex, including shape, folding, thickness, and density. Slice thickness = 1.0mm; FOV = 26cm; Matrix size: 256 × 256. A Sensitivity Encoding (SENSE) coil was used to address geometric distortion artifacts due to macroscopic magnetic susceptibility effects that can cause signal dropout at the air-tissue interface. Cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu).
Regional measures of cortical thickness, surface area and volume, as well as regional measures of adjacent white matter volume were obtained using automated methods within Freesurfer (Fischl et al., 2004). Total cerebral volume was also measured using Freesurfer. Freesurfer used a fully automated method to perform pre-processing steps including Talairach alignment, intensity normalization, and removal of skull and non-brain tissue with a hybrid watershed/surface deformation procedure, separation of the cerebellum and brainstem from the cerebrum and splitting of the left and right hemispheres (Dale, Fischl, & Sereno, 1999; Segonne et al., 2004). A deformable surface algorithm was used to define inner (gray-white) and outer (gray-cerebrospinal fluid) cortical surfaces (Dale et al., 1999). Automated topological correction, surface inflation and registration to a spherical atlas were also included in the processing stream (Dale et al., 1999; Fischl, Sereno, & Dale, 1999). An automated labeling system was then used to parcellate the cortex into 34 gyral-based regions of interest (ROIs) (Desikan et al., 2006) and a probabilistic labeling algorithm (Fischl et al., 2004) was used to apply this parcellation to the cortical surfaces of each participant. ROIs were summed to obtain lobar cortical volume, surface area and associated white matter volume. Weighted averages were used to obtain lobar cortical thickness. More detailed methods are outlined in a previously published manuscript examining older, school-age populations (Wolosin, Richardson, Hennessey, Denckla, & Mostofsky, 2007).
The basal ganglia structures were traced manually by experienced raters who were blind to participant diagnostic group, using MIPAV (Medical Image Processing and Visualization) (McAulliffe, 2001). The caudate nucleus (excluding the nucleus accumbens), putamen, and globus pallidus were outlined on contiguous coronal slices. Detailed tracing guidelines are described in a previously published manuscript examining older, school-age populations (Qiu et al., 2009). For the initial protocol, intrarater reliability was established for each region on five scans measured twice by the same rater (Rater 1). Inter-rater reliability was established by a second rater and calculated using the time 2 measures from Rater 1. Intraclass correlation coefficients (ICC) revealed high rates of intra- and inter-rater reliability for the caudate (intra = 0.96; inter = 0.92), putamen (intra = 0.98; inter = 0.93) and globus pallidus (intra = 0.96; inter = 0.90).
Data Analysis
Group differences (ADHD versus control) in lobar (gray matter, white matter) and basal ganglia volumes were examined using independent samples t-tests. Zero-order and partial correlations between regional volumes and ADHD symptom severity measures were used to examine brain-behavior relationships. As this is an initial exploratory study, significance was set at p < 0.05, although, given the small sample size, those analyses with effect size η2 > 0.10 were also considered in the interpretation of findings.
Results
Sample Information
Demographic characteristics of all 26 participants are summarized in Table 1. There were 13 children with ADHD in the sample (8 boys) and 13 controls (5 boys) in the sample and the mean age was 5.0 years (standard deviation = 0.6). The racial composition of the sample was 85% Caucasian, 8% African-American, 4% biracial and 3% Asian; 88% percent of the sample was right-handed and 12% left- or mixed-handed. There were no significant differences between ADHD and control groups in age [F(1,24) = 0.05, p = 0.947], Full Scale IQ [F(1,24) = 0.04, p = 0.850], sex distribution (χ2 = 1.38, p = 0.434), racial composition (χ2 = 4.18, p = 0.242), or handedness (χ2 = 0.38, p = 0.539) (see Table 1). None of the children in the ADHD group had begun taking stimulant medication at the time of participation in the study, although several began treatment shortly after participation.
Table 1.
Demographic Information
| Control (n=13) |
ADHD (n=13) |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | η2p | |
| Age (years) | 5.0 | 0.5 | 5.0 | 0.6 | .947 | .000 |
| WPPSI-3 Full Scale IQ | 110.9 | 12.9 | 109.8 | 17.6 | .850 | .002 |
| Total Cerebral Volume (mm3) | 996219.4 | 79327.6 | 950060.4 | 59997.5 | .133 | .104 |
| CPRS-R Scale L T-score | 48.7 | 7.4 | 68.2 | 14.5 | .001 | .447 |
| CPRS-R Scale M T-score | 52.3 | 8.0 | 70.7 | 14.6 | .001 | .409 |
Note: ADHD = Attention-deficit/Hyperactivity Disorder; WPPSI-3 = Wechsler Preschool and Primary Scale of Intelligence, Third Edition; CPRS-R = Conners’ Parent Rating Scale-Revised, Scale L (DSM-IV Inattentive); Scale M (DSM-IV Hyperactive/Impulsive); η2p = effect size, partial eta-squared.
Cortical and total cerebral volume measurements were available for 23 children, including 12 controls (5 boys) and 11 children with ADHD (7 boys). The missing cortical measurements for three children (2 ADHD, 1 control) were due to software processing errors in Freesurfer in which there were topographical anomalies in the data that could not be rectified by the surface definition algorithm. Among the 23 children with cortical measurements, there were no significant differences between groups in age, IQ, sex distribution, handedness, or racial distribution. There were also no significant group differences in total cerebral volume [F(1,21) = 2.44, p = 0.133].
Basal ganglia measurements were available for 24 children, including 13 controls (5 boys) and 11 children with ADHD (7 boys). Due to excessive motion artifact, basal ganglia measurements were not acquired for two children (both ADHD) as the boundaries of the basal ganglia could not be reliably assessed. Among the 24 children with basal ganglia measurements, there were no significant differences between groups in age, IQ, sex distribution, handedness, or racial distribution.
Behavioral Training for Scans
The behavioral training protocol was effective for teaching 87% (26 of 30) of the study participants to remain sufficiently motionless for MR image acquisition without sedation. The success rates for ADHD (81%) and control participants (93%) did not differ significantly.
Group Differences in Cortical Volume, Thickness, and Surface Area
Statistical difference maps for cortical volume, surface area and thickness between groups were also generated and displayed on an average brain generated from all subjects (Fischl et al., 1999). A false discovery rate of p = 0.05 was applied to the statistical difference maps to correct for multiple comparisons. No significant differences in cortical volume, surface area or thickness were detected between the ADHD group and control group in statistical difference maps.
Means and standard deviations for gray and white matter lobar volumes for the ADHD and control groups are listed in Table 2. There were no significant group differences in total cortical gray matter volume in any of the four lobes, for either hemisphere, using t-tests or non-parametric analyses (Mann-Whitney U). For white matter, there were also no significant group differences in lobar volumes (using t-tests or non-parametric analyses), although large effect sizes were observed (η2p > 0.10) for right parietal and left temporal white matter—in both cases controls showing larger volumes than children with ADHD.
Table 2.
Regional Lobar Cortical Gray and White Matter Volumes (mm3)
| Gray Matter | White Matter | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Control (n=12) |
ADHD (n=11) |
Control (n=12) |
ADHD (n=11) |
||||||||
| Mean | SD | Mean | SD | p | η2p | Mean | SD | Mean | SD | p | η2p | |
| Frontal Lobe (Left) | 95974.5 | 5805.1 | 92758.4 | 10743.2 | .376 | .037 | 64102.5 | 7245.6 | 60379.3 | 4923.7 | .168 | .088 |
| Frontal Lobe (Right) | 98392.8 | 6250.6 | 94406.7 | 10025.2 | .261 | .060 | 64103.6 | 7670.3 | 60127.5 | 5704.9 | .176 | .085 |
| Temporal Lobe (Left) | 70634.9 | 4977.3 | 68397.9 | 5780.8 | .330 | .045 | 34297.8 | 3904.6 | 32043.3 | 2324.2 | .111 | .116 |
| Temporal Lobe (Right) | 70669.3 | 5139.2 | 67876.7 | 6982.1 | .284 | .054 | 34768.7 | 4332.5 | 32516.6 | 3398.8 | .183 | .083 |
| Parietal Lobe (Left) | 82923.2 | 7310.1 | 81195.2 | 6575.9 | .559 | .017 | 51327.3 | 7378.9 | 48556.7 | 4754.6 | .302 | .051 |
| Parietal Lobe (Right) | 85468.7 | 5943.9 | 82500.8 | 5316.9 | .222 | .070 | 54116.9 | 7336.3 | 49817.7 | 3868.3 | .098 | .125 |
| Occipital Lobe (Left) | 20757.2 | 2855.5 | 20091.3 | 3095.2 | .597 | .014 | 12344.3 | 1857.3 | 11602.4 | 1901.4 | .355 | .041 |
| Occipital Lobe (Right) | 20340.5 | 2810.9 | 20736.2 | 3330.3 | .760 | .005 | 11870.8 | 1668.4 | 11509.1 | 1333.8 | .574 | .015 |
Note. ADHD = Attention-deficit/Hyperactivity Disorder; η2p = effect size, partial eta-squared
Means and standard deviations for lobar cortical thickness and surface area for the ADHD and control groups are listed in Table 3. There were no significant group differences in regional thickness for any of the lobar regions bilaterally. Similarly, there were no significant differences between groups in cortical surface area for any of the lobar regions of interest; although for right parietal lobe surface area, the group difference approached significance (p = 0.053), with a large effect size (η2p = 0.167), showing greater surface area for controls. Using non-parametric analysis however (given the small sample size), the differences in group distribution for right parietal surface area were not statistically significant (Mann-Whitney U = −1.72, p = 0.091).
Table 3.
Regional Cortical Thickness (mm) and Surface Area (mm2)
| Cortical Thickness | Surface Area | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Control (n=12) |
ADHD (n=11) |
Control (n=12) |
ADHD (n=11) |
||||||||||||
| Mean | SD | Mean | SD | p | η2p | Mean | SD | Mean | SD | p | η2p | |||||
| Frontal Lobe (Left) | 2.70 | 0.12 | 2.68 | 0.14 | .684 | .008 | 30217 | 2282 | 29298 | 2636 | .416 | .032 | ||||
| Frontal Lobe (Right) | 2.78 | 0.14 | 2.78 | 0.16 | .959 | .000 | 30685 | 3044 | 29173 | 2159 | .188 | .081 | ||||
| Temporal Lobe (Left) | 2.88 | 0.17 | 2.90 | 0.19 | .800 | .003 | 20898 | 1639 | 20215 | 1088 | .257 | .061 | ||||
| Temporal Lobe (Right) | 2.92 | 0.17 | 2.94 | 0.20 | .864 | .001 | 21187 | 1894 | 20180 | 1193 | .146 | .098 | ||||
| Parietal Lobe (Left) | 2.64 | 0.13 | 2.70 | 0.15 | .311 | .049 | 27246 | 2782 | 25994 | 1465 | .197 | .078 | ||||
| Parietal Lobe (Right) | 2.62 | 0.10 | 2.67 | 0.13 | .277 | .056 | 28452 | 2815 | 26618 | 936 | .053 | .167 | ||||
| Occipital Lobe (Left) | 2.30 | 0.13 | 2.35 | 0.10 | .303 | .050 | 8088 | 814 | 7719 | 918 | .318 | .048 | ||||
| Occipital Lobe (Right) | 2.30 | 0.12 | 2.32 | 0.17 | .512 | .021 | 7980 | 819 | 7988 | 792 | .982 | .000 | ||||
Note. ADHD = Attention-deficit/Hyperactivity Disorder; η2p = effect size, partial eta-squared
Group Differences in Basal Ganglia Volumes
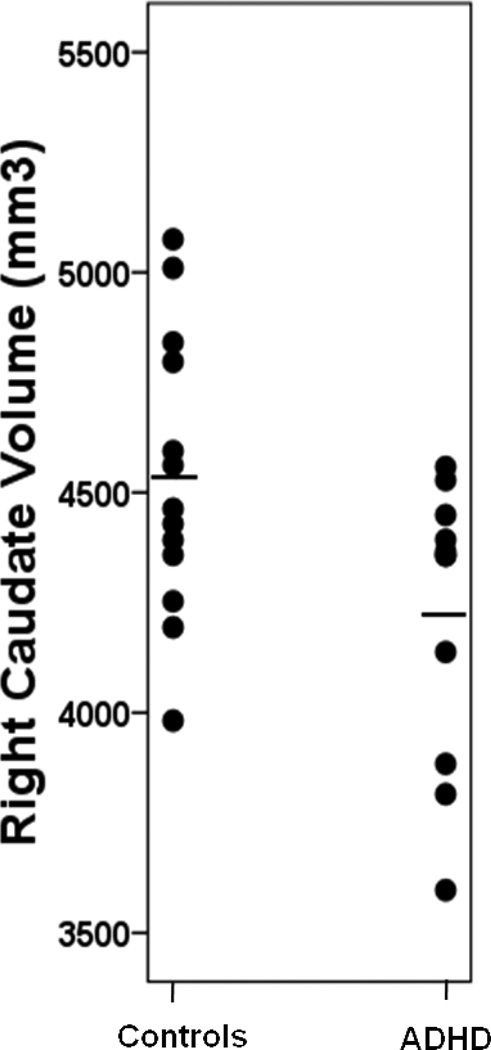
Means and standard deviations for basal ganglia volumes are presented in Table 4. There were no significant differences between ADHD and control groups in putamen or globus pallidus volumes. In contrast, the ADHD group had significantly reduced right caudate volumes, compared to controls (p = 0.027, η2p = 0.204), with large effect size (Figure 1). While not statistically significant (p = 0.123), the reductions in left caudate volumes observed in children with ADHD also had moderate-to-large effect size (η2p = 0.095). Given the small sample size, group comparisons were also made using non-parametric statistics for the right caudate, yielding similar results (Mann-Whitney U = −2.06, p = 0.041).
Table 4.
Basal Ganglia Volumes (mm3)
| Control (n=13) |
ADHD (n=11) |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | η2p | |
| Caudate Nucleus (Left) | 4440.7 | 252.1 | 4231.1 | 416.7 | .143 | .095 |
| Caudate Nucleus (Right) | 4534.6 | 323.2 | 4222.0 | 319.7 | .027 | .204 |
| Putamen (Left) | 4508.8 | 523.5 | 4267.2 | 354.6 | .208 | .071 |
| Putamen (Right) | 4446.2 | 562.2 | 4216.8 | 322.8 | .245 | .061 |
| Globus Pallidus (Left) | 1580.8 | 170.0 | 1501.3 | 138.4 | .227 | .066 |
| Globus Pallidus (Right) | 1641.9 | 163.7 | 1575.4 | 127.0 | .285 | .052 |
Note. ADHD = Attention-deficit/Hyperactivity Disorder; η2p = effect size, partial eta-squared
Figure 1.
Right Caudate Volumes (without Nucleus Accumbens) in Preschoolers with and without ADHD (Controls > ADHD p = 0.03). Horizontal lines represent means.
Correlations between Imaging Variables and ADHD Symptoms
The relationships between ADHD symptoms and regional (cortical, basal ganglia) volumes were explored using Pearson correlations, collapsing data across diagnostic groups. Parent ratings from two ADHD symptom scales were used to measure ADHD symptoms severity in a dimensional manner across groups (CPRS-R Scale L: DSM-IV Inattentive and CPRS-R Scale M: DSM-IV Hyperactive/Impulsive).
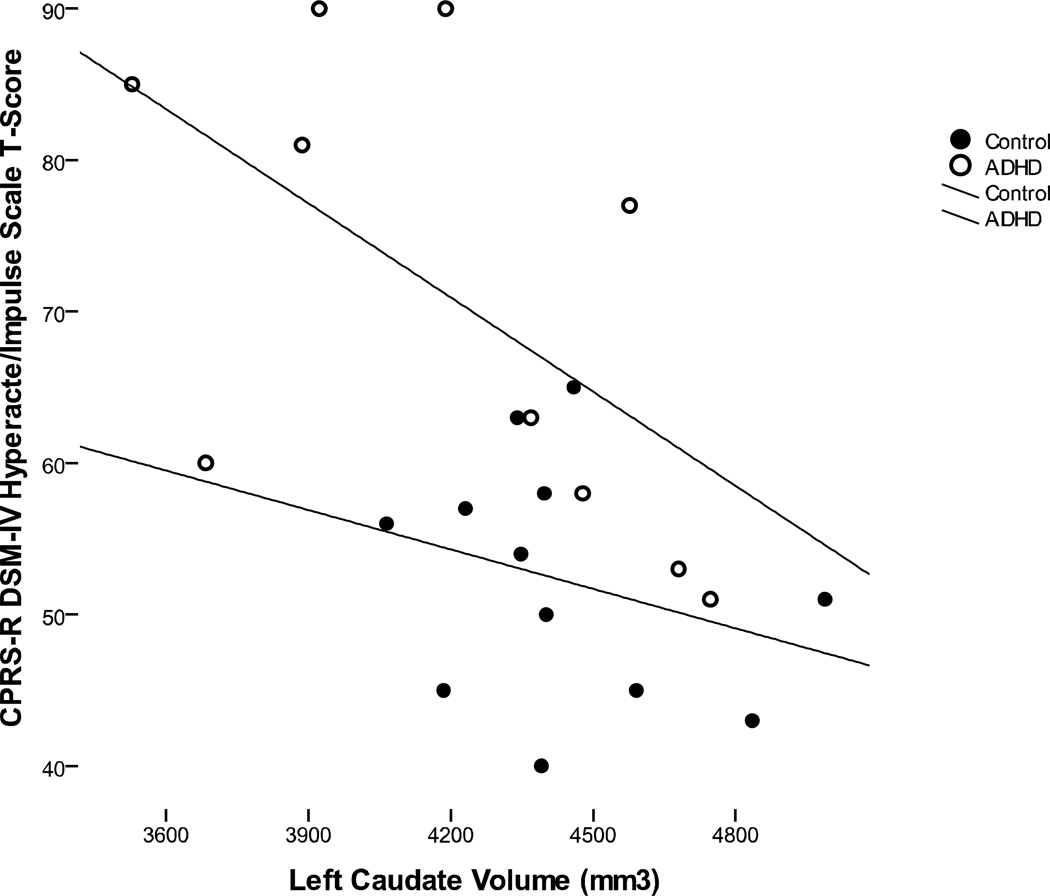
None of the regional cortical lobar volumes (left/right, gray/white), were significantly correlated with either hyperactive/impulsive or inattentive symptoms. Similarly, none of the lobar regional measures of surface area or cortical thickness were significantly associated with hyperactive/impulsive or inattentive symptoms. For the basal ganglia, neither putamen, nor globus pallidus volumes were significantly associated with ADHD symptom severity. However, both right (r = −0.52, p = 0.013) and left (r = −0.57, p = 0.006) caudate volumes were significantly correlated with parent ratings of hyperactive/impulsive symptoms. Further, when analyzed separately by group, the association between left caudate volume and parent rating of hyperactive/impulsive symptoms remained significant within ADHD group (r = −0.58, p = 0.039), but not within the control group (Figure 2). The associations (across groups, but not within groups) also remained after controlling for age and total cerebral volume—right (rp = −0.50, p = 0.014) and left (rp = −0.58, p = 0.004). Neither right caudate (rp = −0.32, p = 0.123) nor left caudate (rp = −0.34, p = 0.109) volumes were significant predictors of inattentive symptoms, within or across groups. For these partial correlations, missing data for total cerebral volume values for the three children (2 ADHD, 1 control) who had basal ganglia measurements but not cortical measurements were imputed by expectation maximization method.
Figure 2.
Association between left caudate volumes (without nucleus accumbens) and parent ratings of hyperactive/impulsive symptoms. Within the ADHD group, the association was significant (R2 = 0.34, p =0.039), but not within the control group (R2 = 0.08, p = 0.19).
Discussion
This is the first study to directly examine regional cortical and subcortical brain volumes in preschool children presenting with early symptoms of ADHD. Our preliminary results reveal early anomalies (reduced volumes) in the development of the caudate nucleus among preschool children presenting with symptoms of ADHD, and a strong association between bilateral caudate volumes and severity of hyperactive/impulsive (but not inattentive) symptoms. Conversely, cortical volumes were less atypical than caudate volumes (i.e., showing less reduction) in this age range among children with symptoms of ADHD, and were not associated with either hyperactive/impulsive or inattentive symptom severity.
Although early anatomical MRI studies identified anomalies in cortical (frontal) regions (Casey et al., 1997; Castellanos et al., 1996; Durston et al., 2004; Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002) as well as basal ganglia (Aylward et al., 1996; Castellanos et al., 1996; Singer et al., 1993), recent research has argued that the subcortical brain regions may provide answers to the early etiology of ADHD (Halperin & Schulz, 2006; Valera et al., 2007). For example, among children with early prefrontal lesions, functional impairment often does not manifest until later childhood (Anderson, 1999), and then tends to get worse upon entry into adolescence (Denckla & Cutting, 2004); in contrast, symptoms of ADHD are almost always evident during the preschool years (Barkley, 2006), and the severity of symptoms (most notably hyperactive/impulsive symptoms) tend to diminish with age (Hinshaw, Owens, Sami, & Fargeon, 2006). This pattern has led some researchers to hypothesize that the prefrontal cortex may be more involved in recovery from ADHD, rather than development of the disorder, and that early disruption to other regions, notably the basal ganglia, may be involved in etiology of ADHD (Halperin & Schulz, 2006; Soliva et al., 2010). This hypothesis has considerable merit, as the basal ganglia serve as the nexus through which prefrontal, premotor and motor signals inhibit competing motor programs and disinhibit intended behaviors (Mink, 1996; Nachev, Kennard, & Husain, 2008). Furthermore, the number of cells that project from the basal ganglia to the supplementary motor complex (SMC; a region critical to response control which shows anomalous structure and function in older children with ADHD) (Mahone et al., 2009; Ranta,; Suskauer, McNally, Crocetti, & Mostofsky, 2009; Suskauer et al., 2008a; Suskauer et al., 2008b), is 3–4 times the number that project from the cerebellum to the SMC, unlike the pattern for other cortical motor areas (Akkal, Dum, & Strick, 2007).
Additionally, consistent with this hypothesis, a recent meta-analysis among children ages 9–14 years, showed the largest ADHD-related reductions, compared to controls, in the right caudate (Valera et al., 2006). These findings, along with animal models (Hsu et al., 2010), highlight the importance of early striatal reductions (those that may actually stabilize later in life), in the development of ADHD. Since normalization of reduced caudate volume often occurs by puberty in children with ADHD (Castellanos et al., 2002), the developmental trajectory of caudate anomalies in ADHD appears to parallel the reduction of hyperactive/impulsive symptoms (Biederman, 2000). For example, Carmona and colleagues reported bilateral reductions in ventral striatum, and observed that volume of right ventral striatum was correlated with maternal ratings of hyperactivity (Carmona et al., 2009); and in a study of nine monozygotic twin pairs discordant for ADHD, Castellanos reported that the affected children had smaller total caudate volumes (Castellanos et al., 2003).
The basal ganglia may also contribute to the pathophysiology of ADHD via reward-based modulation of cortical activity (Shadmehr & Krakauer, 2008) which is critical to development of mechanisms involving response selection and control. Recent finding using transcranial magnetic stimulation (Gilbert et al., 2011) reveal children with ADHD show reduced short interval cortical inhibition (SICI), a mechanism critical to response selection that is enhanced by dopamine and related reward-based input. The combined findings from these studies suggest that early abnormalities in dopamienrgic input to the basal ganglia may contribute to impaired development of cortical mechanisms necessary for efficient response selection and control.
Clinically, as a neurodevelopmental disorder affecting motor intentional systems that are mediated in part by frontostriatal circuitry (Durston, van Belle, & de Zeeuw, 2010), these early neuroanatomic anomalies in children with ADHD set the stage for deficits in motor coordination, processing speed, and response control, which, when carefully assessed, are ubiquitous to the disorder, and contribute to persistent cognitive and academic dysfunction (Jacobson et al., 2011). Indeed, school-aged children with ADHD commonly exhibit deficits in controlled behavior including difficulties with inhibition and delay aversion (Sonuga-Barke, Bitsakou, & Thompson, 2010), that are supported by circuits link specific regions of the frontal lobes to subcortical structures (in particular, the caudate), that supply modality-specific mechanisms for interaction with the environment, and provide the framework for understanding the neurobiological substrate of ADHD in childhood.
The present findings highlight insights gained by examining early brain development among children with ADHD, and how findings among younger children may differ from those observed in older children. To date, the preponderance of neuroimaging studies of children with ADHD have included those ages 6 years and older. The emphasis on school-age children is likely, in part, practical. MRI scanning with preschoolers is challenging, as is the diagnosis of ADHD. Nevertheless, using systematic behavioral desensitization and training procedures such as those employed in the present study, MRI studies of younger children are clearly possible without sedation, in a majority of children.
Strengths of the study include the careful characterization of the sample using methods adapted from the PATS studies (Kollins et al., 2006), as well as the exclusion of comorbid conditions that might confound the interpretation of data specific to ADHD, and the simultaneous examination of cortical and subcortical regions. At the same time, the strict entry criteria limit the generalization of results to young children with ADHD who present with a wider range of comorbidities (i.e., those more likely to visit psychiatry clinics in the preschool years). The absence of IQ score reductions (relative to controls) commonly observed in samples of children with ADHD (Jepsen, Fegerlund & Mortensen, 2009) may be an artifact of the strict sampling criteria used in the current study, and may have contributed to the relative lack of cortical reductions observed in our ADHD group. On the other hand, the Jepsen et al. meta-analysis suggested that reduced IQ scores among individuals with ADHD relative to typically developing controls may be specifically related to attentional dysregulation and poor test-taking behavior rather than deficits in “intelligence,” per se. Also, the research highlighting lower IQ scores in ADHD samples has been conducted on ADHD samples of at least school age. It is less clear that these findings hold true in preschoolers, or when IQ is measured by the WPPSI-III rather than the WISC-IV.
An additional limitation is that teacher ratings were not available on all participants, in order to better capture the cross-situational impairment required for DSM-IV diagnosis of ADHD. Of note, collection of teacher ratings in samples of preschool children as young as age 4 years is a challenge, as many of these participants are not yet in school. In addition, the small sample size limited the exploration of a variety of brain-behavior relationships at this time. Further, while the largest effect sizes in this sample involved the caudate nucleus, several cortical regions showed moderate effect sizes that, given larger samples, may show statistically significant group differences in this age range. Questions remain regarding the early neurodevelopmental course of ADHD, and factors associated with better (and worse) outcome in children at risk as they reach school age. Continued examination of brain development in this age range with larger samples using available MRI technologies (anatomic, functional, diffusion, spectroscopy) is clearly warranted, especially examining development longitudinally. Indeed, the current findings represent the initial phase of an ongoing longitudinal study of cortical and subcortical development in preschool children who present with symptoms of ADHD. This type of design should allow for characterization of the early developmental changes in preschoolers that contribute to the behavioral phenotype, and ultimately the formal diagnosis of ADHD, as well as those factors (anatomic, cognitive, behavioral) that are associated with “protection from” the disorder.
In summary, reductions in regional subcortical brain volumes are observable in preschoolers with ADHD, with largest effects observed in caudate volumes. Anomalous basal ganglia development may play an important role in early development of ADHD. In particular, these preliminary findings suggest that early anomalous caudate development (more than cortical development) is associated with onset of ADHD symptoms in preschoolers.
Acknowledgements
A portion of this study was presented at the 2010 meeting of the American Academy of Clinical Neuropsychology (AACN) on June 17, 2010, in Chicago, Illinois. Supported by HD-24061 (Intellectual and Developmental Disabilities Research Center), R01MH078160, R01MH085328, the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program, UL1-RR025005, and the Johns Hopkins Brain Sciences Institute.
References
- Abikoff HB, Vitiello B, Riddle MA, Cunningham C, Greenhill LL, Swanson JM, et al. Methylphenidate effects on functional outcomes in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) Journal of Child & Adolescent Psychopharmacology. 2007;17:581–592. doi: 10.1089/cap.2007.0068. [DOI] [PubMed] [Google Scholar]
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. Journal of Neuroscience. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105:1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Armstrong MB, Nettleton SK. Attention deficit hyperactivity disorder and preschool children. Seminars in Speech Language. 2004;25:225–232. doi: 10.1055/s-2004-833670. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Reiss AL, Reader MJ, Singer HS, Brown JE, Denckla MB. Basal ganglia volumes in children with attention-deficit hyperactivity disorder. Journal of Child Neurology. 1996;11:112–115. doi: 10.1177/088307389601100210. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention Deficit Hyperactivity Disorder: A handbook for diagnosis and treatment. 3rd. ed. New York, NY: Guilford Press; 2006. [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. American Journal of Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Bush G. Neuroimaging of attention deficit hyperactivity disorder: can new imaging findings be integrated in clinical practice? Child & Adolescent Psychiatric Clinics of North America. 2008;17:385–404. doi: 10.1016/j.chc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Bussing R, Mason DM, Bell L, Porter P, Garvan C. Adolescent outcomes of childhood attention-deficit/hyperactivity disorder in a diverse community sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:595–605. doi: 10.1016/j.jaac.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Proal E, Haekzema E, Gispert J, Picado M, Moreno I, et al. Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;66:972–977. doi: 10.1016/j.biopsych.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Geidd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Geidd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sharp WS, Gottesman RF, Greenstein DK, Giedd JN, Rapoport JL. Anatomic brain abnormalities in monozygotic twins discordant for attention deficit hyperactivity disorder. American Journal of Psychiatry. 2003;160:1693–1696. doi: 10.1176/appi.ajp.160.9.1693. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners' Rating Scales - Revised. North Tonawanda, New York: Multi-Health Systems Inc.; 1997. [Google Scholar]
- Connor DF. Preschool attention deficit hyperactivity disorder: a review of prevalence, diagnosis, neurobiology, and stimulant treatment. Journal of Developmental & Behavorial Pediatrics. 2002;23:S1–S9. doi: 10.1097/00004703-200202001-00002. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: segmentation and cortical surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Cutting LE. Genetic Disorders with a High Incidence of Learning Disabilities. Learning Disabilities Research & Practice. 2004;19:131–132. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, McGoey KE, Eckert TL, VanBrakle J. Preschool children with attention-deficit/hyperactivity disorder: impairments in behavioral, social, and school functioning. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:508–515. doi: 10.1097/00004583-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Durston S, van Belle J, de Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Egger HL, Kondo D, Angold A. The epidemiology and diagnostic issues in preschool attention-deficit/hyperactivity disorder: A review. Infants and Young Children. 2006;19:109–122. [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76:615–621. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill LL, Muniz R, Ball RR, Levine A, Pestreich L, Jiang H. Efficacy and safety if dexmethylphenidate extended-release capsules in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:817–823. doi: 10.1097/01.chi.0000220847.41027.5d. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Posner K, Vaughan BS, Kratochvil CJ. Attention deficit hyperactivity disorder in preschool children. Child and Adolescent Psychiatric Clinics of North America. 2008;17:347–366. doi: 10.1016/j.chc.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Hagberg BS, Miniscalco C, Gillberg C. Clinic attenders with autism, attention-deficit/hyperactivity disorder: Cognitive profile at school age and its relationship to preschool indicators of language delay. Research in Developmental Disabilities. 2010;31:1–8. doi: 10.1016/j.ridd.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychology Bulletin. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Sami N, Fargeon S. Prospective follow-up of girls with attention deficit/hyperactivity disorder into adolescence: Evidence for continuing cross-domain impairment. Journal of Consulting and Clinical Psychology. 2006;74:489–499. doi: 10.1037/0022-006X.74.3.489. [DOI] [PubMed] [Google Scholar]
- Hsu JW, Lee LC, Chen RF, Yen CT, Chen YS, Tsai ML. Striatal volume changes in a rat model of childhood attention-deficit/hyperactivity disorder. Psychiatry Research. 2010;179:338–341. doi: 10.1016/j.psychres.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Annals of Neurology. 1999;46:755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jacobson LA, Ryan M, Martin RB, Ewen J, Mostosfky SH, Denckla MB, Mahone EM. Verbal working memory influences processing speed and reading fluency in ADHD. Child Neuropsychology. 2011 doi: 10.1080/09297049.2010.532204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen JRM, Fagerlund B, Mortensen EL. Do attention deficits influence IQ in children and adolescents with ADHD? Journal of Attention Disorders. 2009;12:551–562. doi: 10.1177/1087054708322996. [DOI] [PubMed] [Google Scholar]
- Kollins S, Greenhill L, Swanson J, Wigal S, Abikoff H, McCracken J, Bauzo A. Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1275–1283. doi: 10.1097/01.chi.0000235074.86919.dc. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Kipp H, Ehrhardt A, Lee SS, Willcutt EG, Hartung CM, Chronis A, Massetti G. Three-year predictive validity of DSM-IV attention deficit hyperactivity disorder in children diagnosed at 4–6 years. American Journal of Psychiatry. 2004;161:2014–2020. doi: 10.1176/appi.ajp.161.11.2014. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Stein MA, Loney J, Trapani C, Nugent K, Kipp H, Schmidt E, Lee S, Cale M, Gold E, Hartung CM, Willcutt E, Baumann B. Validity of DSM-IV attention deficit/hyperactivity disorder for younger children. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:695–702. doi: 10.1097/00004583-199807000-00008. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Owens EB, Hinshaw SP. Few preschool boys and girls with ADHD are well-adjusted during adolescence. Journal of Abnormal Child Psychology. 2007;36:373–383. doi: 10.1007/s10802-007-9184-6. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Haidar H, Huppi PS, Bassan H, Warfield SK, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116:844–850. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Fisher P, Luby JL. Young Child DISC-IV Research Draft: Diagnostic Interview Schedule for Children. New York, NY: Columbia University, Division of Children Psychiatry, Joy and William Ruane Center to Identify and Treat Mood Disorders; 1998. [Google Scholar]
- Lucas CP, Fisher P, Luby JL. Young Child DISC-IV: Diagnostic Interview Schedule for Children. New York, NY: Columbia University, Division of Children Psychiatry, Joy and William Ruane Center to Identify and Treat Mood Disorders; 2008. [Google Scholar]
- Mahone EM, Richardson ME, Crocetti D, O’Brien JW, Kaufmann WE, Denckla MB, et al. Manual MRI Parcellation of Frontal Lobe in Boys and Girls with ADHD; Paper presented at the Presented at the 15th Annual Meeting of the Organization for Human Brain Mapping; San Francisco, CA. 2009. [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Massetti GM, Lahey BB, Pelham WE, Loney J, Ehrhardt A, Lee SS, et al. Academic Achievement Over 8 Years Among Children Who Met Modified Criteria for Attention-deficit/Hyperactivity Disorder at 4–6 Years of Age. Journal of Abnormal Child Psychology. 2007;36:399–410. doi: 10.1007/s10802-007-9186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAulliffe M, Lalonde E, McGarry D, Gandler W, Csaky K, Trus B. Medical image processing, analysis and visualization in clinical research; Paper presented at the IEEE Symposium on computer-based medical systems; Bethesda, MD. July 2001.2001. [Google Scholar]
- McGee R, Partridge F, Williams S, Silva PA. A twelve-year follow-up of preschool hyperactive children. Journal of the American Academy of Child & Adolescent Psychiatry. 1991;30:224–232. doi: 10.1097/00004583-199103000-00010. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mostofsky S, Cooper K, Kates W, Denckla M, Kaufmann W. Smaller prefrontal and premotor volumes in boys with ADHD. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. NIH consensus statement: diagnosis and treatment of attention deficit hyperactivity disorder (ADHD) 1998;16:1–37. November 16-18. [PubMed] [Google Scholar]
- Posner K, Melvin GA, Murray DW, Gugga SS, Skrobala A, Cunningham C, Greenhill LL. Clinical presentation of attention-deficit/hyperactivity disorder in preschool children: The Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment (PATS) Study. Journal of Child and Adolescent Psychopharmacology. 2007;17:547–562. doi: 10.1089/cap.2007.0075. [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller M, et al. Basal ganglia volume and shape in children with ADHD. American Journal of Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta ME, Crocetti D, Clauss JA, Kraut MA, Mostofsky SH, Kaufmann WE. Manual MRI parcellation of the frontal lobe. Psychiatry Research. 2009;172:147–154. doi: 10.1016/j.pscychresns.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Experimental Brain Research. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS, Reiss AL, Brown JE, Aylward EH, Shih B, Chee E, et al. Volumetric MRI changes in basal ganglia of children with Tourette's syndrome. Neurology. 1993;43:859–861. doi: 10.1212/wnl.43.5.950. [DOI] [PubMed] [Google Scholar]
- Slifer KJ, Cataldo M, Gerson AC, Tucker C. Teaching movement control for neuro-imaging in children with acquired neuromuscular disorders post-head trauma; Paper presented at the 20th Annual Convention of the Association for Behavior Analysis; Atlanta, GA. 1994. [Google Scholar]
- Smidts DP, Oosterlaan J. How common are symptoms of ADHD in typically developing preschoolers? A study on prevalence rates and prenatal/demographic risk factors. Cortex. 2007;43:710–717. doi: 10.1016/s0010-9452(08)70500-8. [DOI] [PubMed] [Google Scholar]
- Soliva JC, Fauquet J, Bielsa A, Rovira M, Carmona S, Ramos-quiroga JA, et al. Quantitative MR analysis of caudate abnormalities in pediatric ADHD: proposal for a diagnostic test. Psychiatry Research: Neuroimaging. 2010;182:238–243. doi: 10.1016/j.pscychresns.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Bitsakou P, Thompson M. Beyond the dual pathway model: Evidence for the dissociation of timing, inhibitory control, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Halperin JM. Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: Potential targets for early intervention? Journal of Child Psychological Psychiatry. 2010;51:368–389. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Suskauer S, McNally MA, Crocetti D, Mostofsky SH. Correlations between callosal segmental circumference and measures of response control in children with ADHD and typically developing children; Paper presented at the Presented at the 15th Annual Meeting of the Organization for Human Brain Mapping; San Francisco, CA. 2009. [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, et al. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. Journal of Cognitive Neuroscience. 2008a;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar SG, Blankner JG, Pekar JJ, Denckla MB, et al. FMRI evidence for abnormalities in response selection in ADHD: differences in activation associated with response inhibition but not habitual motor response. Journal of Cognitive Neuroscience. 2008b;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Greenhill L, Wigal T, Kollins S, Stehli A, Davies M, et al. Stimulant-related reductions of growth rates in the PATS. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:1304–1313. doi: 10.1097/01.chi.0000235075.25038.5a. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2006;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Abikoff HB, Chuang SZ, Kollins SH, McCracken JT, Riddle MA, et al. Effectiveness of methylphenidate in the 10-month continuation phase of the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) Journal of Child & Adolescent Psychopharmacology. 2007;17:593–604. doi: 10.1089/cap.2007.0058. [DOI] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler Preschool and Primary Scale of Intelligence. Third Edition. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- Wiig E, Secord W, Semel E. Clinical evaluations of language fundamentals: preschool-second edition (CELF-P-2) San Antonio, TX: Psychological Corporation; 2004. [Google Scholar]
- Wilens TE, Biederman J, Brown S, Monuteaux M, Prince J, Spencer TJ. Patterns of psychopathology and dysfunction in clinically referred preschoolers. J Dev Behav Pediatr. 2002;23:S31–S36. doi: 10.1097/00004703-200202001-00006. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Human Brain Mapping. 2007;30:175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]