Abstract
Objective
We evaluated a new device designed to clean the endotracheal tube (ETT) in mechanically ventilated patients: the Mucus Shaver.
Design
Prospective, randomized trial.
Setting
University hospital intensive care unit.
Patients
We enrolled 24 patients, expected to remain ventilated for more than 72 hours.
Interventions
The Mucus Shaver is a concentric, inflatable catheter for the removal of mucus and secretions from the interior surface of the ETT. The Mucus Shaver is advanced to the distal ETT tip, inflated and subsequently withdrawn over a period of 3–5 seconds. Patients were prospectively randomized, within 2 hours of intubation, to receive standard ETT suctioning treatment or standard suctioning plus Mucus Shaver use, until extubation.
Measurements and Main Results
During the study period, demographic data, recent medical history, adverse events and staff evaluation of the Mucus Shaver were recorded. At extubation, each ETT was removed, cultured and analyzed by Scanning Electron Microscopy (SEM). 12 patients were assigned to the study group and 12 to the control group. No adverse events related to the use of the Mucus Shaver were observed. At extubation, only 1 ETT from the Mucus Shaver group was colonized, while in the control group, 10 ETTs were colonized (8% vs. 83%; p<0.001). SEM showed little secretions on the ETTs from the study group, while thick bacterial deposits were present on all the ETTs from the control group (p<0.001 by Fisher’s exact test, using a maximum biofilm thickness of 30 µm as cut-off). The nursing staff was satisfied by the overall safety, feasibility, and efficacy of the Mucus Shaver.
Conclusions
The Mucus Shaver is a safe, feasible and efficient device for ETT cleaning in the clinical setting. The Mucus Shaver is helpful in preventing ETT colonization by potentially harmful microorganisms.
Keywords: Endotracheal tube, secretion removal, endotracheal tube suctioning, endotracheal tube occlusion, Mucus Shaver, mechanical ventilation, bacterial biofilm, ventilator associated pneumonia
Introduction
An endotracheal tube (ETT) is generally required for the management of critically ill, mechanically ventilated patients. Patency of ETTs is commonly maintained by using suctioning catheters. Nevertheless, it has been shown that intraluminal volume loss due to the accumulation of secretions on the inner wall of ETTs is not prevented by standard suctioning treatment or by optimal humidification (1–4). This vicious process of secretion accumulation can lead to life threatening occlusion of the ETT. Yet, even partial occlusion of the ETT might be a cause of increased work of breathing, requiring higher level of ventilator support and difficulty in weaning, resulting in prolonged mechanical ventilation and ICU stay (1, 3). Furthermore, thick secretions on the walls of the ETT often serve as a source for continued aspiration of microorganisms into the lungs, leading to an increased incidence of infection (5–8). Shah et al. demonstrated that a greater degree of intraluminal ETT-narrowing caused by secretions is associated with longer duration of mechanical ventilation and higher incidence of ventilator-associated pneumonia (VAP) (1).
We developed a novel medical device, the Mucus Shaver, to more efficiently remove secretions from the inner lumen of ETTs in mechanically ventilated patients and therefore to prevent formation of the thick mucus-layer on the lumen of the ETT (9). We previously tested the Mucus Shaver in mechanically ventilated sheep. In this animal model, the Mucus Shaver was found to be easy to use and effective in removing secretions from the ETT lumen (10). In a subsequent study, the Mucus Shaver showed no bacterial colonization in the ventilator breathing circuit when silver-coated ETTs were used in sheep ventilated for 72 hours (11).
In this randomized trial, we assessed the efficacy, safety and feasibility of the Mucus Shaver in patients expected to be mechanically ventilated for more than 72 hours. We hypothesized that the use of the Mucus Shaver after suctioning, as compared to the standard care, a) would result in significant reduction of ETT colonization rate in the clinical setting, as suggested by previous evidence; b) would not result in significant changes in blood pressure, oxygen saturation or ventilatory parameters, requiring change of ventilator settings; c) would be easy to learn and positively evaluated by the nursing staff.
Materials|Methods
Study Location and Patients
We conducted a randomized, controlled clinical trial in the medical intensive care unit of the Department of Perioperative and Critical Care Medicine, San Gerardo Hospital, Monza, Italy. The study was approved by the San Gerardo Hospital (FWA00002213, IRB00002289) Institutional Review Board (IRB-510) and the NHLBI Institutional Review Board (NIH 04H/N290). The Italian Ministry of Health was similarly informed, as requested by EU regulation (93/42/EEC). Twenty-four patients were enrolled from November 2004 to April 2005. Written informed consent was obtained either from the subjects or, when incompetent, from their next of kin. Inclusion criteria were: a) age of 18 years or more; b) expected to remain mechanically ventilated for 72 hours or more; c) intubated orally with an ETT of 7.5 or 8.0 mm of internal diameter for less than 2 hours. Patients unable to tolerate short disconnections from the ventilator (e.g. hemodynamically unstable or presence of severe hypoxia: PaO2/FiO2 ≤200 at PEEP ≥5 cmH20) were excluded. Patients were then randomized into two study groups using a computer algorithm. Patients assigned to the control group (n=12) received routine ETT suctioning with a small disposable flexible plastic catheter every 6 hours. Patients assigned to the study group (n=12) received standard suctioning every 6 hours followed by one cleaning maneuver with the Mucus Shaver. Patients were treated accordingly to the randomization until extubation which was performed, when ordered, by the attending physician. A continuous heated humidification system was used during the entire period of intubation for all the patients.
The Mucus Shaver
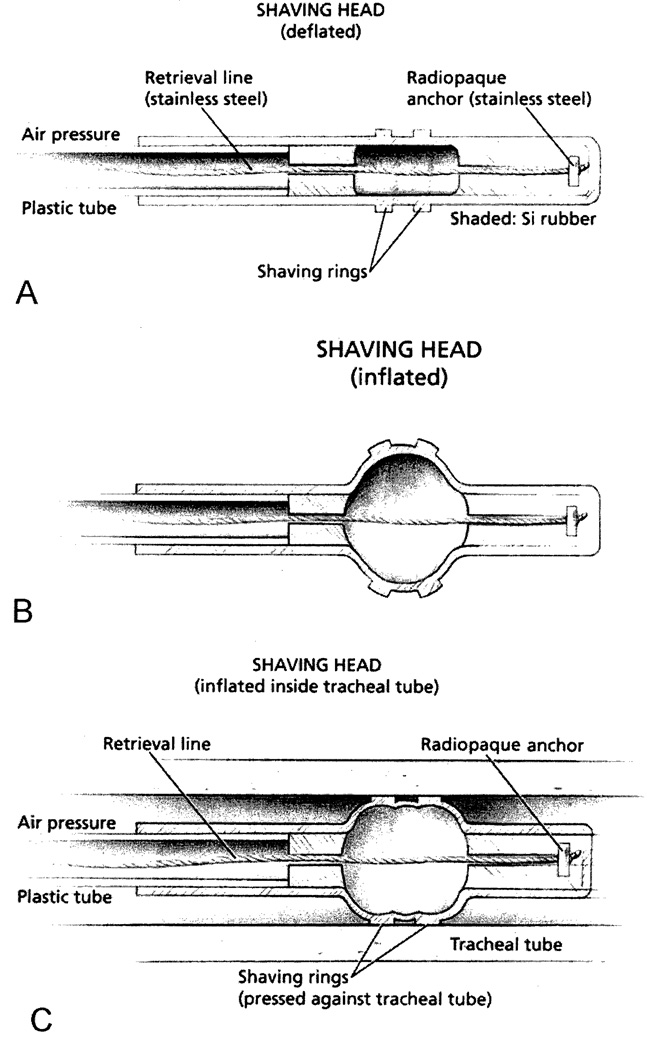
The Mucus Shaver was assembled as previously described (10). Briefly, we used a molded silicon rubber tube (GE CE-4524) 2.0 cm in length (3.5 mm I.D. and 4.5 mm O.D.) with two molded silicon rubber “shaving rings” attached, 1.0 mm wide and 0.5 mm thick, to provide the shaving edge against the ETT lumen. This structure was fastened to a 28 cm long plastic tube (2.0 mm I.D. and 3.0 mm O.D.), as illustrated in Figure 1A. As an additional safety feature, we incorporated a radio opaque stainless steel bead into the distal end of the device. The bead is securely attached to a fine braided stainless steel wire, to facilitate Mucus Shaver head retrieval in the unlikely event of detachment (Figure IB). Prior to use the Mucus Shaver was sent to the hospital sterilization facility for gas sterilization.
Figure 1. The “Mucus Shaver”.
(A) Schematic representation of the Mucus Shaver.
(B) Mucus Shaver inflation.
(C) Mucus Shaver inflated after being introduced into an endotracheal tube. Shaped areas = silicon rubber.
(Permission to reprint by Kolobow T, Anesthesiology 2005;102:1063-5).
In the present study, the Mucus Shaver was introduced through the connector piece of the ETT, until the stopper (designed to fit in the proper position for each ETT size) was against the proximal ETT tip, thus positioning the Mucus Shaver head just beyond the distal end of the ETT. The balloon was then manually inflated with 20 ml of air, through a syringe. In this way, the “shaving” polyurethane rings (and not the balloon) made contact with the inner lumen of the ETT (Figure 1C). The catheter was then withdrawn from the ETT over a period of 3 to 6 seconds along with all accumulated mucus. The Mucus Shaver was used only after standard suctioning, to avoid pushing large aggregates of secretions into the lungs.
Data collection
Age, sex, diagnosis, Simplified Acute Physiology Score 11(12) (SAPS II) and reason for intubation were collected at enrollment. Blood pressure, heart rate, pulse oximetry, and ventilatory settings were recorded continuously by an electronic chart monitoring system. Immediately after randomization, the lumens of the ETTs were brushed with a cotton swab that was sent to the microbiology laboratory for standard bacterial count to assess baseline bacterial colonization. Then, at extubation, ETTs were excised longitudinally into two halves for visual inspection and photographs. A section of the distal ETT was cut (28–29 cm from the ETT connector piece), fixed in 2.5% glutaraldehyde solution, stored at 4°C, and sent for Scanning Electron Microscopy (SEM) at the Laboratoire de Microscopie Electronique, University of Reims, INSERM, France. Secretions from the lumen of the ETT were collected, placed in a sterile vial, and delivered to the microbiology laboratory for quantitative bacteriological analyses.
Major adverse events possibly associated with use of the Mucus Shaver (e.g. desaturation, tachycardia, hypotension, ETT displacement requiring reintubation, tracheal injury evidenced by suctioning of bloodstained secretions, pneumothorax, need of ventilatory settings change after maneuver) were recorded.
The number of days of intubation, VAP incidence diagnosed by bronchoalveolar fluids cultures as previously described (13) and mortality after 28 days from enrollment were recorded for all study patients.
Scanning Electron Microscopy (SEM)
The ETT sections preserved in 2.5% glutaraldehyde solution were dehydrated in graded alcohol and then treated with 50% - 50 % Hexamethyldisilazane (HMDS) (EM Sciences, Fort Washington, PA, USA), followed by 100% HMDS treatment and air-drying. Next the samples were sputter-coated (MED 010, Balzers Union) with 15nm of gold and examined using a Hitachi S-4500 scanning electron microscope equipped with a cold cathode field emission gun at an accelerating voltage of 1.0 kV. All images were recordings using the secondary electron detector. Micrographs were recorded on a Personal Computer using quartz cards.
Nursing staff evaluation of Mucus Shaver use
Certified Critical Care Registered Nurses (CC-RN) were trained in the use of Mucus Shaver during a 1 hour course and were assisted by one of the investigators for the first three shaving maneuvers; they then started using the Mucus Shaver without supervision. Trained CC-RNs were able to perform the suctioning and the shaving maneuver while disconnecting the patient from the ventilator in less than 10 seconds.
To evaluate the feasibility and efficacy of the Mucus Shaver, we administered a standardized questionnaire to the Certified Critical Care Registered Nurses (CC-RN) who used the device during the study. CC-RNs were asked to assign a score (1-worst to 10-best scale) on 3 clinical issues: efficacy in cleaning the ETT of the Mucus Shaver, safety of the new maneuver, and feasibility for future ICU implementation of the Mucus Shaver.
Statistical analysis
This study was designed to show efficacy and safety of the Mucus Shaver in a clinical setting. The primary endpoint was the reduction of bacterial colonization of the ETT lumen in the Mucus Shaver group. Based on prior animal studies, we hypothesized a 20% incidence of ETT colonization in the treatment group and 80% colonization in the control group. We calculated that a sample size of 12 patients per group would provide power > 80 % to detect a, reduction in ETT colonization in the Mucus Shaver group with a 1 sided significance level α=0.05. Statistical analyses were performed using Stata statistical package 8.0 (Stata Corporation, College Station, TX, USA). Continuous measurements are expressed as mean +/− SD. Comparisons of the mean values of continuous variables between the two study groups were performed using Student’s T test. Comparisons of non-normally distributed variables were performed using the Wilcoxon rank sum test. Ratios and proportions among different classes were compared using the chi-square test or Fisher’s exact test, as appropriate. P values less than 0.05 were considered significant. Data are reported as means ± standard deviations, unless otherwise indicated.
Results
Demographics and clinical outcomes
Twenty-four mechanically ventilated patients (n=24) with Pao2/Fio2 >200 at PEEP 5, who were expected to remain intubated for more than 72 hours were enrolled; no patient was subsequently excluded, and none withdrew consent. Twelve patients were randomly assigned to the standard ETT cleaning care group (n = 12, control group), while twelve were treated with the Mucus Shaver in addition to standard suctioning (n = 12, Mucus Shaver group).
Baseline characteristics of the study population are shown in Table 1. No significant differences in demographics between the two study groups were recorded. No tube occlusion occurred during study period; the ETTs in the treatment groups appeared free from secretions (Figure 2). No tube change was needed due to partial obstruction. Additionally, there were no differences between the two groups in VAP incidence, days of intubation, and mortality at 28 days after enrollment between the 2 groups (Table 2).
Table 1.
Baseline characteristics of the study population
| Characteristic | Mucus Shaver Group n=12 |
Control Group n=12 |
p-value |
|---|---|---|---|
| Age, mean (SD) | 53 (19) | 49 (22) | 0.61 |
| Female Sex, No. (%) | 6 (50) | 6 (50) | 1.00 |
| SAPS II, mean (SD) | 43 (15) | 36 (13) | 0.34 |
| Hospital admission category. No. (%) | 0.63 | ||
| Medical | 7 (58) | 9 (75) | |
| Elective surgery | 1 (8) | 1 (8) | |
| Emergency surgery | 4 (33) | 2 (17) | |
| Primary Reason for Intubation, No.(%) | 0.92 | ||
| Acute respiratory failure | 4 (33) | 3 (25) | |
| Cardiovascular failure | 1 (8) | 2 (17) | |
| Neurological failure | 2 (17) | 2 (17) | |
| Trauma | 2 (17) | 1 (8) | |
| Sepsis | 3 (25) | 4 (33) |
Percentages may not sum to 100 because of rounding.
Abbreviations: SAPS II, Simplified Acute Physiologic Score II (12)
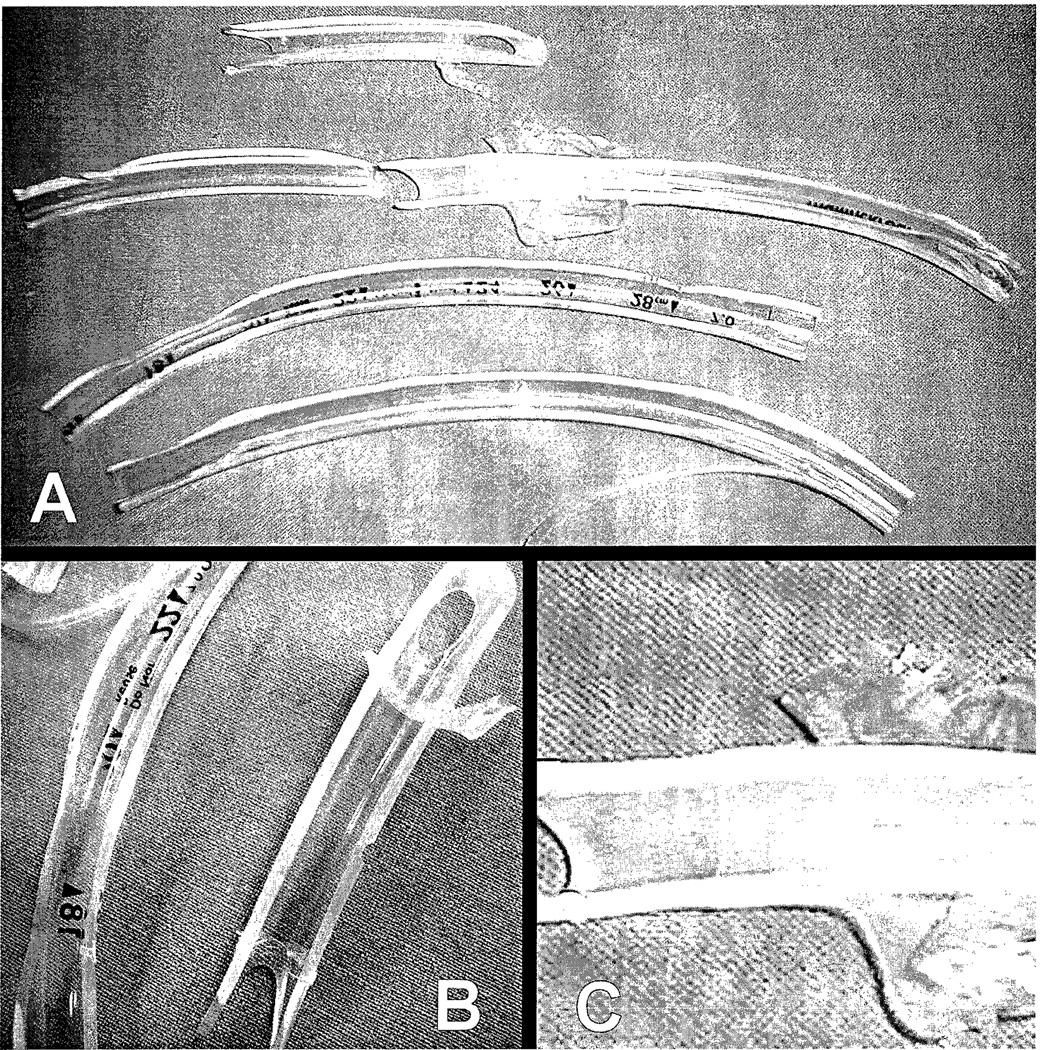
Figure 2. Appearance of an acute occlusion of the ETT lumen.
(A) Appearance of the inner lumen of an ETT after extubation from patients in the Mucus Shaver group intubated for 4 to 19 days. Note the absence of any mucus or secretion deposit. Note that even at the tip of the ETT, no secretions are apparent.
(B) and (C) Higher magnification of ETTs in picture (A).
Table 2.
Microbiology, clinical findings, and outcomes
| Microbiology and Clinical Findings | Mucus Shaver Group (n = 12) | Control Group (n = 12) | p |
|---|---|---|---|
| Microbiology | |||
| ETT at intubation, No. (%) positive | 0 (0) | 0 (0) | 1.00 |
| cultures | |||
| ETT at extubation, No. (%) positive | 1 (8) | 10 (83) | <.001 |
| cultures | |||
| ETT biofilm thickness range (minimum- maximum) at extubation, No. (%) |
<.001a | ||
| 0–5 µm | 7 (58) | 0 | |
| 0–10 µm | 4 (33) | 0 | |
| 0–30 µm | 1 (8) | 0 | |
| 50–150 µm | 0 | 3 (25) | |
| 50–300 µm | 0 | 5 (41) | |
| 100–400 µm | 0 | 3 (25) | |
| 150–500 µm | 0 | 1 (8) | |
| Ventilator-associated pneumonia, confirmed by bronchoalveolar lavage, No. (%) |
1 (8) | 3 (25) | .59 |
| Days of intubation, median value (range) | 6 (4–19) | 6 (3–14) | .78 |
| Mortality at day 28, No. (%) | 2 (17) | 2 (17) | 1.00 |
ETT, endotracheal tube
Comparison of the two groups was performed using Fisher exact test and a maximum biofilm thickness of 30 µm as cut-off.
Microbiology
At enrollment, all ETTs in both groups were sterile. At extubation, 8 out of 12 ETTs were colonized in the control group (range between 104 and 107 CFU/ml) while only one of the ETTs from the Mucus Shaver group was colonized (83% vs. 8%, p<0.001) (Table 2). The most common aerobic bacteria colonizing the lumen of the ETTs was Pseudomonas aeruginosa. In addition, Methicillin-resistant Staphylococcus aureus (MRSA), Neisseriae species, Escherichio coli, Haemophilus influenzae, Candida albicans and Aspergillus flavus were only found in the ETT lumens of the control group (Table 3).
Table 3.
Characteristics of microorganisms found on ETT at extubation
| Microorganism | Patients affected No. (%) |
Growth (median) Cfu/ml |
Multi-drug Resistant No. (%) |
|---|---|---|---|
| P. aeruginosa | 4 (17) | 105 | 4 (100) |
| MRSA | 2 (67) | 105 | 2 (100) |
| N. species | 1 (8) | 106 | 0 (0) |
| E. coli | 1 (8) | 104 | 1 (100) |
| H. influenzae | 1 (8) | 104 | 1 (100) |
| C. albicans | 1 (8) | 105 | 1 (100) |
| A. flavus | 1 (8) | 104 | 1 (100) |
In the control group eight out of twelve ETTs were colonized with Pseudomonas aeruginosa (P.aeruginosa), Methicillin-resistant Staphylococcus aureus (MRSA), Neisseriae species (N.species), Escherichia coli (E. coli), Haemophilus influenzae [H. influenzae), Candida albicans (C albicans) and Aspergillus flavus [A. flavus). One of the ETTs from the Mucus Shaver group was colonized with P. aeruginosa.
Safety
The Mucus Shaver was used in each of the 12 patients in the study group for a total of 104 days of intubation (median 6 days, range 4–19 days). No major adverse events related to the use of the Mucus Shaver (e.g. tube displacement requiring reintubation, tracheal injury evidenced by bloodstained secretions or delayed extubation, pneumothorax) were observed after nearly 400 maneuvers performed by nursing staff. In addition, no intervention attributable to the Mucus Shaver use was required because of significant changes in blood pressure, oxygen saturation or ventilatory parameters.
Microscopy
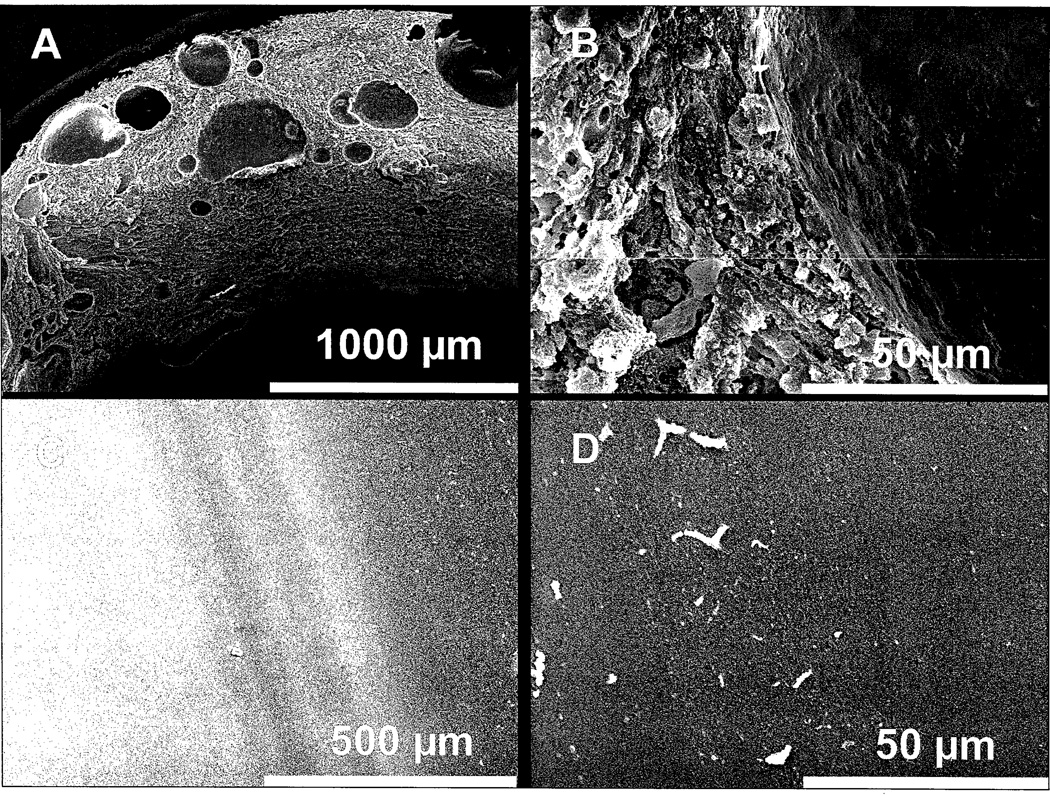
Using SEM, we measured the range of biofilm thickness for each of the ETTs (Figure 3). Biofilm thickness was nonhomogeneous varying according to the site of measurement. Treatment with the Mucus Shaver was associated with few secretion deposits as assessed by SEM (p<0.001 by Fisher’s exact test, using a maximum biofilm thickness of 30 µm as cut-off, Table 2). All the ETTs cleaned with the Mucus Shaver showed absence of biofilm at least in one of the measurement sites, while none of the controls did (p<0.001 by Fisher’s exact test).
Figure 3. Scanning electron microscopy of ETT lumen at extubation.
(A) and (B): Electron micrograph of the ETT lumen in a patient group in which the Mucus Shaver was not used (control group). Note the thick, continuous deposit of secretions on the lumen of the endotracheal tube containing cells. (C) and (D): Electron micrograph of the inner surface of an ETT where Mucus Shaver was used. Few small drops (few to 20 µm) are noted on the ETT lumen.
Nursing staff evaluation of the use of the Mucus Shaver
We collected questionnaires from 13 CC-RNs who took care of patients in the Mucus Shaver group throughout the study period. Overall, CC-RNs were satisfied with the Mucus Shaver safety (median 8, range 5–10) and feasibility (median 8, range 4–9). They found the Mucus shaver more efficient in cleaning the tube compared to standard suctioning (median 9, range 6–10 vs. median 5, range 2–8, p<0.001, Table 4).
Table 4.
Nurses' evaluations of the Mucus Shaver safety, feasibility, and efficacy
| Item Evaluated (1–10 Score) | Response, Median (Range) | p |
|---|---|---|
| Safety of Mucus Shaver use | 8 (5–10) | |
| Feasibility of Mucus Shaver maneuver | 8 (4–9) | |
| Efficacy in cleaning tube | <.001a | |
| Standard suctioning | 5 (2–8) | |
| Mucus Shaver | 9 (6–10) |
Menn-Whitney test is used to compare the efficacy of the two treatments.
Discussion
This study evaluated in a clinical setting the use of the Mucus Shaver, a tool built to remove endoluminal secretions from ETTs. Twenty-four intubated and mechanically ventilated patients were enrolled and randomized to receive standard ETT cleaning solely with a commercially available, flexible suctioning catheter or with the addition of the Mucus Shaver. Patients treated with the Mucus Shaver had less bacterial colonization of the ETT lumen (p<0.001), trivial biofilm development (p<0.01) and lower mucus secretion accumulation than the control group. However, there was no difference in terms of number of intubation days or bacterial pneumonia among the two groups. No major adverse events occurred due to the use of the Mucus Shaver. The nursing staff found the Mucus Shaver to be easy to use and efficient in removing ETT secretions.
In the end of 2003, Kolobow designed and fabricated the Mucus Shaver in a laboratory at the National Institutes of Health, Bethesda Maryland (10). At that time, we conducted studies in an animal model which showed that secretions continuous drain outward from the respiratory tract by muco-ciliary clearance and gravity (14,15). We concluded from those studies on secretion clearance that orientation of the ETT, humidification of the gas flow, cleaning of the ETT and avoidance of biofilm formation in the ETT lumen were critical to avoid colonization of the lower airways and occlusion of the plastic tube (16).
Within hours following tracheal intubation, a bacterial biofilm forms on the polyvinyl-chloride ETT and the respiratory tubing which represents a significant obstruction to respiratory airflow and a source of bacterial inoculation of the lungs. Fragments of the ETT biofilm can detach spontaneously or become dislodged and enter the lungs with the inspiratory gas flow (17–19). Interestingly, the bacterial secretions within the ETT are resistant to antibacterial agents because immune defenses do not reach the inner lumen of the tube, and bacteria may organize in dense multibacterial plaques promoting multidrug resistance, changing gene expression, morphology, and phenotype, according to the pH differences and oxygen deprivation (20–23). Simultaneously, the thickened debris in the ETT lumen hampers the respiratory airflow due to significant alterations in the ETT configuration (2). Despite best practices in respiratory care and medical treatment, the ETT may entrap a fair amount of secretions and microbes, which can cause intraluminal narrowing of the ETT and an increase in airway resistance (24), leading to increased work of breathing and prolonged weaning from the ventilator (1, 3). Standard suctioning of tracheal secretions or bronchoscopy may delay, but not prevent, the accumulation of bacterial secretions inside the ETT, which may prolong weaning protocols, increase VAP incidence and ICU stay (2, 25, 26). Moreover, secretions may abruptly obstruct the ETT, resulting in an acute life-threatening condition requiring emergent re-intubation(27).
The Mucus Shaver aimed to address these problems, and in 2004, we tested the Mucus Shaver in sheep endotracheally intubated and ventilated for a period of 72 hours (10). No technical difficulties were encountered and all the ETTs cleaned with the Mucus Shaver were free of visible secretions. We also appreciated a decrease in peak inspiratory pressure in the Mucus Shaver group as well as absence of biofilm or proteinaceous material on scanning electron microscopy in the lumen of the ETT cleaned by the Shaver, compared with extensive biofilm formation in the control group. In 2004, we repeated the study in sheep intubated with silver sulfadiazine-coated ETTs and ventilated for up to 168 hours. We showed that antimicrobial coated ETTs, when meticulously cleaned with the Mucus Shaver, retained throughout the study excellent bactericidal properties preventing deposit of secretions and bacterial colonization.
In recent years, several researchers and companies have focused their efforts on improving artificial airways with novel designs (e.g.: antimicrobial ETT, ETT cuffs), aiming to decrease infection rate and improve outcome of mechanically ventilated patients (28). The Rescue Cath device has been proposed to remove mucus plugs in case of ETT obstruction (29), but no data are available about prevention of biofilm formation. The Mucus Slurper is effective in maintaining the ETT free from secretions (30), but has been studied only on animal models. To our knowledge, the Mucus Shaver, as presented in this trial, is the first device that shows, in a clinical setting, efficacy, safety and feasibility of removing secretions from the ETT.
There were some limitations to this study. First, a small number of patients (n=24) were recruited and blinding was not provided, due to the nature of the device; a “placebo” effect on biofilm formation is unlikely. Moreover, the study was not powered to detect differences in VAP rates or to evaluate the impact of the device on weaning and time on the ventilator. This trial was designed to assess the safety and efficacy of this novel medical device to address the common and unsolved problem of secretion accumulation and biofilm formation in intubated patients in the ICU. Additional studies in larger populations are needed to validate our findings and determine whether the use of Mucus Shaver decreases the incidence of VAP, reduces work of breathing and time on ventilator and finally, improves patient outcomes. Secondly, we used the Mucus Shaver in 7.5 or 8.0 mm internal diameter ETT, because these are the ETT sizes used in our ICU for adults. The device is functional in smaller ETTs, but clinical data are lacking. Finally, the prototype of Mucus Shaver tested in this study requires disconnection of the ventilator for a period of time longer than standard suctioning. A closed system suction catheter with a built-in Mucus Shaver may be an appropriate solution to avoid disconnection from the ventilator while cleaning the ETT in patients requiring high PEEP and high Fio2.
In conclusion, we propose the Mucus Shaver as a device able to improve care of mechanically ventilated patients by (a) reducing bacterial colonization of the inner ETT lumen, suggesting a potential role in infection prevention; (b) reducing secretion deposits, preventing possible partial or complete ETT occlusion, leading to increased airway resistance and work of breathing.
Acknowledgments
We thank Jessica Hines, staff assistant at the Massachusetts General Hospital Surgical Intensive Care Unit, for reviewing, preparing and coordinating the submission of this manuscript. We thank Hui Zheng, Assistant Professor, Biostatistics Center, Massachusetts General Hospital, Harvard Medical School, for statistical review.
Financial support:
Financial support was provided by the National Heart, Lung, and Blood Institute, Division of International Research, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland, USA and by the Department of Perioperative Medicine and Intensive Care, San Gerardo Hospital, Monza, Italy. JM and TK were supported by the Intramural Research Program, National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda, Maryland, USA.
Footnotes
This study was performed in the Intensive Care Unit of the Department of Perioperative Medicine and Intensive Care at San Gerardo Hospital, Monza, Italy, while microscopy was performed at the Electron Microscopy Laboratory of the University of Reims, Reims, France.
References
- 1.Shah C, Kollef MH. Endotracheal tube intraluminal volume loss among mechanically ventilated patients. Crit Care Med. 2004;32:120–125. doi: 10.1097/01.CCM.0000104205.96219.D6. [DOI] [PubMed] [Google Scholar]
- 2.Villafane MC, Cinnella G, Lofaso F, et al. Gradual reduction of endotracheal tube diameter during mechanical ventilation via different humidification devices. Anesthesiology. 1996;85:1341–1349. doi: 10.1097/00000542-199612000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Boque MC, Gualis B, Sandiumenge A, et al. Endotracheal tube intraluminal diameter narrowing after mechanical ventilation: use of acoustic reflectometry. Intensive Care Med. 2004;30:2204–2209. doi: 10.1007/s00134-004-2465-4. [DOI] [PubMed] [Google Scholar]
- 4.Jaber S, Pigeot J, Fodil R, et al. Long-term effects of different humidification systems on endotracheal tube patency: evaluation by the acoustic reflection method. Anesthesiology. 2004;100:782–788. doi: 10.1097/00000542-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Sottile FD, Marrie TJ, Prough DS, et al. Nosocomial pulmonary infection: possible etiologic significance of bacterial adhesion to endotracheal tubes. Crit Care Med. 1986;14:265–270. [PubMed] [Google Scholar]
- 6.Feldman C, Kassel M, Cantrell J, et al. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur Respir J. 1999;13:546–551. doi: 10.1183/09031936.99.13354699. [DOI] [PubMed] [Google Scholar]
- 7.Adair CG, Gorman SP, Feron BM, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25:1072–1076. doi: 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- 8.Inglis TJ, Millar MR, Jones JG, et al. Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol. 1989;27:2014–2018. doi: 10.1128/jcm.27.9.2014-2018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolobow T, Berra L. United States Patent 7,051,737 May 30, 2006 [Google Scholar]
- 10.Kolobow T, Berra L, Li Bassi G, et al. Novel system for complete removal of secretions within the endotracheal tube: the Mucus Shaver. Anesthesiology. 2005;102:1063–1065. doi: 10.1097/00000542-200505000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Berra L, Curto F, Li Bassi G, et al. Antibacterial-coated tracheal tubes cleaned with the Mucus Shaver: a novel method to retain long-term bactericidal activity of coated tracheal tubes. Intensive Care Med. 2006;32:888–893. doi: 10.1007/s00134-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 12.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 13.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 14.Bassi GL, Zanella A, Cressoni M, et al. Following tracheal intubation, mucus flow is reversed in the semirecumbent position: possible role in the pathogenesis of ventilator-associated pneumonia. Crit Care Med. 2008;36:518–525. doi: 10.1097/01.CCM.0000299741.32078.E9. [DOI] [PubMed] [Google Scholar]
- 15.Trawoger R, Kolobow T, Cereda M, et al. Tracheal mucus velocity remains normal in healthy sheep intubated with a new endotracheal tube with a novel laryngeal seal. Anesthesiology. 1997;86:1140–1144. doi: 10.1097/00000542-199705000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Berra L, Sampson J, Fumagalli J, et al. Alternative approaches to ventilator-associated pneumonia prevention. Minerva Anestesiol. 2011;77:323–333. [PubMed] [Google Scholar]
- 17.Craven DE, Connolly MG, Jr, Lichtenberg DA, et al. Contamination of mechanical ventilators with tubing changes every 24 or 48 hours. N Engl J Med. 1982;306:1505–1509. doi: 10.1056/NEJM198206243062501. [DOI] [PubMed] [Google Scholar]
- 18.Christopher KL, Saravolatz LD, Bush TL, et al. The potential role of respiratory therapy equipment in cross infection. A study using a canine model for pneumonia. Am Rev Respir Dis. 1983;128:271–275. doi: 10.1164/arrd.1983.128.2.271. [DOI] [PubMed] [Google Scholar]
- 19.Berra L, De Marchi L, Yu ZX, et al. Endotracheal tubes coated with antiseptics decrease bacterial colonization of the ventilator circuits, lungs, and endotracheal tube. Anesthesiology. 2004;100:1446–1456. doi: 10.1097/00000542-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Suci PA, Mittelman MW, Yu FP, et al. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 1994;38:2125–2133. doi: 10.1128/aac.38.9.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whiteley M, Bangera MG, Bumgarner RE, et al. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413:860–864. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- 22.Steinberger RE, Allen AR, Hansa HG, et al. Elongation correlates with nutrient deprivation in Pseudomonas aeruginosa-unsaturates biofilms. Microb Ecol. 2002;43:416–423. doi: 10.1007/s00248-001-1063-z. [DOI] [PubMed] [Google Scholar]
- 23.Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416:740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 24.Rumbak MJ, Walsh FW, Anderson WM, et al. Significant tracheal obstruction causing failure to wean in patients requiring prolonged mechanical ventilation: a forgotten complication of long-term mechanical ventilation. Chest. 1999;115:1092–1095. doi: 10.1378/chest.115.4.1092. [DOI] [PubMed] [Google Scholar]
- 25.Lorente L, Blot S, Rello J. New issues and controversies in the prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2010;182:870–876. doi: 10.1164/rccm.201001-0081CI. [DOI] [PubMed] [Google Scholar]
- 26.Wilson AM, Gray DM, Thomas JG. Increases in endotracheal tube resistance are unpredictable relative to duration of intubation. Chest. 2009;136:1006–1013. doi: 10.1378/chest.08-1938. [DOI] [PubMed] [Google Scholar]
- 27.Pingleton SK. Complications of acute respiratory failure. Am Rev Respir Dis. 1988;137:1463–1493. doi: 10.1164/ajrccm/137.6.1463. [DOI] [PubMed] [Google Scholar]
- 28.Coppadoro A, Berra L, Bigatello LM. Modifying endotracheal tubes to prevent ventilator-associated pneumonia. Curr Opin Infect Dis. 2011;24:157–162. doi: 10.1097/QCO.0b013e328343b733. [DOI] [PubMed] [Google Scholar]
- 29.Stone B, Bricknell S. A New Method for Removing Mucous Plugs From an Obstructed Endotracheal Tube. Respir Care. 2011;56:520–522. doi: 10.4187/respcare.00642. [DOI] [PubMed] [Google Scholar]
- 30.Kolobow T, Li Bassi G, Curto F, et al. The Mucus Slurper: A novel tracheal tube that requires no tracheal tube suctioning. A preliminary report. Intensive Care Med. 2006;32:1414–1418. doi: 10.1007/s00134-006-0268-5. [DOI] [PubMed] [Google Scholar]