Abstract
We report a new type of microarray, based on glyconanoparticles (GNPs), to study glycan-lectin interactions. GNPs, synthesized by conjugating carbohydrate ligands on silica nanoparticles, were printed on a photoactive surface followed by covalent immobilization by light activation. The GNP microarrays could be probed by lectins labeled with fluorescein as well as fluorescein-doped silica nanoparticles (FSNPs). Results showed that FSNP as the label enhanced the signals for the higher affinity ligands than the lower ones.
Microarrays, which allow simultaneous interrogation of ligand-receptor interactions in high throughput and at low sample consumption, have significantly increased the efficiencies of bioassays and greatly facilitated the fundamental studies as well as the development of therapeutic and diagnostic tools.1–5 Carbohydrate microarrays,6–15 for example, have been widely used to study glycan-lectin interactions which play an important role in a variety of biological processes, including cell-cell communications, immune responses, and the progression of tumor cells. Carbohydrate microarrays are generally fabricated by spotting and immobilizing glycans on a solid surface either by physical adsorption or covalent coupling.9,16–19 The arrays are then probed with lectins and the affinity of the interactions presented as a physical readout, for example, fluorescence intensity when a fluorescently-tagged lectin is used.
It has been shown that glycans immobilized on surfaces with proper ligand presentation can significantly amplify their binding affinities to lectins.15,20–21 In these cases, the solid surface acts as the multivalent scaffold having multiple copies of ligands when interacting with lectins. On flat surfaces, however, the affinity reaches a limit when the ligand density becomes saturated.15,22 Efforts have been devoted to fabricate 3D glycan surfaces to increase the ligand density.15 For example, Bertozzi and co-workers fabricated glycopolymer microarrays where glycans were covalently attached to a polymer scaffold.23 Pieters et al. fabricated glycodendrimer arrays and studied the binding affinity to lectins with regard to multivalency.24 Miura and coworkers presented dendritic sugar microarrays where glycodendrimers were immobilzed on gold substrate via the click chemistry.25 Gildersleeve and coworkers used bovine serum albumin (BSA)-glycan conjugates to generate arrays and evaluated the affinity to lectins as a function of glycan density.26 Rubina et al. presented a 3D hydrogel glycan microarray where saccharides were immobilized inside a porous polymer gel.27
Here we report a new type of glycan microarray based on glyconanoparticles. The GNP microarrays are fabricated by printing GNPs on a photoactive surface followed by covalent immobilization by light activation. The GNP microarrays were then probed with lectins labeled with fluorescein (FITC) or FITC-doped silica nanoparticles.
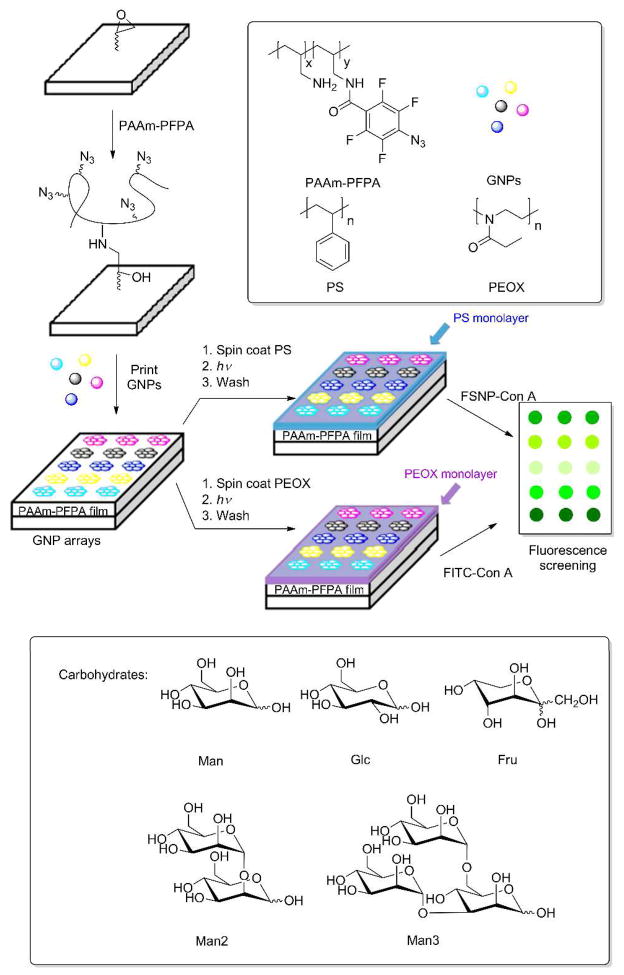
GNPs (diameter 118 nm, see Figure S1, Supporting Information) were synthesized following a previously reported procedure.28 Five carbohydrates, 3,6-di-O-(α-D-mannopyranosyl)-D-mannopyranose (Man 3), 2-O-α-D-mannopyranosyl-D-mannopyranose (Man 2), D-mannose (Man), D-glucose (Glc), and D-fructose (Fru) (Scheme 1) were conjugated onto silica nanoparticles using the photocoupling chemistry developed in our laboratory.11 Briefly, silica nanopartilces prepared by the Stöber method were functionalized with perfluorophenyl azide (PFPA), and carbohydrates were then coupled to the nanoparticles via the CH insertion reaction of the perfluorophenylnitrene generated by photoactivation of PFPA (see Supporting Information for experimental details).29–31 The densities of the immobilized carbohydrates were determined using the colorimetric assay of anthrone/sulfuric acid, from which the coupling yields were also calculated (see Table S1, Supporting Information for results and calculations).
Scheme 1.
Preparation of GNP microarrays and interactions with FSNP-Con A or FITC-Con A.
GNP microarrays were fabricated using the same photocoupling chemistry, however, poly(allyl amine) (PAAm)-PFPA was used in this case (Scheme 1). The polymer matrix is necessary as it ensures the conformal contact with solid materials such as nanoparticles, which is critical in the immobilization of solid materials.32 The process was carried out as shown in Scheme 1 (see Supporting Information for detailed experimental procedures). The epoxy-functionalized wafers33 were first treated with PAAm-PFPA,32 and aqueous solutions of GNPs were then printed on the PAAm-PFPA-functionalized wafers using a robotic printer. A polymer film, polystyrene (PS) or poly(2-ethyl-2-oxazoline) (PEOX), was subsequently introduced by spin-coating the polymer from its chloroform solution. Chloroform was used since GNPs were insoluble and therefore the printed GNPs would remain intact. Subsequent light activation covalently attached both GNPs and the polymer via the surface PFPA (Scheme 1). GNP microarrays were obtained after excess GNPs and the polymer were removed by sonication in chloroform followed by water.
To test the optimal array conditions, Man-conjugated GNPs (Man-GNPs) were printed with varying particle concentrations. The microarray was then treated with Concanavalin A (Con A), a plant lectin that binds specifically to carbohydrates having α-Man and α-Glc structures.34 In the present studies, Con A was labeled with either FSNPs (FSNP-Con A) or FITC (FITC-Con A) to facilitate the visualization of the interactions. FSNP-Con A was prepared by treating FSNPs35 with 3-glycidyloxytrimethoxysilane followed by Con A (see Supporting Information for experimental details). To reduce the non-specific adsorption of the labeled Con A on the microarray, polymer thin films were used as the background coatings. Hydrophilic polymers such as PEOX were highly effective in resisting the adsorption of proteins including FITC-Con A.36–37 For FSNPs, however, the result was the opposite. FNSPs adsorbed strongly on hydrophilic polymers whereas minimal adsorption was observed on hydrophobic polymers such as PS.
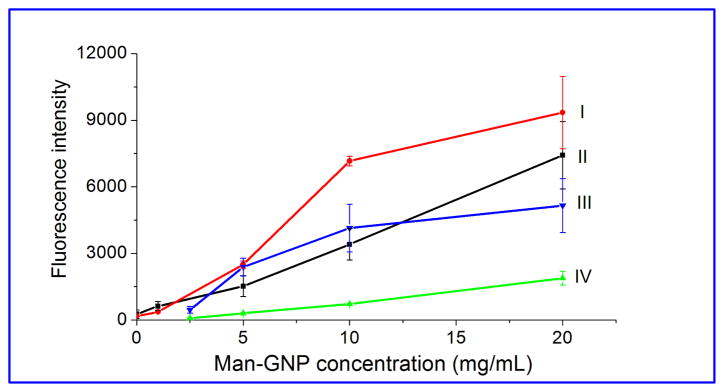
After the Man-GNP microarrays were treated with FSNP-Con A or FITC-Con A, fluorescence signals were clearly visible (Figure S2a, Supporting Information). Additionally, the fluorescence intensity increased with increasing Man-GNP concentration (I, II, Figure 1). Because the photocoupling chemistry occurs at the interface between surface PFPA and GNPs, in principle, only a monolayer of GNPs should result. The binding would therefore saturate and the fluorescence intensity would plateau. However, the signal continued to increase with the Man-GNP printing concentration. A closer examination of the arrayed spots by AFM revealed that the particle density increased with the GNP printing concentration. At 20 mg/mL, agglomerated particles were clearly visible (Figure S3, Supporting Information). The agglomeration increased the GNP density and therefore the binding signals.
Figure 1.
Fluorescence intensity of Man-GNP microarrays probed with FSNP-Con A (I) or FITC-Con A (II). In III and IV, no polymer coating was used and the Man-GNP microarrays were incubated in BSA before treating with FSNP-Con A (III) or FITC-Con A (IV). The data points were connected with lines to aid visualization.
To evaluate the effectiveness of PS and PEOX in reducing the non-specific adsorption, Man-GNP microarrays without the polymer coating were incubated with BSA, a commonly used blocking agent in microarray assays, before they were treated with FSNP-Con A or FITC-Con A. In both cases, lower signals were obtained at all GNP concentrations in comparison to those with the polymer coating (III vs. I, and IV vs. II, Figure 1). Especially at higher GNP concentrations of 10 mg/mL or above, the signals were significantly higher on arrays when the polymer coating was used. The enhanced array sensitivity resulted from the markedly reduced background noises when PS (for FSNP-Con A) or PEOX (for FITC-Con A)36 was used as the antifouling coating. In addition, the signals from FSNP-Con A were higher than those from FITC-Con A. This is consistent with the results from others38–41 and our previous study35 that FSNPs exhibit high fluorescence intensity by entrapping multiple dye molecules inside the silica nanoparticles.
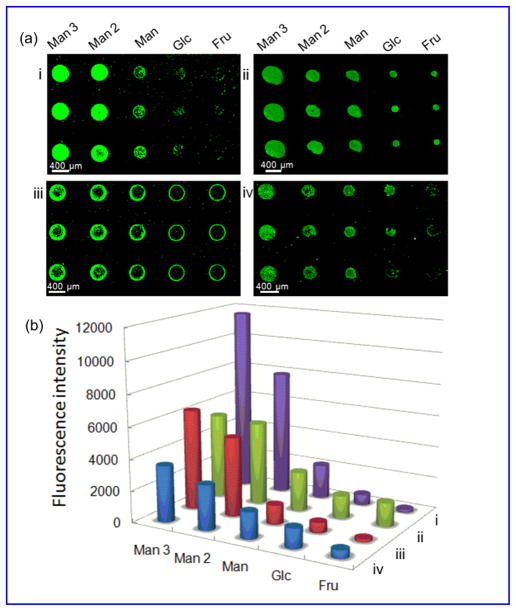
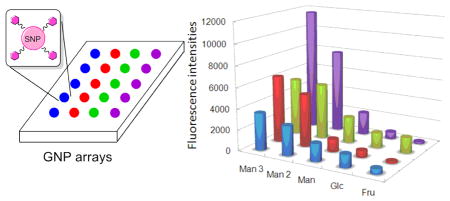
GNP microarrays were next prepared following the procedures and conditions developed above. Five different GNPs were printed on PAAm-PFPA surface at 10 mg/mL particle concentration. PS was then spin-coated and the GNP microarray was obtained after excess GNPs and the polymer were removed by sonication in chloroform followed by water. Figure 2i is the fluorescence image of the GNP microarrays after treating with FSNP-Con A. Man3-GNP showed the highest fluorescence intensity followed by Man2-GNP and Man-GNP. Only weak fluorescence was detected from Glc-GNP, and for Fru-GNP, the signal was minimal. These results are consistent with the affinity rank order of the corresponding free ligands with Con A (Table S2, Supporting Information).42–43
Figure 2.
(a) Fluorescence images of GNP microarrays probed with FSNP-Con A (i) or FITC-Con A (iii), and carbohydrate microarrays probed with FSNP-Con A (ii) or FITC-Con A (iv). (b) Fluorescence intensity of the four arrays in (a). Each data point was the average of the 3 spots in (a), and the error bars were omitted for clarity.
GNP microarrays were then compared with carbohydrate microarrays that were prepared by printing the corresponding free carbohydrate ligands on the PAAm-PFPA surface. Spin-coating PS followed by UV irradiation and solvent extraction gave the carbohydrate microarray. The array was then treated with FSNP-Con A. It can be clearly seen that the signals for the higher affinity ligands Man 3 and Man 2 were higher on the GNP microarray (Figure 2i) than those on the carbohydrate microarray (Figure 2ii). Since the background coating (PS) and the probe (FSNP-Con A) were the same for both arrays, the signal enhancement should come from GNPs as compared to the carbohydrate ligands.44–46 For the weaker ligands Man and Glc, on the other hand, the signals were lower on the GNP microarray (Figure 2i) than those on the carbohydrate microarray (Figure 2ii). This selective affinity amplification for high affinity ligands was observed previously on Au NPs21,47–48 as well as on dendrimers49 and neoglycopolymers.50 Note that Fru, a non-binding ligand, showed a relatively high fluorescence on the carbohydrate microarray (Figure 2ii). This could be due to the non-specific adsorption of FSNP-Con A nanoparticles on the hydrophilic Fru surface.
The GNP and carbohydrate microarrays were further evaluated using FITC-Con A. In this case, PEOX was used as the polymer coating to reduce the non-specific adsorption of FITC-Con A. The overall signal intensities were lower in this case (Figure 2iii) than when FSNP-Con A was used (Figure 2iv), similar to the result shown in Figure 1. Again, GNP microarray displayed higher signals for Man 3 and Man 2, and lower signals for Man and Glc (Figure 2iii) than the carbohydrate microarray (Figure 2iv). Also, higher residual fluorescence was seen for the Fru spots on carbohydrate microarray (Figure 2iv).
CONCLUSIONS
In summary, a new microarray platform, based on glyconanoparticles, was developed to study glycan-lectin interactions. The GNP microarrays were fabricated using a general photocoupling chemistry whereby the printed GNPs were covalently immobilized on the substrates with high efficiency. The GNP microarrays, when probed with FNSP-labeled lectin, gave higher signals as compared to FITC-labeled lectin or carbohydrate microarrays. In addition, the signal enhancement was greater for higher affinity ligands than for the lower affinity ones. This new GNP microarray platform should find a wide range of applications in bioanalysis facilitating the development of new diagnostic and therapeutic tools.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of General Medical Science (NIGMS) under NIH Award Numbers R01GM080295 and 2R15GM066279.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Allison DB, Cui XQ, Page GP, Sabripour M. Nat Rev Genet. 2006;7:55–65. doi: 10.1038/nrg1749. [DOI] [PubMed] [Google Scholar]
- 2.Hoheisel JD. Nat Rev Genet. 2006;7:200–210. doi: 10.1038/nrg1809. [DOI] [PubMed] [Google Scholar]
- 3.Kingsmore SF. Nat Rev Drug Discov. 2006;5:310–320. doi: 10.1038/nrd2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupuy A, Simon RM. J Natl Cancer Inst. 2007;99:147–157. doi: 10.1093/jnci/djk018. [DOI] [PubMed] [Google Scholar]
- 5.LaFratta CN, Walt DR. Chem Rev. 2008;108:614–637. doi: 10.1021/cr0681142. [DOI] [PubMed] [Google Scholar]
- 6.Houseman BT, Mrksich M. Chem Biol. 2002;9:443–454. doi: 10.1016/s1074-5521(02)00124-2. [DOI] [PubMed] [Google Scholar]
- 7.Feizi T, Fazio F, Chai WC, Wong CH. Curr Opin Struct Biol. 2003;13:637–645. doi: 10.1016/j.sbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez RA, Blixt O. Glycobiology. Vol. 415. Elsevier Academic Press Inc; San Diego: 2006. pp. 292–310. [Google Scholar]
- 9.de Paz JL, Seeberger PH. QSAR Comb Sci. 2006;25:1027–1032. [Google Scholar]
- 10.Paulson JC, Blixt O, Collins BE. Nat Chem Biol. 2006;2:238–248. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- 11.Stevens J, Blixt O, Paulson JC, Wilson IA. Nat Rev Microbiol. 2006;4:857–864. doi: 10.1038/nrmicro1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent N, Voglmeir J, Flitsch SL. Chem Commun. 2008:4400–4412. doi: 10.1039/b806983m. [DOI] [PubMed] [Google Scholar]
- 13.Liang PH, Wu CY, Greenberg WA, Wong CH. Curr Opin Chem Biol. 2008;12:86–92. doi: 10.1016/j.cbpa.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Palma AS, Feizi T. Biol Chem. 2009;390:647–656. doi: 10.1515/BC.2009.071. [DOI] [PubMed] [Google Scholar]
- 15.Oyelaran O, Gildersleeve JC. Curr Opin Chem Biol. 2009;13:406–413. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Liu S, Shah D, Wang D. Methods Mol Biol. 2005;310:241–252. doi: 10.1007/978-1-59259-948-6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia B, Kawar ZS, Ju T, Alvarez RA, Sachdev GP, Cummings RD. Nat Methods. 2005;2:845–850. doi: 10.1038/nmeth808. [DOI] [PubMed] [Google Scholar]
- 18.Shin I, Tae J, Park S. Curr Chem Biol. 2007;1:187–199. [Google Scholar]
- 19.Wang X, Liu LH, Ramström O, Yan M. Exp Biol Med. 2009;234:1128–1139. doi: 10.3181/0904-MR-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S, Lee MR, Shin I. Nat Protoc. 2007;2:2747–2758. doi: 10.1038/nprot.2007.373. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Ramström O, Yan M. Anal Chem. 2010;82:9082–9089. doi: 10.1021/ac102114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyukova VI, Shilova NV, Galanina OE, Rubina AY, Bovin NV. Biochim Biophys Acta-Gen Subj. 2006;1760:603–609. doi: 10.1016/j.bbagen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Godula K, Bertozzi CR. J Am Chem Soc. 2010;132:9963–9965. doi: 10.1021/ja103009d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branderhorst HM, Ruijtenbeek R, Liskamp RMJ, Pieters RJ. Chembiochem. 2008;9:1836–1844. doi: 10.1002/cbic.200800195. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda T, Onogi S, Miura Y. Thin Solid Films. 2009;518:880–888. [Google Scholar]
- 26.Oyelaran O, Li Q, Farnsworth D, Gildersleeve JC. J Proteome Res. 2009;8:3529–3538. doi: 10.1021/pr9002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyukova VI, Dementieva EI, Zubtsov DA, Galanina OE, Bovin NV, Rubina AY. Anal Biochem. 2005;347:94–105. doi: 10.1016/j.ab.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Ramström O, Yan M. J Mater Chem. 2009;19:8944–8949. doi: 10.1039/B917900C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu LH, Yan M. Acc Chem Res. 2010;43:1434–1443. doi: 10.1021/ar100066t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norberg O, Deng L, Aastrup T, Yan M, Ramström O. Anal Chem. doi: 10.1021/ac102781u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HB, Zhang YM, Yuan X, Chen Y, Yan M. 2010;83:1000–1007. [Google Scholar]; Bioconjugate Chem. 2011;22:26–32. doi: 10.1021/bc100251f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo T, Wang X, Tong Q, Yan M. Langmuir. 2011;27:9372–9378. doi: 10.1021/la201324h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsukruk VV, Luzinov I, Julthongpiput D. Langmuir. 1999;15:3029–3032. [Google Scholar]
- 34.Loris R, Hamelryck T, Bouckaert J, Wyns L. BBA-PROTEIN STRUCT M. 1998;1383:9–36. doi: 10.1016/s0167-4838(97)00182-9. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Ramström O, Yan M. Chem Commun. 2011;47:4261–4263. doi: 10.1039/c0cc05299j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Ren J, Hlaing A, Yan M. J Colloid and Interface Sci. 2011;354:160–167. doi: 10.1016/j.jcis.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Li LL, Tong Q, Yan M. Acs Appl Mater Interface. 2011;3:3463–3471. doi: 10.1021/am200690s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santra S, Dutta D, Walter GA, Moudgil BM. Technol Cancer Res Treat. 2005;4:593–602. doi: 10.1177/153303460500400603. [DOI] [PubMed] [Google Scholar]
- 39.Sharrna P, Brown S, Walter G, Santra S, Moudgil B. Adv Colloid Interface Sci. 2006;123:471–485. doi: 10.1016/j.cis.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Yao G, Wang L, Wu YR, Smith J, Xu JS, Zhao WJ, Lee EJ, Tan WH. Anal Bioanal Chem. 2006;385:518–524. doi: 10.1007/s00216-006-0452-z. [DOI] [PubMed] [Google Scholar]
- 41.Yan JL, Estevez MC, Smith JE, Wang KM, He XX, Wang L, Tan WH. Nano Today. 2007;2:44–50. [Google Scholar]
- 42.Schwarz FP, Puri KD, Bhat RG, Surolia A. J Biol Chem. 1993;268:7668–7677. [PubMed] [Google Scholar]
- 43.Mandal DK, Kishore N, Brewer CF. Biochemistry. 1994;33:1149–1156. doi: 10.1021/bi00171a014. [DOI] [PubMed] [Google Scholar]
- 44.Lin CC, Yeh YC, Yang CY, Chen GF, Chen YC, Wu YC, Chen CC. Chem Commun. 2003:2920–2921. doi: 10.1039/b308995a. [DOI] [PubMed] [Google Scholar]
- 45.Tsai CS, Yu TB, Chen CT. Chem Commun. 2005:4273–4275. doi: 10.1039/b507237a. [DOI] [PubMed] [Google Scholar]
- 46.Lyu YK, Lim KR, Lee BY, Kim KS, Lee WY. Chem Commun. 2008:4771–4773. doi: 10.1039/b807438k. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Matei E, Deng L, Ramström O, Gronenborn A, Yan M. Chem Commun. 2011 doi: 10.1039/c1cc12981c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Ramstrom O, Yan M. Adv Mater. 2010;22:1946–1953. doi: 10.1002/adma.200903908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolfenden ML, Cloninger MJ. J Am Chem Soc. 2005;127:12168–12169. doi: 10.1021/ja053008n. [DOI] [PubMed] [Google Scholar]
- 50.Mortell KH, Weatherman RV, Kiessling LL. J Am Chem Soc. 1996;118:2297–2298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.